Summary
Introduction
Reperfusion results in a proinflammatory cytokine response, as has been observed following resuscitation from cardiac arrest. Variations in the inflammatory response have been shown to be gender dependent and mediated by steroid hormones. The purpose of this study was to determine whether the tumour necrosis factor-alpha response following resuscitation was gender dependent.
Methods
Anaesthetized swine (15 males and 15 females, weights 32-47 kg) underwent 7 min of electrically induced cardiac arrest, followed by conventional resuscitation and then measurement of tumour necrosis factor-alpha by enzyme-linked immunosorbent assay at intervals for up to 3h. Testosterone and 17-estradiol were measured in 8 males and 8 females.
Results
In all animals 17-estradiol was undetectable. Testosterone exceeded the lower limit of detection in 3 females and 1 male. Levels of tumour necrosis factor-alpha were higher in males than females, from 30 min after resuscitation to 3 h. In females, tumour necrosis factor-alpha levels were significantly higher than control values only at 15 min following restoration of circulation; the levels in males demonstrated marked inter-animal variation.
Conclusions
In this swine model, males demonstrated an augmented post-resuscitation tumour necrosis factor-α response when compared with females. This difference was not related to steroid hormone levels.
Keywords: Cardiac arrest, Cardiopulmonary resuscitation, Inflammatory response, Gender
Introduction
Following oxidant injury as in cardiac arrest and resuscitation, activation of characteristic metabolic cascades responsible for reperfusion injury, and the accompanying inflammatory response, are to be expected.1,2 The latter is likely to play a role in the multiorgan failure that also commonly follows resuscitation from cardiac arrest.3 Increases in proinflammatory cytokines and soluble receptors have been reported among humans hours after resuscitation from cardiac arrest, and detectable plasma levels of endotoxin appear within days, presumably due to gut translocation.4 An association between ischaemia, reperfusion and the innate inflammatory response has been suggested, and non-survivors have greater cytokine elevations at some time following resuscitation than survivors.5 Similarly, cytokine levels have been shown to be predictive of post-resuscitation neurological function among the clinical population.6-8 Laboratory investigations confirm that activation of proinflammatory cytokine pathways is evident early after resuscitation from cardiac arrest.9,10
Studies in animal models, primarily rodents, and in the clinical population, including both healthy and injured people, suggest that there are gender differences in the cytokine response to stress.11-16 In animal models, males appear to be more likely to develop sepsis than females and females have a lower mortality rates than males. These differences are generally ascribed to 17-estradiol. Clinical studies have yielded conflicting data but, in general, support gender differences in the proinflammatory response. To our knowledge, gender differences in the ‘sepsis-like’ syndrome following cardiac arrest and resuscitation have not been assessed. Sample sizes in clinical observations studies have been small, with a predominance of males; generally any females included have been older and postmenopausal.
Domestic swine have become the standard or established large animal model for the study of cardiac arrest and resuscitation. Swine used are typically 12-16 weeks of age, weigh 30-45 kg and are often stated to be immature. Domestic swine do not become sexually mature until approximately 10-12 months of age. Steroid hormone levels in the usual resuscitation swine model have not been previously reported, nor has the impact of steroid hormone levels on the stress response to cardiac arrest and resuscitation/reperfusion been evaluated. There are no published studies demonstrating a difference in outcome between males and females in the resuscitation laboratory setting.
The purpose of this study was to evaluate the proinflammatory cytokine response, involving specifically tumour necrosis factor-α (TNF- α), to cardiac arrest and resuscitation, and determine if whether this response differs between male and female swine. Testosterone and 17-estradiol were measured in study animals to determine whether these steroid hormones had a role in such differences.
Methods
This investigation was approved by the Animal Care and Utilization Review Committee of our institution and adheres to the American Physiological Society's Guiding Principles in the Care and Use of Animals.
Domestic swine of both genders (15 males, mean weight 41 ± 4 kg, and 15 females, mean weight 39 ± 5 kg) were premedicated with ketamine (20 mg/kg) and xylazine (2 mg/kg). General anaesthesia was induced with isoflurane via nose cone and, following endotracheal intubation, was maintained with inhaled isoflurane (MAC 1.0-2.5%) and nitrous oxide in a 1:1 mixture with oxygen. End-tidal carbon dioxide was continuously monitored using a side-stream capnometer (LifePak 12, PhysioControl, Redmond, WA) and minute ventilation was adjusted to maintain a value of 35-45 mmHg. Standard lead II of the surface electrocardiogram was monitored continuously.
