Abstract
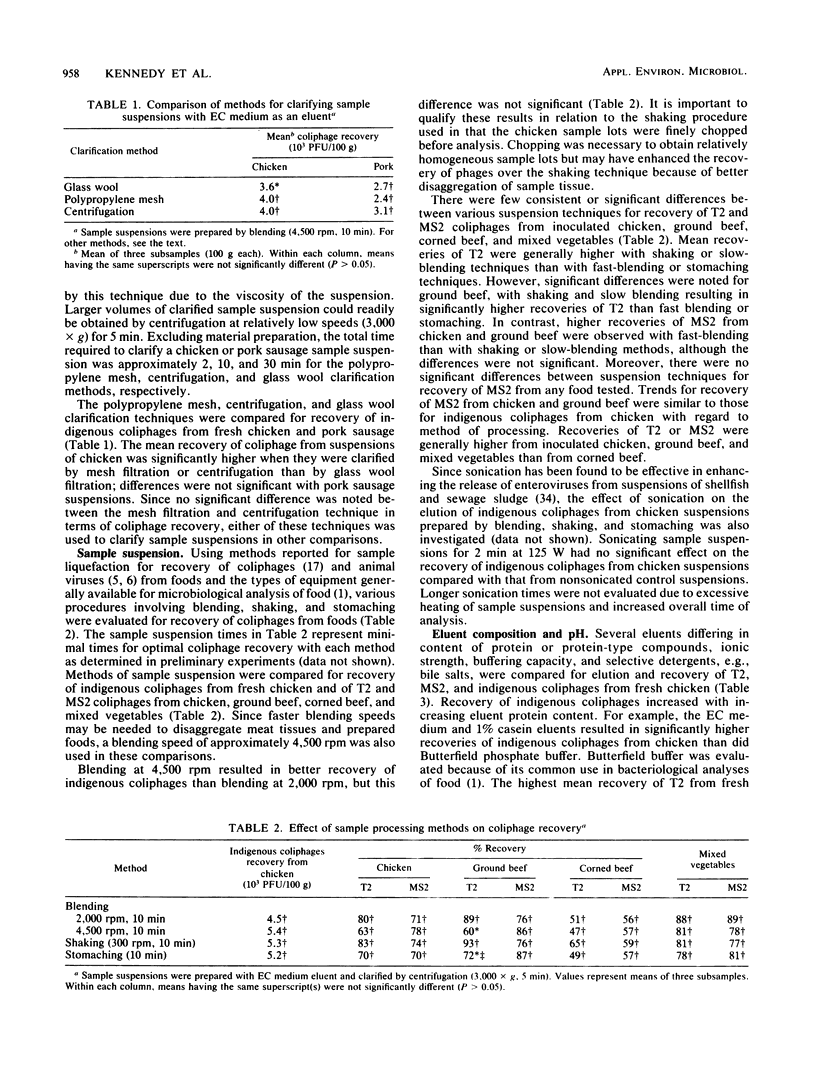
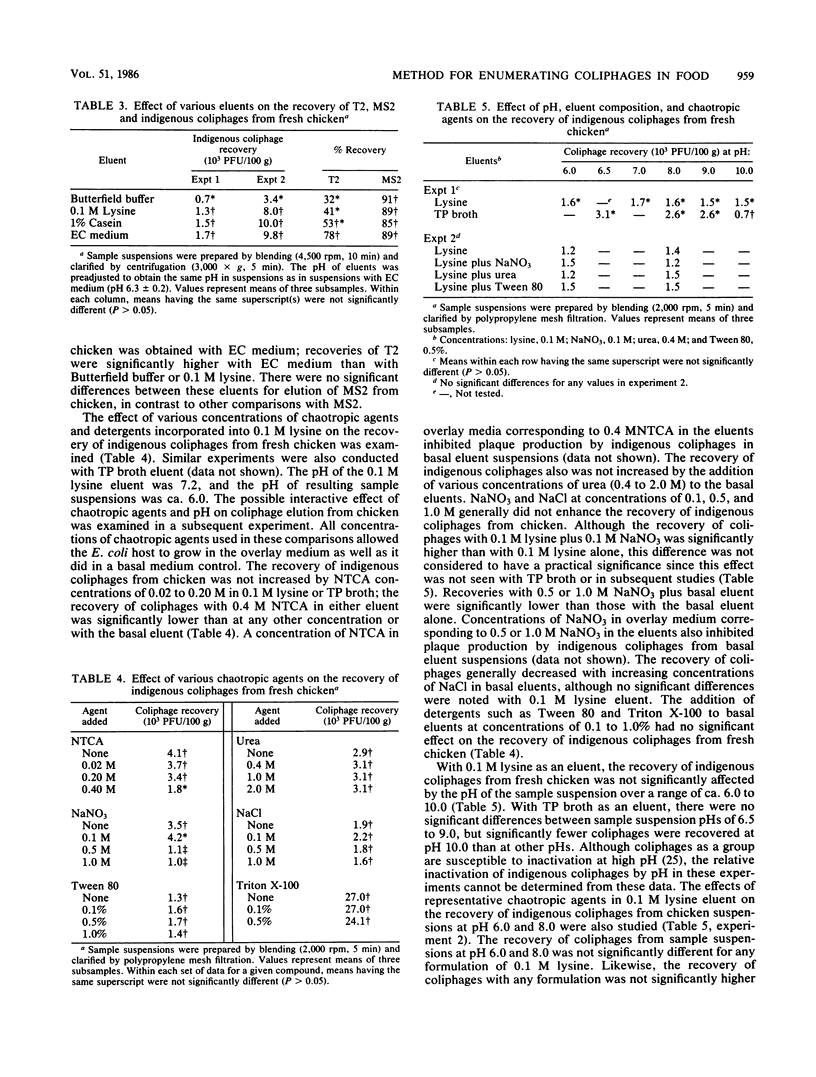
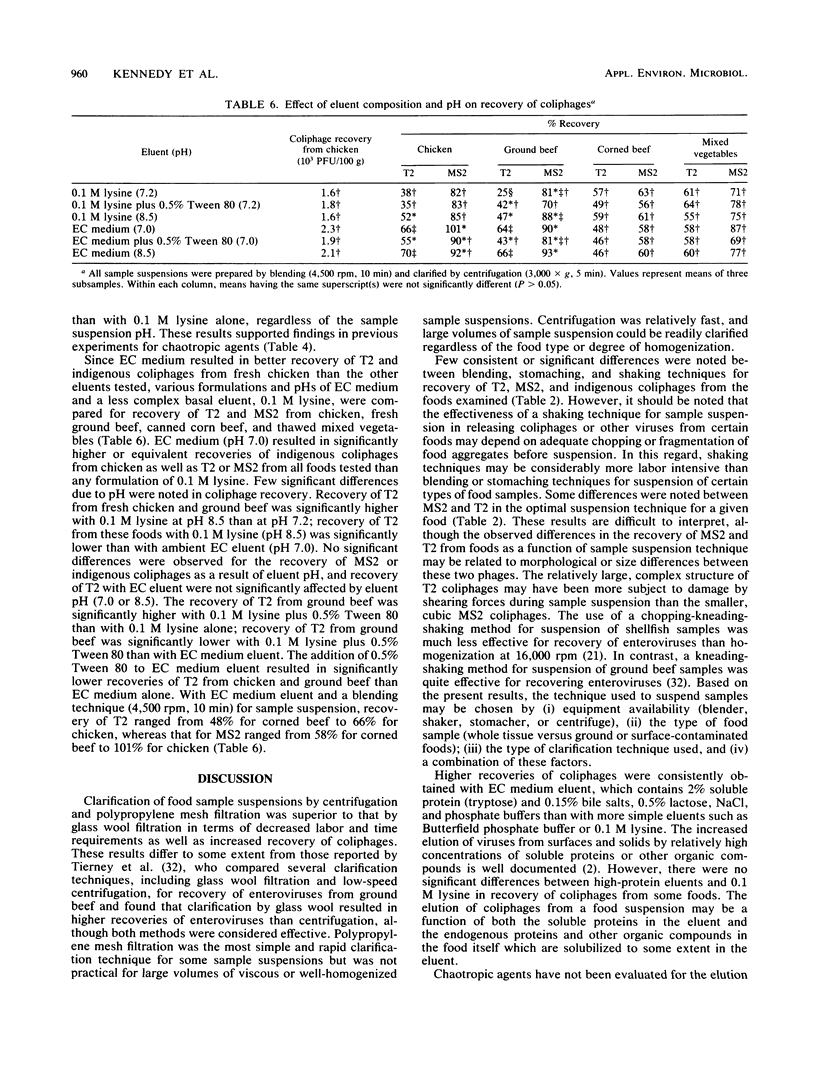
The effects of eluent composition, pH, and chaotropic agents on the recovery of T2, MS2, and indigenous coliphages from various foods were investigated. Additionally, methods of sample suspension and clarification were evaluated for coliphage recovery and application to various foods. Clarified sample suspensions were assayed for coliphages with a modified agar layer technique and appropriate Escherichia coli hosts. Centrifugation and polypropylene mesh filtration were more rapid and effective than glass wool filtration for clarification of sample suspensions and subsequent recovery of coliphages. Blending, stomaching, and shaking procedures were generally comparable for sample liquefaction and release of coliphages from foods. Complex basal eluents, EC medium and 1% casein, were generally more effective than a less complex eluent, phosphate buffer, for elution of coliphages from foods. For most foods, incorporation of sodium chloride or chaotropic agents, i.e., sodium trichloroacetate, urea, Tween 80, Triton X-100, and sodium nitrate, into basal eluents did not enhance recovery of coliphages. Indigenous coliphage recovery was not affected by sample suspension pH over a range of 6.0 to 9.0. With an optimal procedure, i.e., EC medium eluent, blending, and centrifugation, the recovery of T2 and MS2 ranged from 48 to 81% and from 58 to 100%, respectively, depending on the food type.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitton G., Charles M. J., Farrah S. R. Virus detection in soils: a comparison of four recovery methods. Can J Microbiol. 1979 Aug;25(8):874–880. doi: 10.1139/m79-130. [DOI] [PubMed] [Google Scholar]
