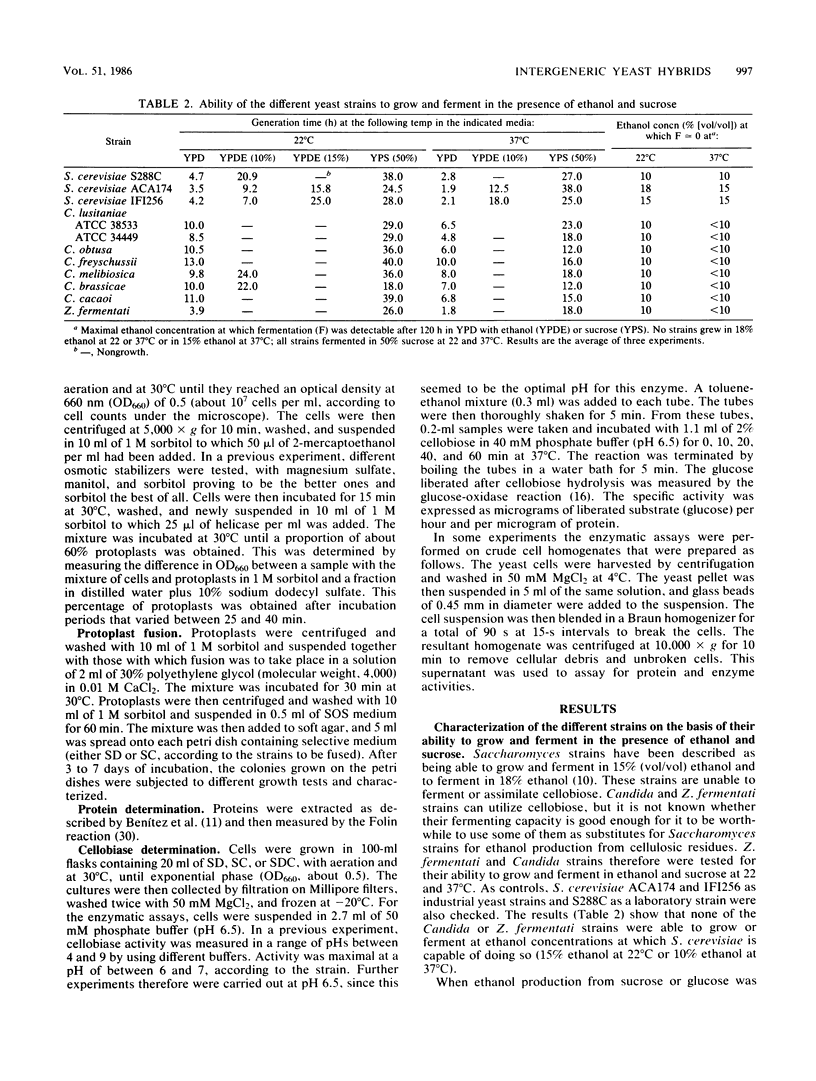
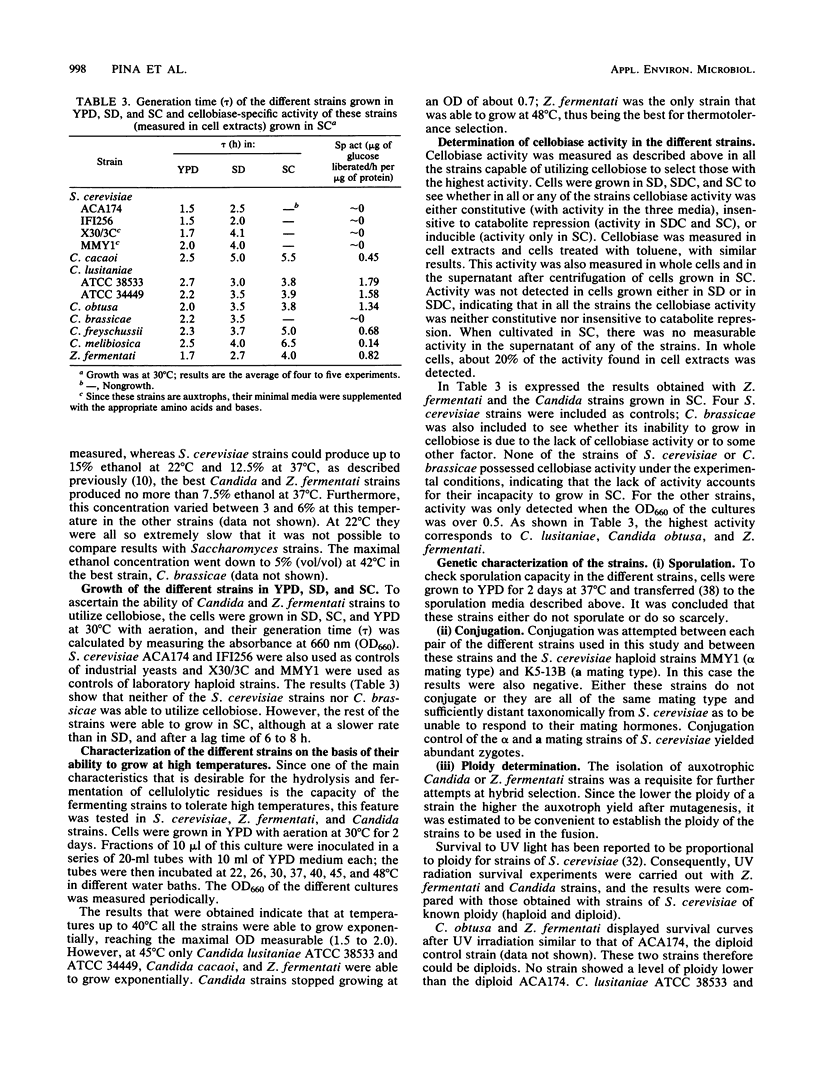
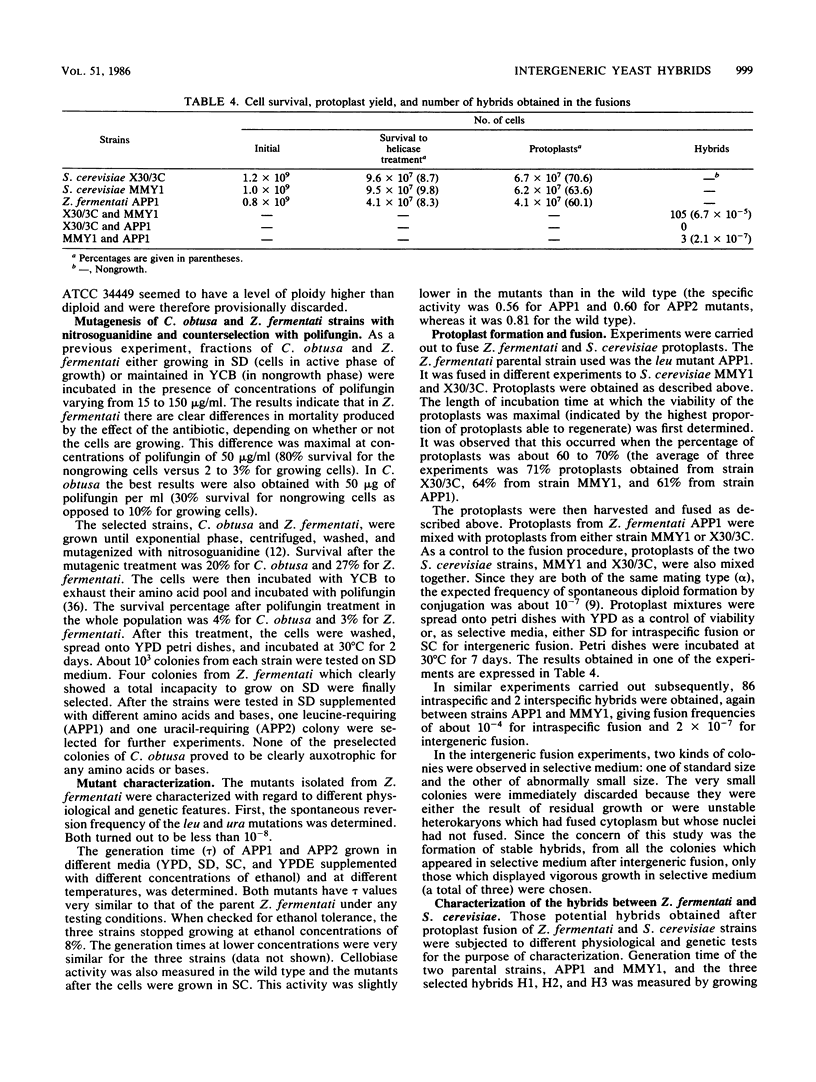
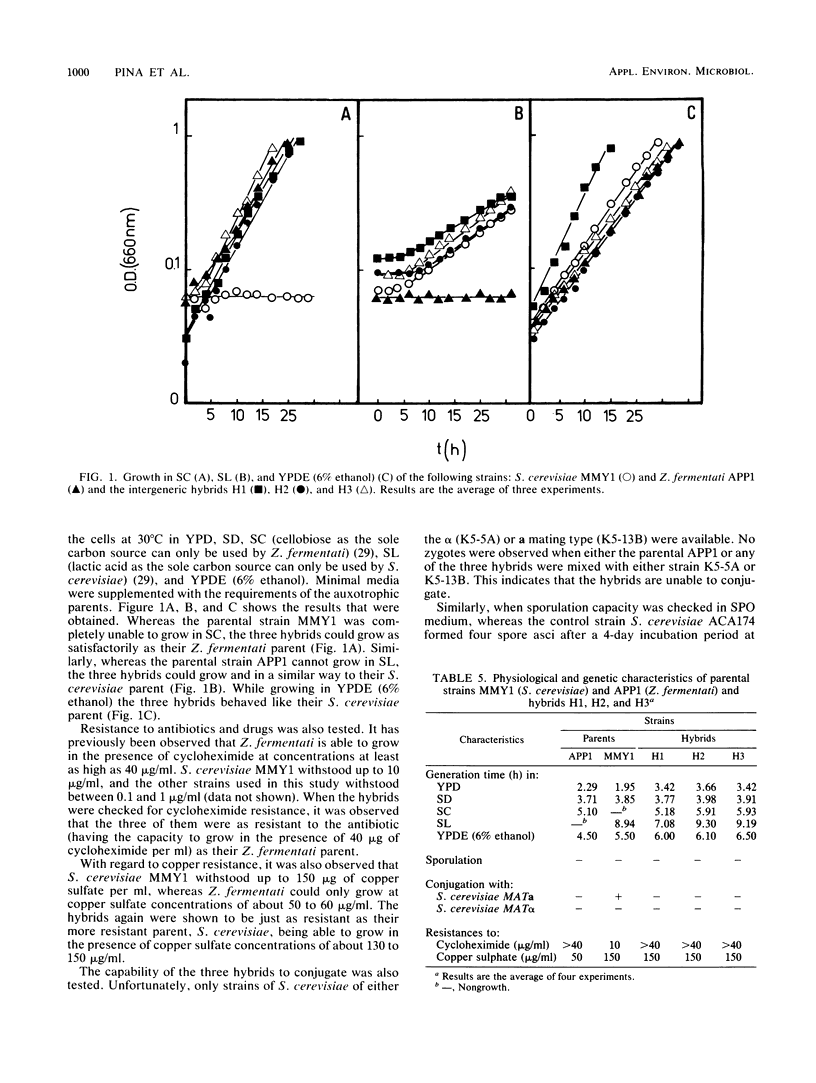
Abstract
To obtain strains that are able to efficiently produce ethanol from different carbohydrates, mainly cellulose hydrolysates, several species of the genus Candida and a Zygosaccharomyces fermentati strain were examined for their ability to utilize cellobiose and produce ethanol, as well as for their thermotolerance and the possibility of genetic manipulation. Candida obtusa and Zygosaccharomyces fermentati tolerated the maximal temperature for growth, possessed the highest cellobiase activity, and offered the possibility of genetic manipulation, although neither of them proved to be a good producer of ethanol. Intergeneric hybrids of Saccharomyces cerevisiae and Z. fermentati were obtained after protoplast fusion. They were selected as prototrophic strains, after isolation of auxotrophic mutants from Z. fermentati and fusion with an S. cerevisiae strain which was also auxotrophic. The hybrids, which appeared at a frequency of 2 X 10(-7), presented characteristics of both parents, such as resistance to certain drugs and the ability to grow with either cellobiose or lactic acid as the sole carbon source; they were very stable, even under nonselective conditions. These hybrids may have important industrial