Under fluoroscopic guidance, high-fidelity, micromanometer-tipped catheters (Millar Instruments, Houston, TX) were positioned in the ascending aorta via a femoral artery and in the right atrium via a jugular vein. A standard 7F bipolar pacing catheter was placed in the jugular vein and advanced to the apex of the right ventricle. Haemodynamic data were recorded and stored in a laptop computer using PowerLab Chart v. 5.2 (ADInstruments, Castle Hill, Australia). Standard adhesive defibrillation electrode patches (Quick-Combo) were applied to the left and right lateral aspects of the thorax. Transthoracic impedance was measured using a tetrapolar constant current impedance measuring system (THRIM®, Morro Bay, CA). A non-inductive resistor (30 Ω) was then placed in series with the impedance compensating truncated exponential biphasic defibrillation waveform defibrillator (LifePak 12, PhysioControl, Redmond, WA).
Following instrumentation, ventricular failure (VF) was induced electrically by passing 60 Hz of AC current for approximately 0.5 s through the electrodes of a bipolar catheter positioned in the right ventricular apex. After 7 min of untreated VF, manual closed-chest compressions were begun with the animal in the supine position, and were administered at a rate of approximately 100/min with force sufficient to depress the sternum 3.5-5.0 cm; 1 min after starting chest compressions, a transthoracic countershock at 200 J was given. If VF persisted, additional shocks in an escalating energy sequence (300 J, 360J) were administered. Successful defibrillation was defined as termination of VF, regardless of the post-shock cardiac rhythm or haemodynamic outcome, i.e. spontaneous QRS complexes with or without associated arterial pressure pulses, determined 5 s after a defibrillation shock.17 Chest compressions were performed between shocks and positive pressure ventilations (FiO2 = 1.00) were performed at a rate of 8-10 ventilations/min. If VF persisted, epinephrine was administered at doses of 0.5 mg 150 mg, cardiopulmonary resuscitation (CPR) continued and shocks were repeated until VF was terminated or for 15 min. If asystole or pulseless electrical activity (PEA) followed the shocks, CPR and additional epinephrine were administered until spontaneous arterial pressures of 60 mmHg appeared or for 15 min. At the end of 15 min of CPR, animals remaining in VF, PEA or asystole were considered resuscitation failures and resuscitative efforts were terminated.
In those animals achieving return of spontaneous circulation (ROSC), defined as an arterial systolic pressure >60 mmHg,18 haemodynamic and blood gas measurements were carried out at intervals for 3 h. Before electrical VF induction and at 15, 30, 60, 90, 120 and 180 min following ROSC, arterial blood was sampled, placed in sterile chilled (0°C) heparinised tubes and centrifuged at 5000 rpm for 10 min. Plasma was immediately separated and stored at -80°C until analysis. TNF-α concentrations were determined by quantitative sandwich enzyme-linked immunosorbent assay (ELISA) using a commercially available kit specific for porcine TNF-α (R&D Systems, Minneapolis, MN).
Plasma 17-estradiol was measured in 8 male and 8 female swine by direct radioimmunoassay with reagents from Diagnostic Systems Laboratories (Webster, TX). Plasma testosterone levels were also measured by a specific radioimmunoassay kit from Diagnostic Products (Los Angeles, CA). These assays were previously validated in the Harbor-UCLA Endocrine Research Laboratory.19-22 The lower limit of quantitation for 17-estradiol is 20 pg/ml and for testosterone is 0.025 ng/ml.
Data are presented as the mean ± standard deviation unless otherwise stated. For all comparisons, p<0.05 was considered statistically significant. TNF-α plasma concentrations in males and females at the selected time intervals were compared using the Mann-Whitney rank sum test. Differences over time within gender groups were determined using Friedman repeated measures ANOVA on ranks and Dunnett's test.
Results
Two males and two females could not be resuscitated. For resuscitated animals, there were no significant gender differences with respect to transthoracic impedance, number of countershocks necessary for the first successful defibrillation, total joules administered during resuscitative efforts or time to ROSC (Table 1).
Table 1.