- Bitton G., Chou Y. J., Farrah S. R. Techniques for virus detection in aquatic sediments. J Virol Methods. 1982 Feb;4(1):1–8. doi: 10.1016/0166-0934(82)90048-9. [DOI] [PubMed] [Google Scholar]
- Farrah S. R., Bitton G. Elution of poliovirus adsorbed to membrane filters. Appl Environ Microbiol. 1978 Dec;36(6):982–984. doi: 10.1128/aem.36.6.982-984.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Bitton G. Low molecular weight substitutes for beef extract as eluents for poliovirus adsorbed to membrane filters. Can J Microbiol. 1979 Sep;25(9):1045–1051. doi: 10.1139/m79-160. [DOI] [PubMed] [Google Scholar]
- Farrah S. R. Chemical factors influencing adsorption of bacteriophage MS2 to membrane filters. Appl Environ Microbiol. 1982 Mar;43(3):659–663. doi: 10.1128/aem.43.3.659-663.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Conklin R. H., Wallis C., Melnick J. L., DuPont H. L. A simple method for concentration of enteroviruses and rotaviruses from cell culture harvests using membrane filters. Intervirology. 1978;9(1):56–59. doi: 10.1159/000148921. [DOI] [PubMed] [Google Scholar]
- Farrah S. R., Scheuerman P. R., Bitton G. Urea-lysine method for recovery of enteroviruses from sludge. Appl Environ Microbiol. 1981 Feb;41(2):455–458. doi: 10.1128/aem.41.2.455-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Shah D. O., Ingram L. O. Effects of chaotropic and antichaotropic agents on elution of poliovirus adsorbed on membrane filters. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1229–1232. doi: 10.1073/pnas.78.2.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finance C., Villeval F., Block J. C., Schwartzbrod L. Improved method for virological analysis of food. Appl Environ Microbiol. 1981 Jul;42(1):176–179. doi: 10.1128/aem.42.1.176-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A. H., Hogeboom W. M. Factors affecting the enumeration of coliphages in sewage and sewage-polluted waters. Antonie Van Leeuwenhoek. 1983 Nov;49(4-5):387–397. doi: 10.1007/BF00399318. [DOI] [PubMed] [Google Scholar]