applications as good fermenting strains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anne J., Eyssen H., De Somer P. Somatic hybridisation of Penicillium roquefortii with P. chrysogenum after protoplast fusion. Nature. 1976 Aug 19;262(5570):719–721. doi: 10.1038/262719a0. [DOI] [PubMed] [Google Scholar]
- Anné J., Peberdy J. F. Induced fusion of fungal protoplasts following treatment with polyethylene glycol. J Gen Microbiol. 1976 Feb;92(2):413–417. doi: 10.1099/00221287-92-2-413. [DOI] [PubMed] [Google Scholar]
- Bailey R. B., Benitez T., Woodward A. Saccharomyces cerevisiae Mutants Resistant to Catabolite Repression: Use in Cheese Whey Hydrolysate Fermentation. Appl Environ Microbiol. 1982 Sep;44(3):631–639. doi: 10.1128/aem.44.3.631-639.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal J., Balbin E., Pieniazek N. J. Method for isolating auxotrophic mutants in Aspergillus nidulans using N-glycosyl-polifungin. J Gen Microbiol. 1974 Sep;84(1):111–116. doi: 10.1099/00221287-84-1-111. [DOI] [PubMed] [Google Scholar]
- Barnett J. A. The utilization of sugars by yeasts. Adv Carbohydr Chem Biochem. 1976;32:125–234. doi: 10.1016/s0065-2318(08)60337-6. [DOI] [PubMed] [Google Scholar]