Resuscitation variablesa in the swine model
| Swine | Weight (kg) | Countershocks to defibrillate | Total joules | Time to ROSCb |
|---|---|---|---|---|
| Males | 41 ± 3 | 1 (1,2) | 400 (200,500) | 100 (96,166) |
| Females | 39 ± 5 | 2 (1,2.3) | 500 (200,860) | 120 (96,166) |
ROSC = restoration of spontaneous circulation; statistically significant differences were not observed between genders.
Values are the mean ± SD or median (25-75% interquartile range).
Seconds.
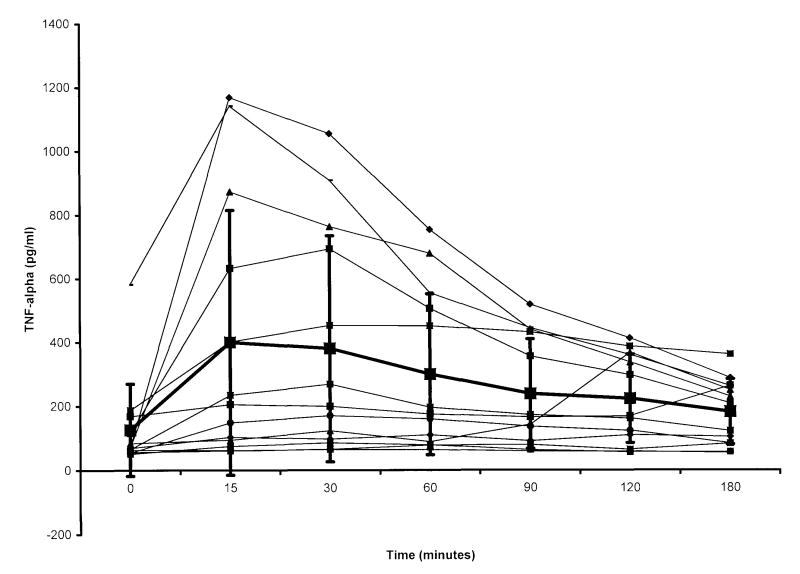
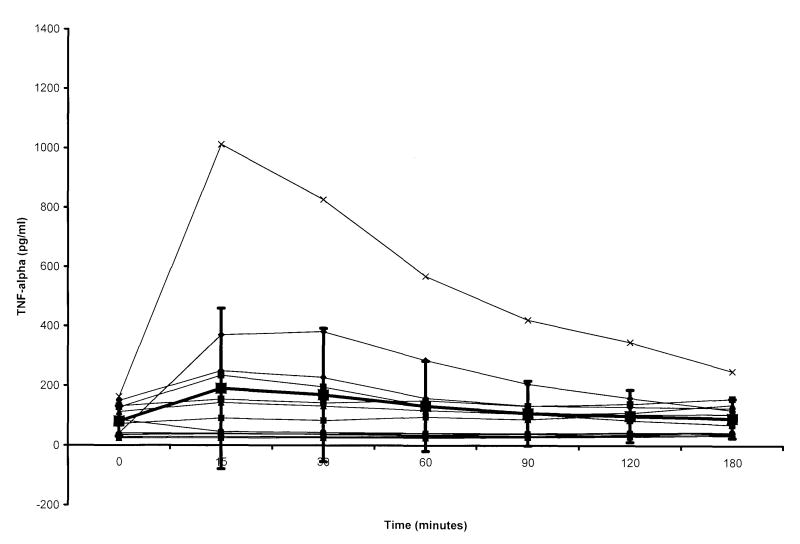
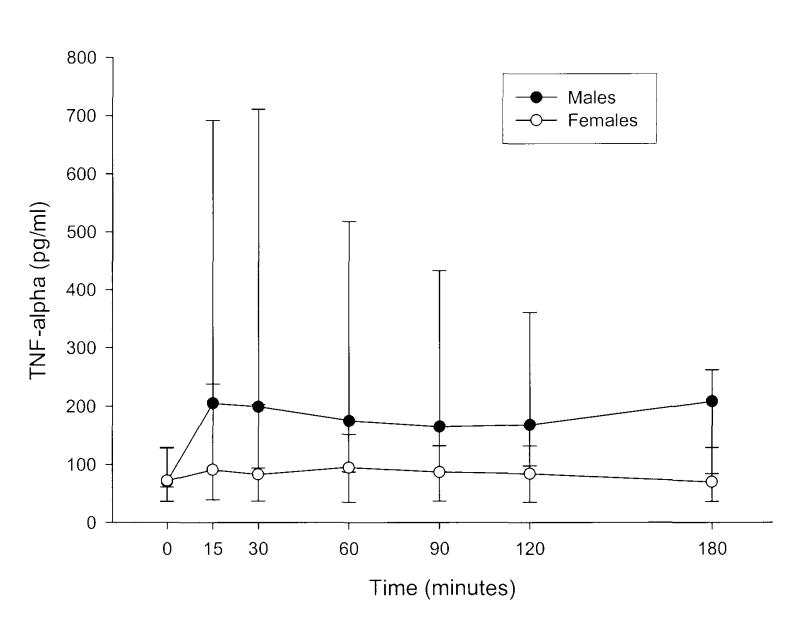
Plasma TNF-α concentrations for individual male and female swine and mean values for the gender groups are shown in Figures 1 and 2. With the exception of the timepoints at 0 and 15 min post-resuscitation, TNF-α levels were higher in males than females during the 180-min sampling interval. Within gender groups, the TNF-α concentration was significantly different from control values in the male group over the first hour of the post-resuscitation period. In females, only the 15-min value was greater than that measured before arrest (Figure 3).