- Kenard R. P., Valentine R. S. Rapid determination of the presence of enteric bacteria in water. Appl Microbiol. 1974 Mar;27(3):484–487. doi: 10.1128/am.27.3.484-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. E., Jr, Bitton G., Oblinger J. L. Comparison of selective media for assay of coliphages in sewage effluent and lake water. Appl Environ Microbiol. 1985 Jan;49(1):33–36. doi: 10.1128/aem.49.1.33-36.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin E. P., Tierney J. T., Sullivan R., Peeler J. T. Collaborative study of the glass wool filtration method for the recovery of virus inoculated into ground beef. J Assoc Off Anal Chem. 1975 May;58(3):576–578. [PubMed] [Google Scholar]
- Lipson S. M., Stotzky G. Effect of proteins on reovirus adsorption to clay minerals. Appl Environ Microbiol. 1984 Sep;48(3):525–530. doi: 10.1128/aem.48.3.525-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkin P. I., Sharpe A. N. Filtering out food debris before microbiological analysis. Appl Environ Microbiol. 1981 Jul;42(1):63–65. doi: 10.1128/aem.42.1.63-65.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino C. M., Maier S. Differential inactivation of three bacteriophages by acid and alkaline pH used in the membrane adsorption-elution method of virus recovery. Can J Microbiol. 1980 Dec;26(12):1403–1407. doi: 10.1139/m80-233. [DOI] [PubMed] [Google Scholar]
- Seeley N. D., Primrose S. B. The isolation of bacteriophages from the environment. J Appl Bacteriol. 1982 Aug;53(1):1–17. doi: 10.1111/j.1365-2672.1982.tb04729.x. [DOI] [PubMed] [Google Scholar]