- Bassel J., Warfel J., Mortimer R. Complementation and genetic recombination in Candida lipolytica. J Bacteriol. 1971 Oct;108(1):609–611. doi: 10.1128/jb.108.1.609-611.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez T., Nurse P., Mitchison J. M. Arginase and sucrase potential in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1980 Dec;46:399–431. doi: 10.1242/jcs.46.1.399. [DOI] [PubMed] [Google Scholar]
- Benítez T., Del Castillo L., Aguilera A., Conde J., Cerdáolmedo E. Selection of wine yeasts for growth and fermentation in the presence of ethanol and sucrose. Appl Environ Microbiol. 1983 May;45(5):1429–1436. doi: 10.1128/aem.45.5.1429-1436.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG C. W., NICKERSON W. J. Polysaccharide syntheses in growing yeasts. J Biol Chem. 1954 May;208(1):395–407. [PubMed] [Google Scholar]
- Calderón I. L., Cerdá-Olmedo E. Induction by N-methyl-N'-nitro-N-nitrosoguanidine of nuclear and cytoplasmic mutations in Saccharomyces cerevisiae. Mutat Res. 1983 Mar;108(1-3):133–146. doi: 10.1016/0027-5107(83)90115-x. [DOI] [PubMed] [Google Scholar]
- DUBOIS M., GILLES K., HAMILTON J. K., REBERS P. A., SMITH F. A colorimetric method for the determination of sugars. Nature. 1951 Jul 28;168(4265):167–167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- DUERKSEN J. D., HALVORSON H. The specificity of induction of beta-glucosidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1959 Nov;36:47–55. doi: 10.1016/0006-3002(59)90068-x. [DOI] [PubMed] [Google Scholar]