Figure 1.
Tumour necrosis factor (TNF)-α concentrations in plasma of male swine achieving return of spontaneous circulation (ROSC), versus time. Time 0 = pre-arrest, i.e. control value; time intervals after 0 represent time from ROSC. Large boxes with error bars represent mean and standard deviation for the group. TNF-α levels at 15, 30 and 60 min were significantly greater than control values. After 60 min, levels were not significantly different from pre-arrest values.
Figure 2.
Tumour necrosis factor (TNF)-α concentrations in plasma of female swine achieving return of spontaneous circulation (ROSC), versus time. Time 0 = pre-arrest, i.e. control value; time intervals after 0 represent time from ROSC. Large boxes with error bars represent mean and standard deviation for the group. In females, only the value at 15 min was greater than pre-arrest concentrations.
Figure 3.
Tumour necrosis factor (TNF)-α concentrations in plasma of male swine achieving return of spontaneous circulation (ROSC) versus those in female swine achieving ROSC, versus time. Time 0 = pre-arrest, i.e. control value. Time intervals after 0 represent time from ROSC. Data are presented as the median and interquartile range (25-75%). Values for males exceed those of females at all time points except for pre-arrest levels and those at 15 s after ROSC.
Testosterone was detectable in only one male (0.072 ng/ml) and in three females (0.0461, 0.0264, and 0.0288 ng/ml); 17-estradiol was not detectable (<0.20 pg/ml) in any animal.
Discussion
In this study, a significantly greater TNF-α response following resuscitation from cardiac arrest was observed in male swine when compared with females. The animals used in this study were typical of those used in cardiac arrest research, i.e. 30-45 kg in weight, approximately 3-4 months of age and sexually immature. Not unexpectedly, no difference in sex steroids was observed between genders. The proinflammatory cytokine response in this typical cardiac arrest and resuscitation model appears to be gender dependent but is not related to plasma sex steroid concentration and is likely to be independent of sex hormone receptors.
Although no significant differences in 17-estradiol and testosterone levels were demonstrated between males and females in our study, the TNF-α response following resuscitation and reperfusion was augmented in males when compared with females. This difference was not related to time to ROSC, total ischaemia time (untreated VF period plus duration of resuscitative efforts) or epinephrine dose. Of note, there was a wide inter-animal variation in the TNF-α response within the male group (Figure 1).
