Abstract
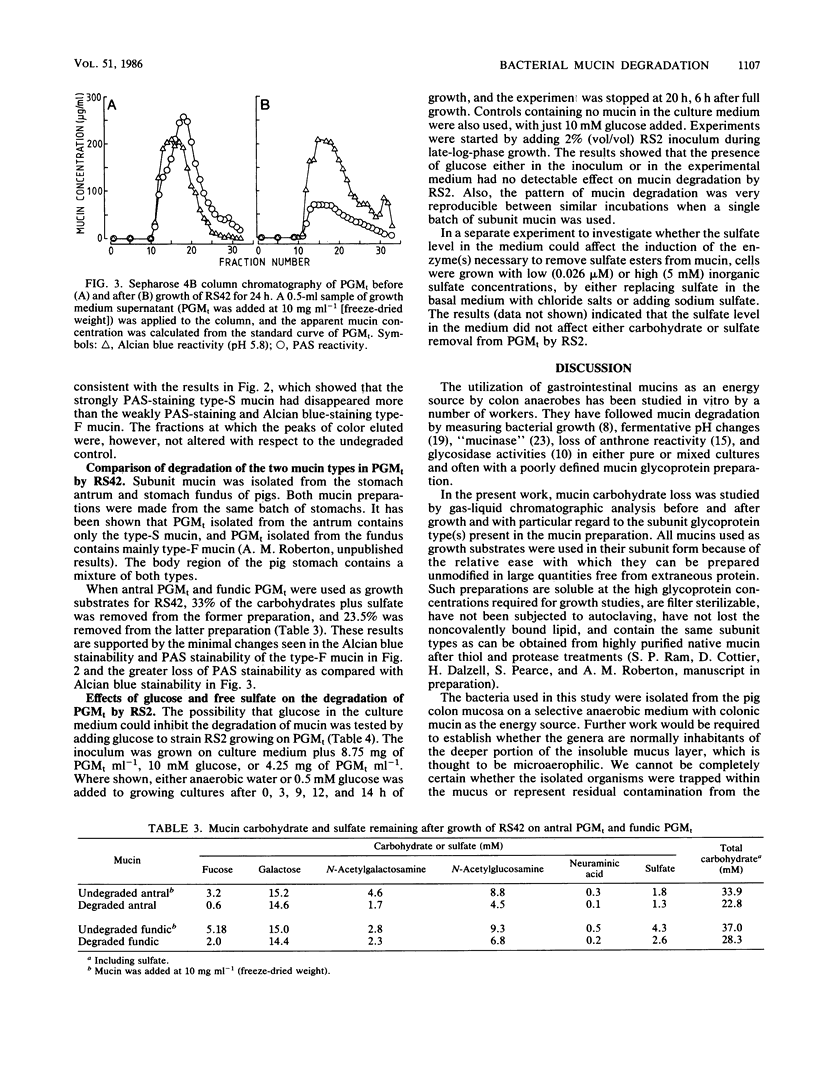
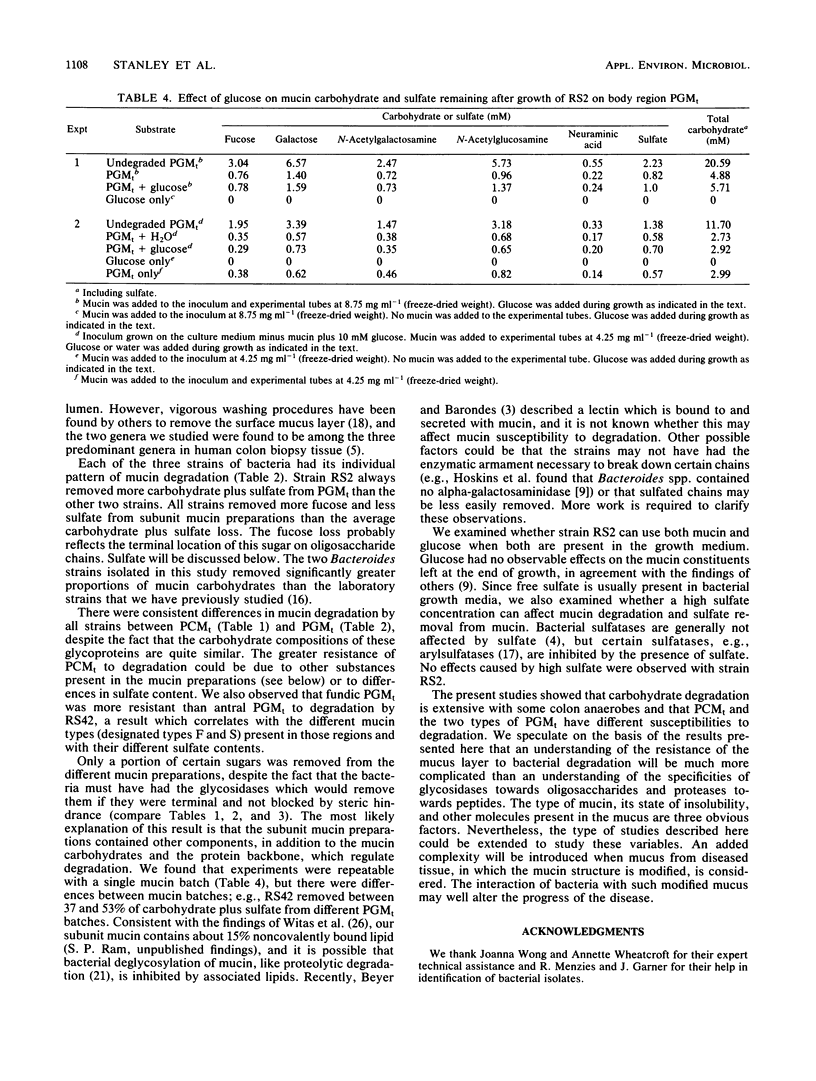
Mucin degradation was studied with one Clostridium (RS42) and two Bacteroides (RS2 and RS13) strains isolated from the pig colon mucosa. Mucins from pig colon and stomach were prepared in their subunit forms for use as growth substrates, and the loss of the individual sugars from the mucins was measured after bacterial growth. Colonic mucin was more resistant to degradation than gastric mucin. The strains differed in their competence in degrading the mucins. Carbohydrate plus sulfate removal from gastric mucin varied from 63 to 76% for RS2, 37 to 46% for RS13, and 37 to 53% for RS42. All three strains removed more fucose (67 to 87%) and less sulfate (22 to 63%) than the average carbohydrate plus sulfate loss. Under the same conditions of growth, a mixed pig fecal culture removed 78% of sulfate and 96% of each sugar. Of the two major glycoprotein types present in the subunit pig gastric mucin preparation (R. A. Stanley, S. P. Lee, and A. M. Roberton, Biochim. Biophys. Acta 760:262-269, 1983), the less highly sulfated mucin was more susceptible to RS42 degradation. The degradation of gastric mucin by RS2 was not affected by glucose or high sulfate concentrations in the growth medium. The results show that the three strains of colon bacteria are