Abstract
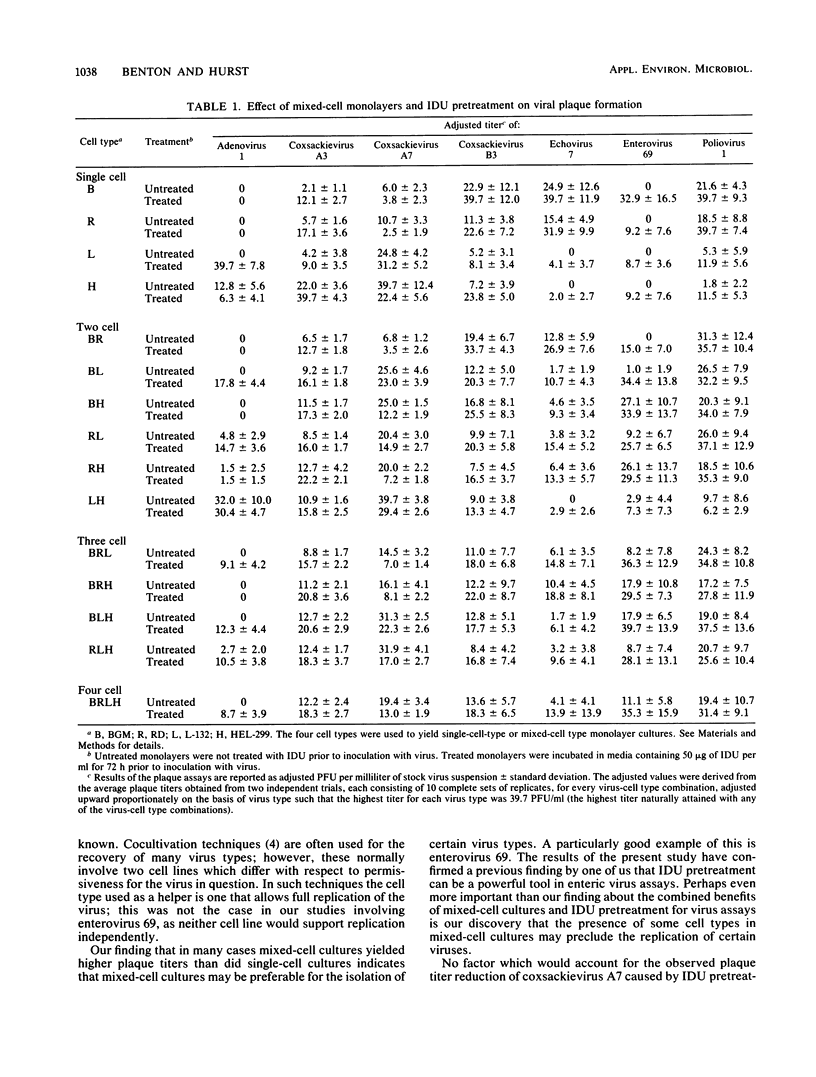
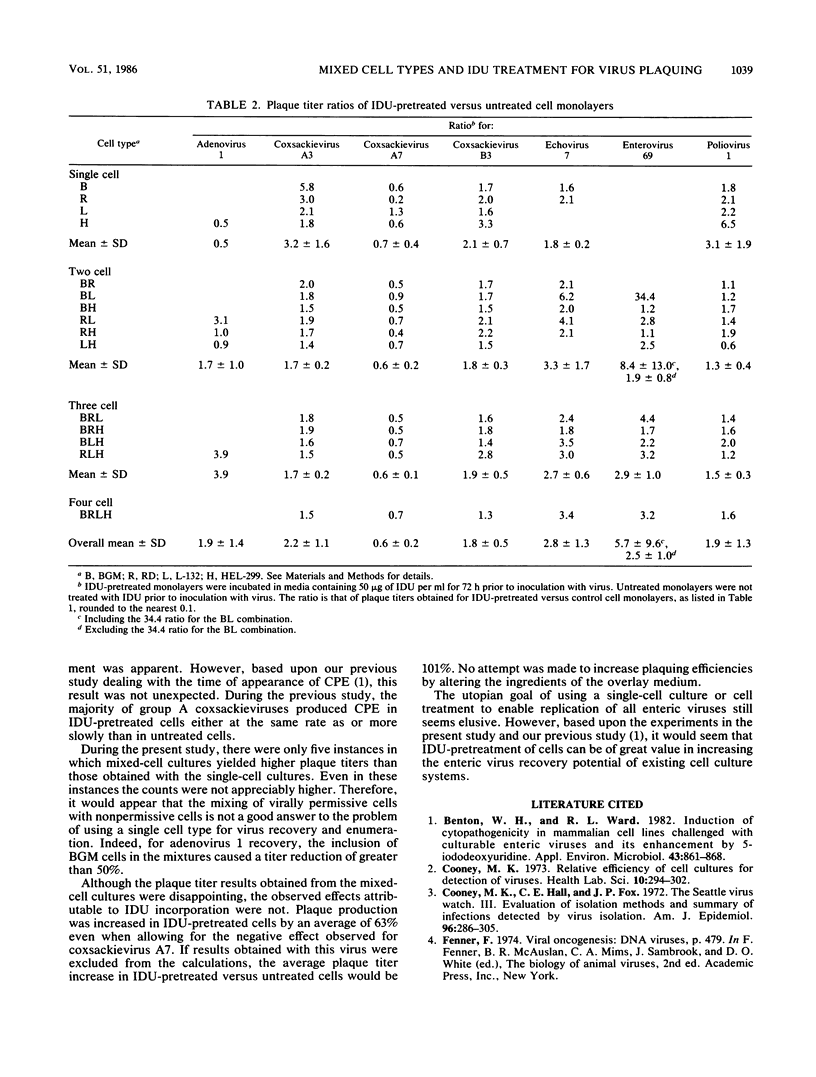
Four continuous cell lines, BGM, L-132, HEL-299, and RD, were compared both when cultured separately and as mixtures for use in plaque assay titrations of human adenovirus 1 and six human enterovirus serotypes. The effect of incubating these cell cultures in media containing 5-iodo-2'deoxyuridine (IDU) prior to inoculation with virus was also studied. The use of mixed-cell cultures revealed cell line-dependent synergistic effects as well as inhibitory effects. These effects were strongly virus dependent. In particular, enterovirus 69 did not form plaques on any of the four cell lines when cultured independently. However, it did form plaques on nearly all of the cell lines when cultured as mixtures. Contrary to this effect, when BGM cells were used in combination with the other cell lines, plaque counts for adenovirus 1 were greatly reduced. The effect of IDU pretreatment was also virus and cell line specific and enabled some viruses to form plaques on cell lines when they otherwise would not. Overall, IDU pretreatment resulted in an approximate twofold increase in plaque titers over those obtained without treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. H., Ward R. L. Induction of cytopathogenicity in mammalian cell lines challenged with culturable enteric viruses and its enhancement by 5-iododeoxyuridine. Appl Environ Microbiol. 1982 Apr;43(4):861–868. doi: 10.1128/aem.43.4.861-868.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney M. K., Hall C. E., Fox J. P. The Seattle virus watch. 3. Evaluation of isolation methods and summary of infections detected by virus isolations. Am J Epidemiol. 1972 Oct;96(4):286–305. doi: 10.1093/oxfordjournals.aje.a121459. [DOI] [PubMed] [Google Scholar]
- Cooney M. K. Relative efficiency of cell cultures for detection of viruses. Health Lab Sci. 1973 Oct;10(4):294–302. [PubMed] [Google Scholar]
- Feorino P. M., Hannon W. H. Use of DEAE dextran in agar overlays to enhance size of ECHO virus plaques. Public Health Rep. 1966 Nov;81(11):1015–1018. [PMC free article] [PubMed] [Google Scholar]
- Green J. A., Baron S. 5-iododeoxyuridine potentiation of the replication in vitro of several unrelated RNA and DNA viruses. Science. 1975 Dec 12;190(4219):1099–1101. doi: 10.1126/science.1188388. [DOI] [PubMed] [Google Scholar]