Trauma, haemorrhage, burns, surgical stress and sepsis have all been shown to induce a proinflammatory cytokine response.23 Data regarding the effect of gender on this response have been conflicting. The effects of sex hormones are dependent upon blood levels, the expression of hormone receptors on immune cells, and the interaction of sex hormones with other hormones with immunomodulator properties. Animal experiments have shown a more robust innate immune response in females, a lower incidence of septic shock, and improved survival. Human studies have demonstrated a variable response, i.e. the response among females is the same, greater or less than among males.24 The effects of sex hormones and the different components of inflammatory response are similarly conflicting. However, in general, estrogen appears to decrease IL-6 levels, and in-vitro stimulation of immune cells results in greater TNF-α production by males.25,26 A number of laboratory investigations suggest that estrogens play a role in regulating or mediating TNF-α production following cell activation by a stimulus. For instance, studies suggest a gender-specific difference in activation of p38 mitogen activated protein kinase via phosphorylation.16,27
Evidence of oxidant injury appears rapidly after resuscitation from cardiac arrest, and activation of characteristic metabolic cascades responsible for reperfusion injury and the accompanying inflammatory response are to be expected.1,2 Observational clinical studies in small series of patients have demonstrated elevated proinflammatory cytokine levels among individuals resuscitated from cardiac arrest.5 These studies did not address the relationship between gender and cytokine response. A difference between men and women would not be immediately expected considering the age of the typical cardiac arrest patient population. The women in this cohort were likely to be postmenopausal, and the impact of exogenous hormones at low doses might not impact the innate immune response.
There were several limitations to this study, which involved domestic, mixed-breed swine weighing approximately 35-45 kg, i.e. similar in weight to those reported by other laboratories. Domestic swine of this weight are typically sexually immature.29,30 The use of sexually mature animals would probably have resulted in different sex steroid levels but not necessarily different TNF-α profiles between genders. We measured only plasma TNF-α as a marker of the post-resuscitation inflammatory response. There may not be gender-dependent differences in other cytokines measured in blood. We likewise did not measure TNF-α or other cytokine concentrations in specific tissues. Lastly, the short-term post-resuscitation period precluded an assessment of the role that TNF-α might play in long-term outcome or post-resuscitation organ dysfunction.
This study suggests sexual dimorphism in the TNF-α response to reperfusion following cardiac arrest and resuscitation. However, this dimorphism was not explained by differences in sex hormones. At the same time, the post-resuscitation TNF-α response in this model of electrically-induced VF in sexually immature swine did not appear to be reliably predictable. In general, the TNF-α response was greater in males and less in females, but there was considerable variation between animals, particularly among males. This may well have been due to genetic TNF-promoter polymorphism, which has been well studied in a number of clinical populations.28 The sexual immaturity of the typical swine model may preclude the detection of outcome differences between genders ascribed entirely, or in part, to sex hormones.
Supplementary Material
Acknowledgments
This study was supported, in part, by a grant from the National Institutes of Health, NHLBI R01 HL076671.
Footnotes
Conflict of interest statement: None of the authors has a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basu S, Nozari A, Liu XL, Rubertsson S, Wiklund L. Development of a novel biomarker of free radical damage in reperfusion injury after cardiac arrest. FEBS Lett. 2000;470:1–6. doi: 10.1016/s0014-5793(00)01279-5. [DOI] [PubMed] [Google Scholar]
- 2.Idris AH, Roberts J, II, Caruso L, et al. Oxidant injury occurs rapidly after cardiac arrest, cardiopulmonary resuscitation, and reperfusion. Crit Care Med. 2005;33:2043–8. doi: 10.1097/01.ccm.0000174104.50799.bd. [DOI] [PubMed] [Google Scholar]
- 3.Negovsky VA. Postresuscitation disease. Crit Care Med. 1988;16:942–6. doi: 10.1097/00003246-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou J, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–12. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 5.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–8. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 6.Mussack T, Biberthaler P, Gippner-Steppert C, et al. Early cellular brain damage and systemic inflammatory response after cardiopulmonary resuscitation or isolated severe head trauma: a comparative pilot study on common pathomechanisms. Resuscitation. 2001;49:193–9. doi: 10.1016/s0300-9572(00)00346-4. [DOI] [PubMed] [Google Scholar]
