Abstract
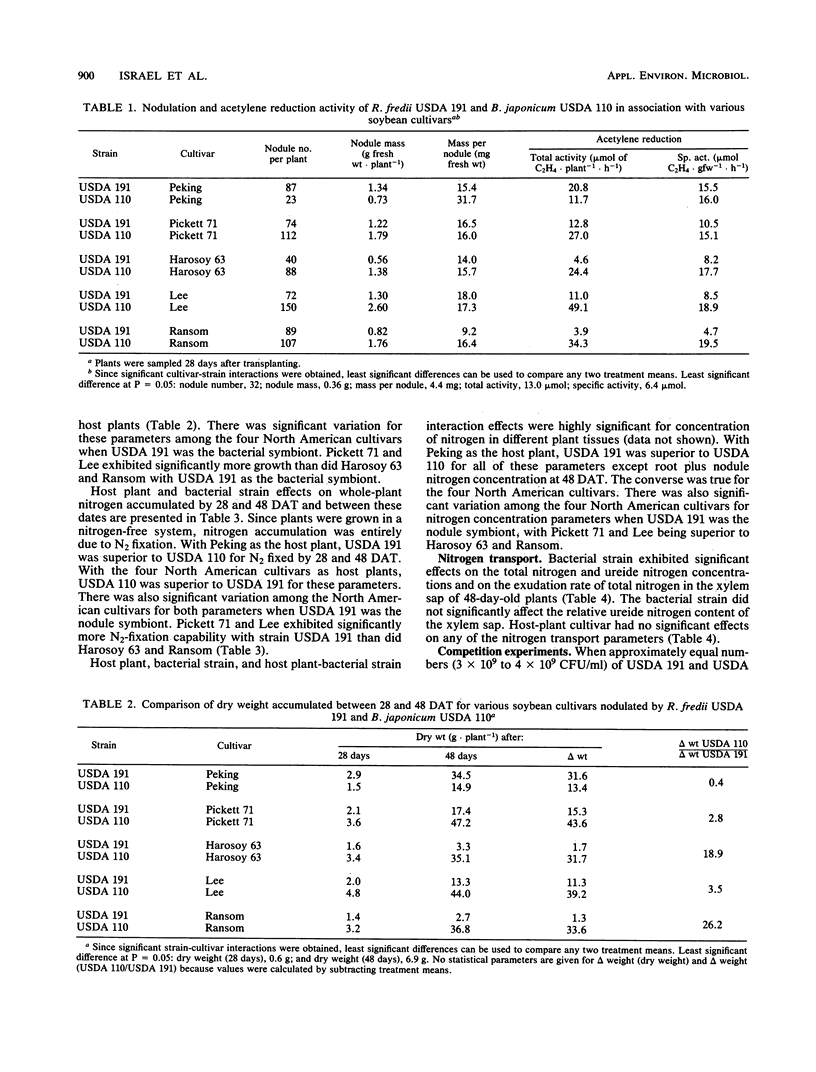
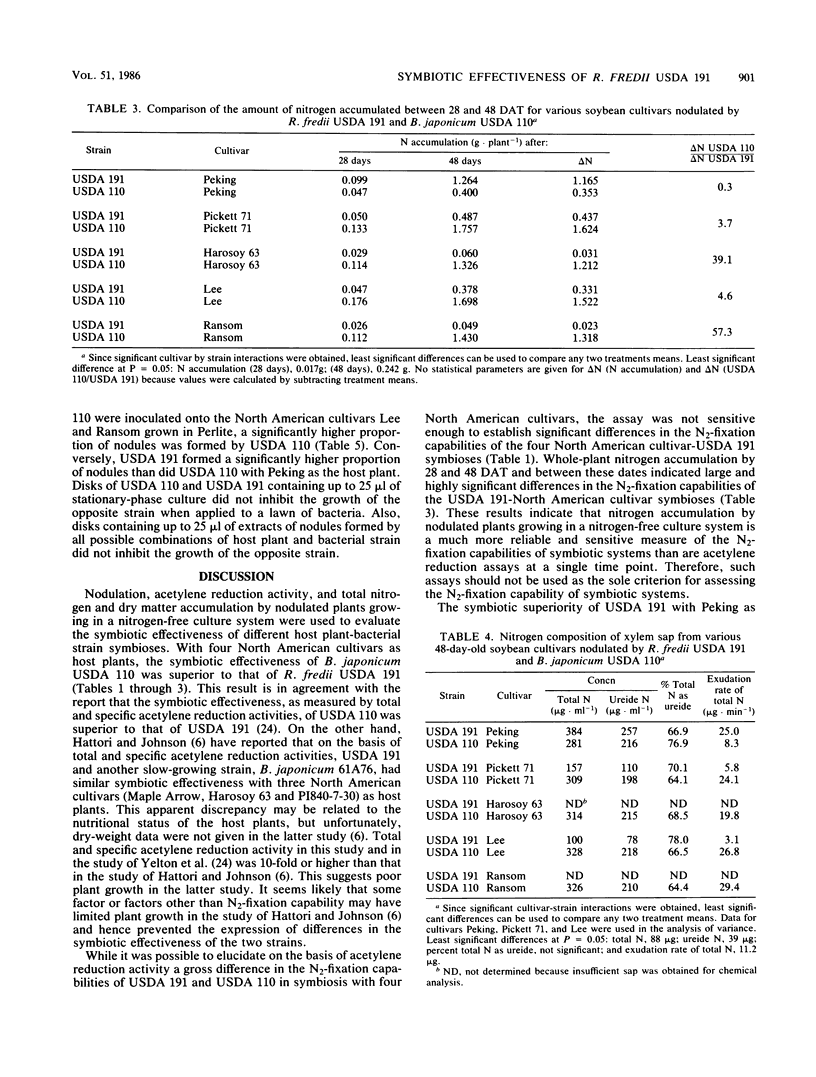
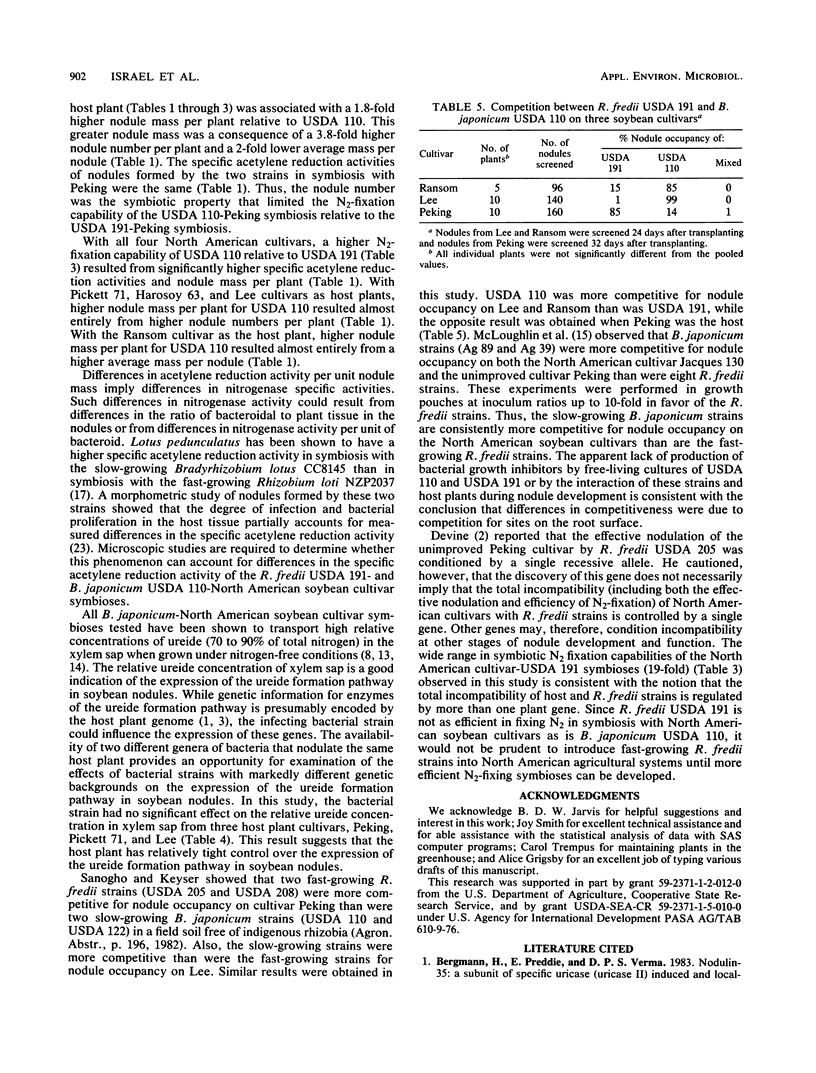
Nodulation, acetylene reduction activity, dry matter accumulation, and total nitrogen accumulation by nodulated plants growing in a nitrogen-free culture system were used to compare the symbiotic effectiveness of the fast-growing Rhizobium fredii USDA 191 with that of the slow-growing Bradyrhizobium japonicum USDA 110 in symbiosis with five soybean (Glycine max (L.) Merr.) cultivars. Measurement of the amount of nitrogen accumulated during a 20-day period of vegetative growth (28 to 48 days after transplanting) showed that USDA 110 fixed 3.7, 39.1, 4.6, and 57.3 times more N2 than did USDA 191 with cultivars Pickett 71, Harosoy 63, Lee, and Ransom as host plants, respectively. With the unimproved Peking cultivar as the host plant, USDA 191 fixed 3.3 times more N2 than did the USDA 110 during the 20-day period. The superior N2 fixation capability of USDA 110 with the four North American cultivars as hosts resulted primarily from higher nitrogenase activity per unit nodule mass (specific acetylene reduction activity) and higher nodule mass per plant. The higher N2-fixation capability of USDA 191 with the Peking cultivar as host resulted primarily from higher nodule mass per plant, which was associated with higher nodule numbers. There was significant variation in the N2-fixation capabilities of the four North American cultivar-USDA 191 symbioses. Pickett 71 and Lee