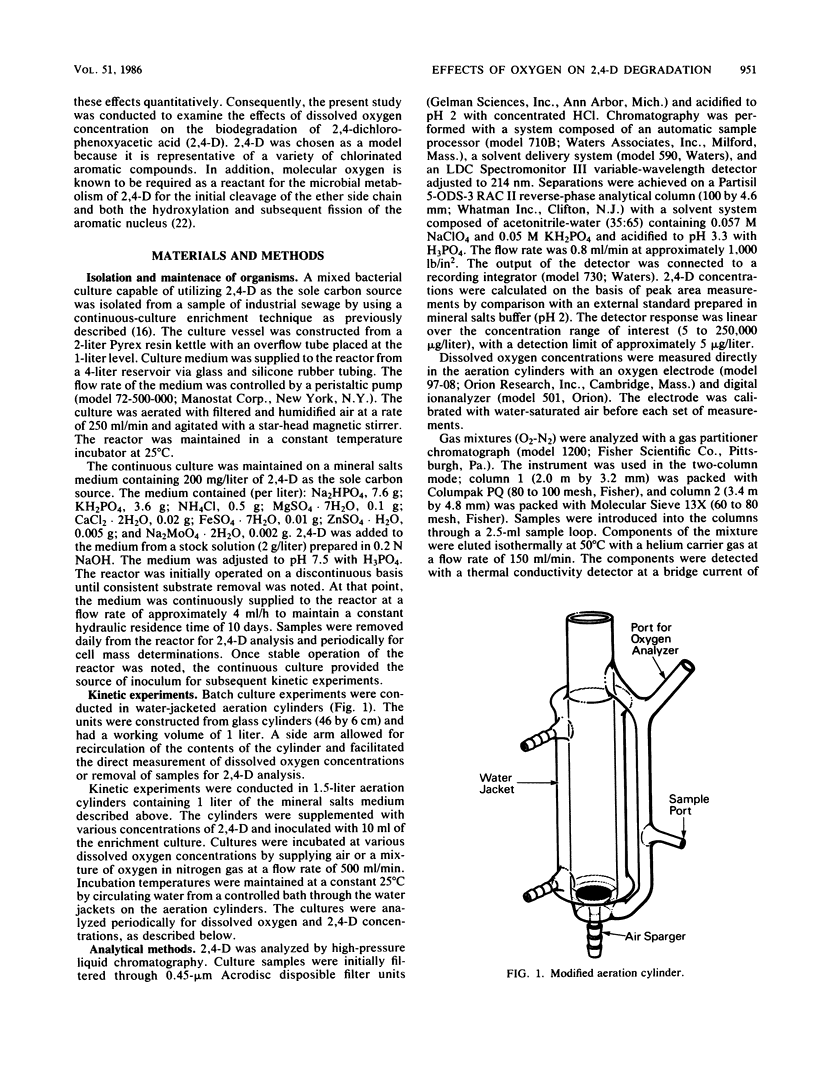
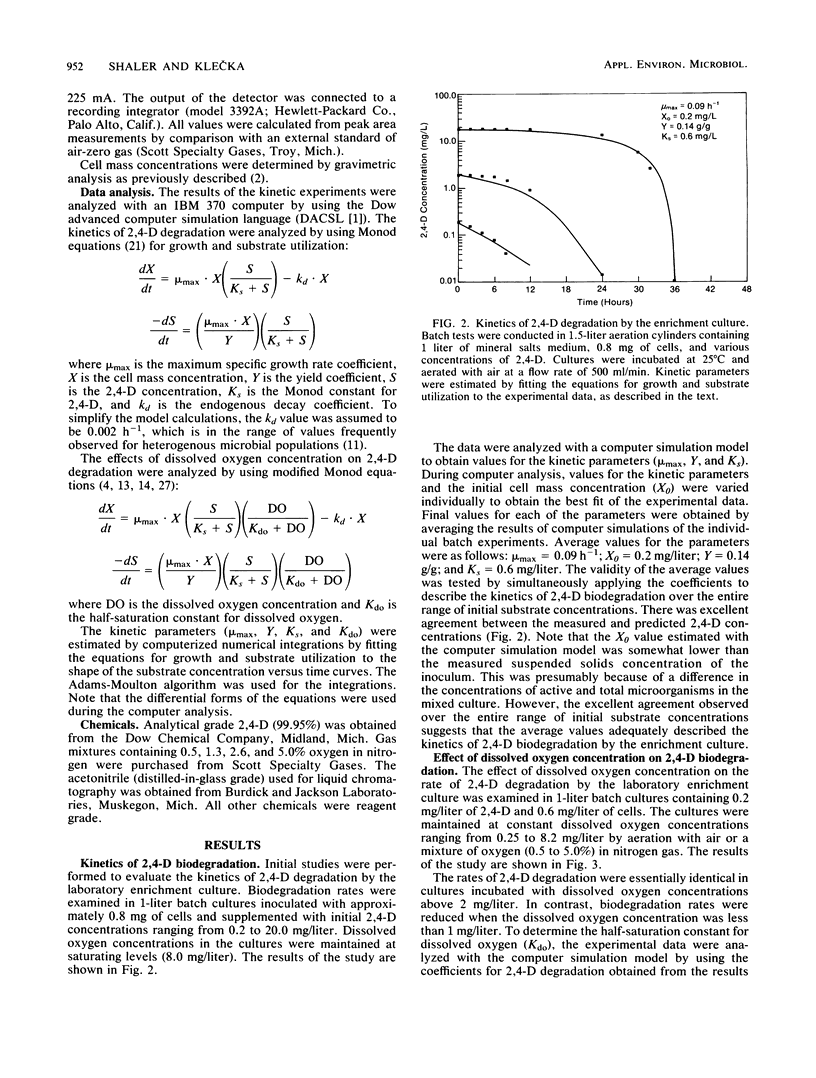
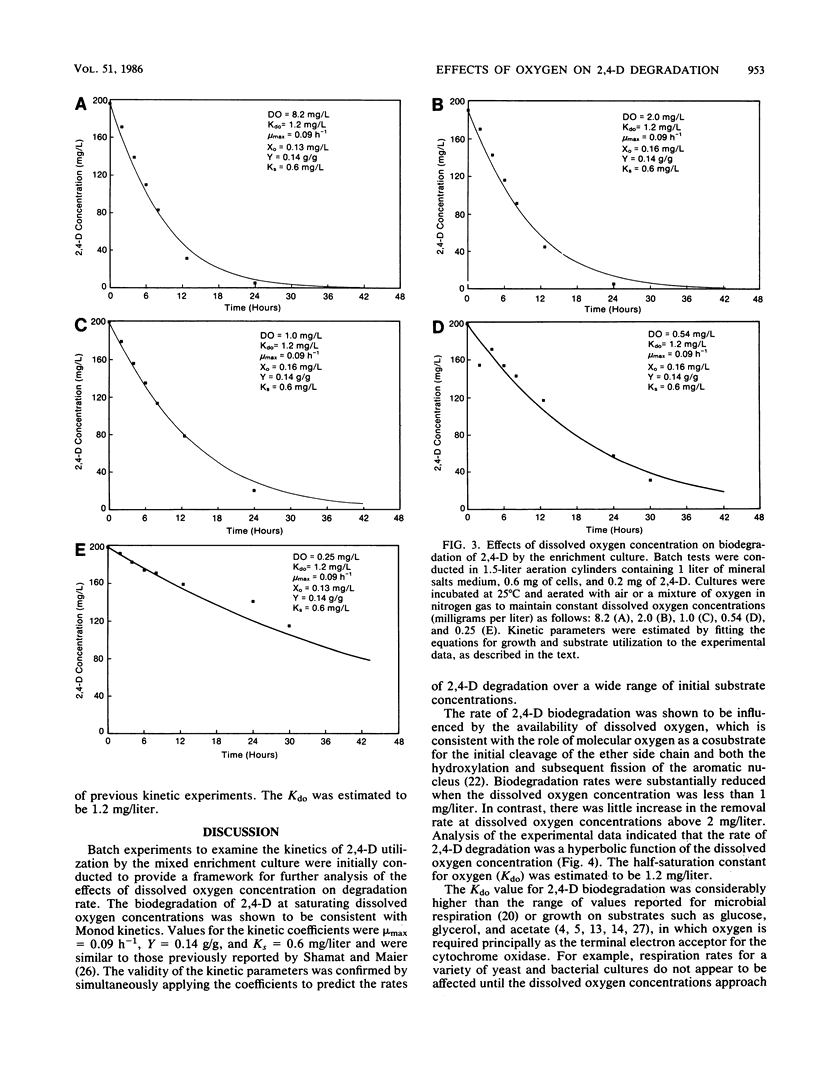
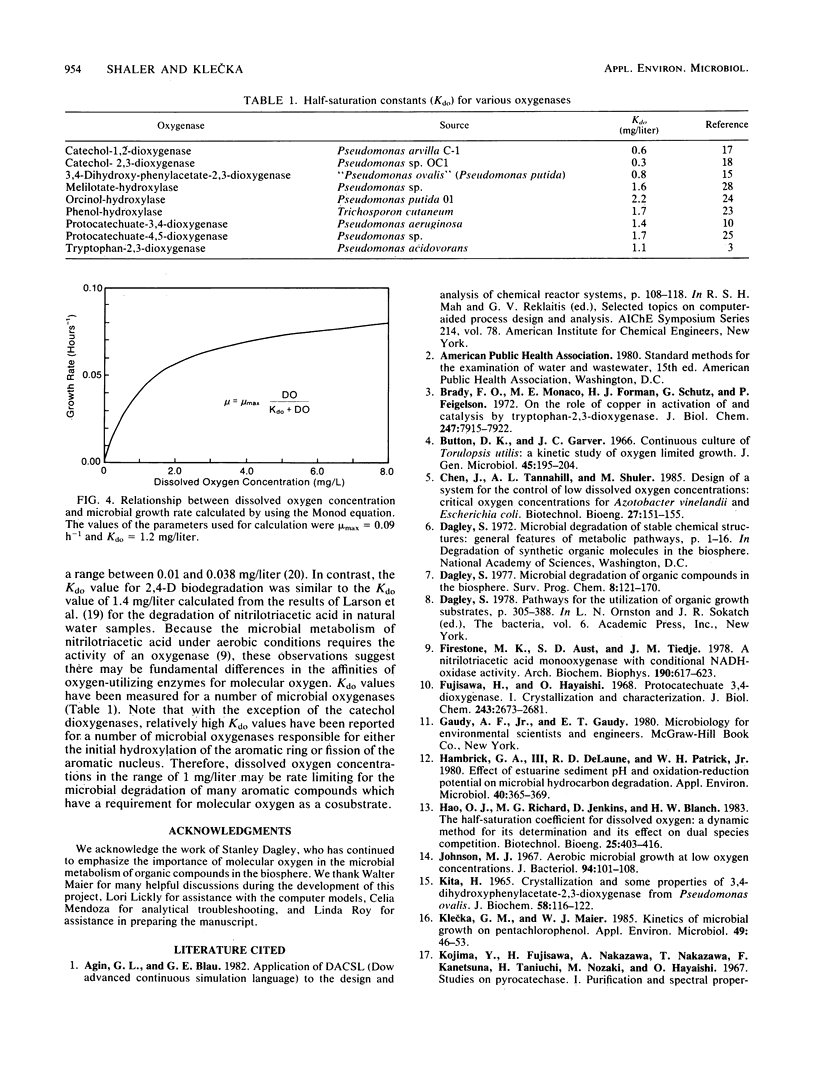
Abstract
Batch experiments were conducted to examine the effects of dissolved oxygen concentration on the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) by an enrichment culture of 2,4-D-utilizing bacteria. A modified Monod equation was found to describe the relationship between the specific growth rate and the concentrations of both the organic substrate and dissolved oxygen. Values for the maximum specific growth rate, yield, and Monod coefficient for growth on 2,4-D were 0.09 h-1, 0.14 g/g, and 0.6 mg/liter, respectively. The half-saturation constant for dissolved oxygen was estimated to be 1.2 mg/liter. These results suggest that dissolved oxygen concentrations below 1 mg/liter may be rate limiting for the biodegradation of chlorinated aromatic compounds such as 2,4-D, which have a requirement for molecular oxygen as a cosubstrate for metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady F. O., Monaco M. E., Forman H. J., Schutz G., Feigelson P. On the role of copper in activation of and catalysis by tryptophan-2,3-dioxygenase. J Biol Chem. 1972 Dec 25;247(24):7915–7922. [PubMed] [Google Scholar]