- 7.Mussack T, Biberthaler P, Kanz KG, et al. Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med. 2002;30:2669–74. doi: 10.1097/00003246-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Saitoh D, Fukuzuka K, et al. Significance of elevated serum interleukin-8 in patients resuscitated after cardiopulmonary arrest. Resuscitation. 2001;51:47–53. doi: 10.1016/s0300-9572(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 9.Niemann JT, Garner D, Lewis RJ. Tumor necrosis factor-α is associated with early postresuscitation myocardial function. Crit Care Med. 2004;32:1753–8. doi: 10.1097/01.ccm.0000132899.15242.d3. [DOI] [PubMed] [Google Scholar]
- 10.Niemann JT, Rosborough J, Thomas J, et al. Proinflammatory cytokine storm follows early reperfusion after resuscitation from prolonged ischemia-associated VF in swine. Circulation. 2006;114:465. [Google Scholar]
- 11.Angele MK, Knoferl MW, Schwacha MG, et al. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol. 1999;277:35–42. doi: 10.1152/ajpcell.1999.277.1.C35. [DOI] [PubMed] [Google Scholar]
- 12.Angele MK, Schwacha MG, Ayala A, Chaudry IH. The effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 13.Hessen M, Bloemeke B, Heussen N, Kunz D. Can the interleukin-6 response to endotoxin be predicted? Studies of the influence of a promoter polymorphism of the interleukin-6 gene, gender, and the density of the endotoxin receptor CD14, and inflammatory cytokines. Crit Care Med. 2002;30:664–9. doi: 10.1097/00003246-200203000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Bouman A, Schipper M, Heineman MJ, Faas MM. Gender differences in the nonspecific and specific immune response in humans. Am J Reprod Immunol. 2004;52:19–26. doi: 10.1111/j.1600-0897.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 15.Kher A, Wang M, Tsai BM, et al. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- 16.Imahara SD, Jelacic S, Junker CE, O'Keefe GE. The influence of gender on human innate immunity. Surgery. 2005;138:275–82. doi: 10.1016/j.surg.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Gliner BE, White RD. Electrocardiographic evaluation of defibrillation shocks delivered to out-of-hospital sudden cardiac arrest patients. Resuscitation. 1999;41:133–44. doi: 10.1016/s0300-9572(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 18.Idris AH, Becker LB, Ornato JP, et al. Utstein-stype guidelines for uniform reporting of laboratory CPR research. Circulation. 1996;94:2324–36. doi: 10.1161/01.cir.94.9.2324. [DOI] [PubMed] [Google Scholar]
- 19.Qoubaitary A, Meriggiola C, Ng CM, et al. Pharmacokinetics of testosterone undecanoate injected alone or in combination with norethisterone enanthate in healthy men. J Androl. 2006;27:853–67. doi: 10.2164/jandrol.106.000281. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Catlin DH, Starcevic B, et al. Low-fat high-fiber diet decreased serum and urine androgens in men. J Clin Endocrinol Metab. 2005;90:355–9. doi: 10.1210/jc.2004-1530. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Iranmanesh A, Berman N, et al. Comparative pharmacokinetics of three doses of percutaneous dihydrotestosterone gel in healthy elderly men—a clinical research center study. J Clin Endocrinol Metab. 1998;83:2749–57. doi: 10.1210/jcem.83.8.4996. [DOI] [PubMed] [Google Scholar]
- 22.Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500–10. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 23.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–9. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Suffredini AF. Systemic inflammation and sexual dimorphism: more than meets the eye. Crit Care Med. 2007;35:1610–2. doi: 10.1097/01.CCM.0000266793.09378.22. [DOI] [PubMed] [Google Scholar]
- 25.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–23. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 26.Moxley G, Posthuma D, Carlson P, et al. Sexual dimorphism in innate immunity. Arthritis Rheum. 2002;46:250–8. doi: 10.1002/1529-0131(200201)46:1<250::AID-ART10064>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Angele MK, Nitsche S, Knoferl MW, et al. Sex-specific p38 MAP kinase activation following trauma-hemorrhage: involvement of testosterone and estradiol. Am J Physiol Endocrinol Metab. 2003;285:189–96. doi: 10.1152/ajpendo.00035.2003. [DOI] [PubMed] [Google Scholar]
- 28.Bayley JP, Ottenhoff THM, Verweij CL. Is there a future for TNF promoter polymorphisms? Genes Immun. 2004;5:315–29. doi: 10.1038/sj.gene.6364055. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Barber DA, Lewis DA, et al. Gender and transcriptional regulation of NO synthetase and ET-1 in porcine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 1997:H1962–7. doi: 10.1152/ajpheart.1997.273.4.H1962. [DOI] [PubMed] [Google Scholar]
- 30.Jayachandran M, Okano H, Chatrath R, Owen WG, McConnell JP, Miller VM. Sex-specific changes in platelet aggregation and secretion with sexual maturity in pigs. J Appl Physiol. 2004;97:1445–52. doi: 10.1152/japplphysiol.01074.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.