cultivars fixed significantly more N2 in symbiosis with USDA 191 than did the Harosoy 63 and Ransom cultivars. This quantitative variation in N2-fixation capability suggests that the total incompatibility (effectiveness of nodulation and efficiency of N2 fixation) of host soybean plants and R. fredii strains is regulated by more than one host plant gene. These results indicate that it would not be prudent to introduce R. fredii strains into North American agricultural systems until more efficient N2-fixing symbioses between North American cultivars and these fast-growing strains can be developed. When inoculum containing equal numbers of USDA 191 and of strain USDA 110 was applied to the unimproved Peking cultivar in Perlite pot culture, 85% of the 160 nodules tested were occupied by USDA 191. With Lee and Ransom cultivars, 99 and 85% of 140 and 96 nodules tested, respectively, were occupied by USDA 110.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F., Künstner P. W., Nguyen T., Verma D. P. Soybean nodulin genes: Analysis of cDNA clones reveals several major tissue-specific sequences in nitrogen-fixing root nodules. Proc Natl Acad Sci U S A. 1983 May;80(9):2594–2598. doi: 10.1073/pnas.80.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori J., Johnson D. A. Fast-Growing Rhizobium japonicum That Effectively Nodulates Several Commercial Glycine max L. Merrill Cultivars. Appl Environ Microbiol. 1984 Jul;48(1):234–235. doi: 10.1128/aem.48.1.234-235.