- Hambling M. H., O'Neill J. J. A comparison of various tissue cultures for the rapid isolation of viruses. Mon Bull Minist Health Public Health Lab Serv. 1967 Dec;26:266–273. [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Nonoyama M., Chang S. Y., Tagamets A., Showalter S. D. Programming of events in Epstein-Barr virus-activated cells induced by 5-iododeoxyuridine. Virology. 1974 Nov;62(1):71–89. doi: 10.1016/0042-6822(74)90304-3. [DOI] [PubMed] [Google Scholar]
- Jerkofsky M., Rapp F. Stimulation of adenovirus replication in simian cells in the absence of a helper virus by pretreatment of the cells with iododeoxyuridine. J Virol. 1975 Feb;15(2):253–258. doi: 10.1128/jvi.15.2.253-258.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K. Studies on the effect of chymotrypsin on reovirions. Virology. 1972 Sep;49(3):700–715. doi: 10.1016/0042-6822(72)90527-2. [DOI] [PubMed] [Google Scholar]
- Lee L. H., Phillips C. A., South M. A., Melnick J. L., Yow M. D. Enteric virus isolation in different cell cultures. Bull World Health Organ. 1965;32(5):657–663. [PMC free article] [PubMed] [Google Scholar]
- Miller D. G., Gabrielson M. O., Horstmann D. M. Clinical virology and viral surveillance in a pediatric group practice: the use of double-seeded tissue culture tubes for primary virus isolation. Am J Epidemiol. 1968 Sep;88(2):245–256. doi: 10.1093/oxfordjournals.aje.a120883. [DOI] [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaier H. P., Wigand R. The influence of iododeoxyuridine pretreatment of various cell cultures on adenovirus multiplication. Med Microbiol Immunol. 1983;171(4):251–258. doi: 10.1007/BF02123499. [DOI] [PubMed] [Google Scholar]
- Simon M., Dömök I. Enhancing effect of human erythrocyte extracts on the susceptibility of monkey kidney cells to certain enteroviruses. Acta Microbiol Acad Sci Hung. 1966;13(3):229–233. [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus replication in cells pretreated with 5-iodo-2'-deoxyuridine. J Virol. 1973 Jun;11(6):986–990. doi: 10.1128/jvi.11.6.986-990.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F. Enhancement of adenovirus plaque formation on HeLa cells by magnesium chloride. J Gen Virol. 1970 Dec;9(3):251–255. doi: 10.1099/0022-1317-9-3-251. [DOI] [PubMed] [Google Scholar]