- Button D. K., Garver J. C. Continuous culture of Torulopsis utilis: a kinetic study of oxygen limited growth. J Gen Microbiol. 1966 Nov;45(2):195–204. doi: 10.1099/00221287-45-2-195. [DOI] [PubMed] [Google Scholar]
- Chen J., Tannahill A. L., Shuler M. L. Design of a system for the control of low dissolved oxygen concentrations: critical oxygen concentrations for Azotobacter vinelandii and Escherichia coli. Biotechnol Bioeng. 1985 Feb;27(2):151–155. doi: 10.1002/bit.260270208. [DOI] [PubMed] [Google Scholar]
- Firestone M. K., Aust S. D., Tiedje J. M. A nitrilotriacetic acid monooxygenase with conditional NADH-oxidase activity. Arch Biochem Biophys. 1978 Oct;190(2):617–623. doi: 10.1016/0003-9861(78)90318-1. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Hayaishi O. Protocatechuate 3,4-dioxygenase. I. Crystallization and characterization. J Biol Chem. 1968 May 25;243(10):2673–2681. [PubMed] [Google Scholar]
- Hambrick G. A., Delaune R. D., Patrick W. H. Effect of Estuarine Sediment pH and Oxidation-Reduction Potential on Microbial Hydrocarbon Degradation. Appl Environ Microbiol. 1980 Aug;40(2):365–369. doi: 10.1128/aem.40.2.365-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. J. Aerobic microbial growth at low oxygen concentrations. J Bacteriol. 1967 Jul;94(1):101–108. doi: 10.1128/jb.94.1.101-108.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJIMA Y., ITADA N., HAYAISHI O. Metapyrocatachase: a new catechol-cleaving enzyme. J Biol Chem. 1961 Aug;236:2223–2228. [PubMed] [Google Scholar]
- Kita H. Crystallization and some properties of 3,4-dihydroxyphenylacetate 2,3-oxygenase from Pseudomonas ovalis. J Biochem. 1965 Aug;58(2):116–122. doi: 10.1093/oxfordjournals.jbchem.a128172. [DOI] [PubMed] [Google Scholar]
- Klecka G. M., Maier W. J. Kinetics of microbial growth on pentachlorophenol. Appl Environ Microbiol. 1985 Jan;49(1):46–53. doi: 10.1128/aem.49.1.46-53.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Fujisawa H., Nakazawa A., Nakazawa T., Kanetsuna F., Taniuchi H., Nozaki M., Hayaishi O. Studies on pyrocatechase. I. Purification and spectral properties. J Biol Chem. 1967 Jul 25;242(14):3270–3278. [PubMed] [Google Scholar]
- LONGMUIR I. S. Respiration rate of bacteria as a function of oxygen concentration. Biochem J. 1954 May;57(1):81–87. doi: 10.1042/bj0570081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neujahr H. Y., Gaal A. Phenol hydroxylase from yeast. Purification and properties of the enzyme from Trichosporon cutaneum. Eur J Biochem. 1973 Jun;35(2):386–400. doi: 10.1111/j.1432-1033.1973.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Higgins I., Ribbons D. W. Metabolism of resorcinylic compounds by bacteria. Purification and properties of orcinol hydroxylase from Pseudomonas putida 01. J Biol Chem. 1975 May 25;250(10):3814–3825. [PubMed] [Google Scholar]
- Ono K., Nozaki M., Hayaishi O. Purification and some properties of protocatechuate 4,5-dioxygenase. Biochim Biophys Acta. 1970 Nov 11;220(2):224–238. doi: 10.1016/0005-2744(70)90008-2. [DOI] [PubMed] [Google Scholar]
- Strickland S., Massey V. The mechanism of action of the flavoprotein melilotate hydroxylase. J Biol Chem. 1973 Apr 25;248(8):2953–2962. [PubMed] [Google Scholar]