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. W., Jackson W. A. Ion balance, uptake, and transport processes in n(2)-fixing and nitrate- and urea-dependent soybean plants. Plant Physiol. 1982 Jan;69(1):171–178. doi: 10.1104/pp.69.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- Kuykendall L. D., Elkan G. H. Rhizobium japonicum derivatives differing in nitrogen-fixing efficiency and carbohydrate utilization. Appl Environ Microbiol. 1976 Oct;32(4):511–519. doi: 10.1128/aem.32.4.511-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis J. N., Barbour W. M., Elkan G. H. Effect of Sym Plasmid Curing on Symbiotic Effectiveness in Rhizobium fredii. Appl Environ Microbiol. 1985 Jun;49(6):1385–1388. doi: 10.1128/aem.49.6.1385-1388.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis J. N., Kuykendall L. D., Elkan G. H. Restriction Endonuclease and nif Homology Patterns of Bradyrhizobium japonicum USDA 110 Derivatives With and Without Nitrogen Fixation Competence. Appl Environ Microbiol. 1986 Mar;51(3):477–480. doi: 10.1128/aem.51.3.477-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W., Volk R. J. Evaluation of the Relative Ureide Content of Xylem Sap as an Indicator of N(2) Fixation in Soybeans: GREENHOUSE STUDIES. Plant Physiol. 1980 Oct;66(4):720–725. doi: 10.1104/pp.66.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloger C. Symbiotic effectiveness and n(2) fixation in nodulated soybean. Plant Physiol. 1969 Dec;44(12):1666–1668. doi: 10.1104/pp.44.12.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]