- Delgado J. M., Herrera L. S. Protoplast fusion in the yeast Candida utilis. Acta Microbiol Acad Sci Hung. 1981;28(4):339–345. [PubMed] [Google Scholar]
- Ferenczy L., Maráz A. Transfer of mitochondria by protoplast fusion in Saccharomyces cerevisiae. Nature. 1977 Aug 11;268(5620):524–525. doi: 10.1038/268524a0. [DOI] [PubMed] [Google Scholar]
- HU A. S., EPSTEIN R., HALVORSON H. O., BOCK R. M. Yeast beta-glucosidase: comparison of the physical-chemical properties of purified constitutive and inducible enzyme. Arch Biochem Biophys. 1960 Dec;91:210–218. doi: 10.1016/0003-9861(60)90492-6. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar S. N., Partridge R. M., Magee P. T. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics. 1983 Jun;104(2):241–255. doi: 10.1093/genetics/104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevei F., Peberdy J. F. Induced segregation in interspecific hybrids of Aspergillus nidulans and Aspergillus rugulosus obtained by protoplast fusion. Mol Gen Genet. 1979 Feb 26;170(2):213–218. doi: 10.1007/BF00337798. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Notario V., Villa T. G., Benítez T., Villanueva J. R. Beta-glucanases in the yeast Cryptococcus albidus var. aerius. Production and separation of beta-glucanases in asynchronous cultures. Can J Microbiol. 1976 Feb;22(2):261–268. doi: 10.1139/m76-035. [DOI] [PubMed] [Google Scholar]