- Shields P. A., Farrah S. R. Influence of salts on electrostatic interactions between poliovirus and membrane filters. Appl Environ Microbiol. 1983 Feb;45(2):526–531. doi: 10.1128/aem.45.2.526-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simková A., Cervenka J. Coliphages as ecological indicators of enteroviruses in various water systems. Bull World Health Organ. 1981;59(4):611–618. [PMC free article] [PubMed] [Google Scholar]
- Stetler R. E. Coliphages as indicators of enteroviruses. Appl Environ Microbiol. 1984 Sep;48(3):668–670. doi: 10.1128/aem.48.3.668-670.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney J. T., Sullivan R., Larkin E. P., Peeler J. T. Comparison of methods for the recovery of virus inoculated into ground beef. Appl Microbiol. 1973 Oct;26(4):497–501. doi: 10.1128/am.26.4.497-501.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wait D. A., Sobsey M. D. Method for recovery of enteric viruses from estuarine sediments with chaotropic agents. Appl Environ Microbiol. 1983 Aug;46(2):379–385. doi: 10.1128/aem.46.2.379-385.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentsel R. S., O'Neill P. E., Kitchens J. F. Evaluation of coliphage detection as a rapid indicator of water quality. Appl Environ Microbiol. 1982 Feb;43(2):430–434. doi: 10.1128/aem.43.2.430-434.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman P. A., Marshall R. T. Isolation of psychrophilic bacteriophage-host systems from refrigerated food products. Appl Microbiol. 1971 Aug;22(2):220–223. doi: 10.1128/am.22.2.220-223.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss U. Dispersal and fate of coliphages in the River Saar. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;174(1-2):160–173. [PubMed] [Google Scholar]