- Olaiya A. F., Sogin S. J. Ploidy determination of Canadida albicans. J Bacteriol. 1979 Dec;140(3):1043–1049. doi: 10.1128/jb.140.3.1043-1049.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peberdy J. F. Fungal protoplasts: isolation, reversion, and fusion. Annu Rev Microbiol. 1979;33:21–39. doi: 10.1146/annurev.mi.33.100179.000321. [DOI] [PubMed] [Google Scholar]
- Pigac J., Hranueli D., Smokvina T., Alacević M. Optimal Cultural and Physiological Conditions for Handling Streptomyces rimosus Protoplasts. Appl Environ Microbiol. 1982 Nov;44(5):1178–1186. doi: 10.1128/aem.44.5.1178-1186.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaina J., Conde J. Use of the polyene antibiotic N-glycosyl-polifungin in counterselecting yeast mutants. Mutat Res. 1981 Mar;91(2):111–114. doi: 10.1016/0165-7992(81)90082-8. [DOI] [PubMed] [Google Scholar]
- Sarachek A., Rhoads D. D., Schwarzhoff R. H. Hybridization of Candida albicans through fusion of protoplasts. Arch Microbiol. 1981 Mar;129(1):1–8. doi: 10.1007/BF00417169. [DOI] [PubMed] [Google Scholar]
- Wickerham L. J., Kurtzman C. P., Herman A. I. Sexual reproduction in Candida lipolytica. Science. 1970 Feb 20;167(3921):1141–1141. doi: 10.1126/science.167.3921.1141. [DOI] [PubMed] [Google Scholar]
- Woods R. A. Nystatin-resistant mutants of yeast: alterations in sterol content. J Bacteriol. 1971 Oct;108(1):69–73. doi: 10.1128/jb.108.1.69-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