capable of significant hydrolysis of mucin carbohydrate and that the extent of degradation seen with pure cultures is determined in part by the subunit glycoprotein type(s) present in the mucin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Cunliffe W. J., Pearson J. P., Sellers L. A., Ward R. Studies on gastrointestinal mucus. Scand J Gastroenterol Suppl. 1984;93:101–113. [PubMed] [Google Scholar]
- BAILEY R. W. The reaction of pentoses with anthrone. Biochem J. 1958 Apr;68(4):669–672. doi: 10.1042/bj0680669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Barondes S. H. Secretion of endogenous lectin by chicken intestinal goblet cells. J Cell Biol. 1982 Jan;92(1):28–33. doi: 10.1083/jcb.92.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston C. E., Jr, Avant G. R., Wilson F. A. Anaerobic bacterial populations on normal and diseased human biopsy tissue obtained at colonoscopy. Appl Environ Microbiol. 1982 May;43(5):1173–1181. doi: 10.1128/aem.43.5.1173-1181.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. L., Miller R. J., Peatfield A. C., Richardson P. S., Williams I., Lampert I. A colorimetric assay for mucous glycoproteins using Alcian Blue [proceedings]. Biochem Soc Trans. 1980 Feb;8(1):72–72. doi: 10.1042/bst0080072. [DOI] [PubMed] [Google Scholar]
- Hoogkamp-Korstanje J. A., Lindner J. G., Marcelis J. H., den Daas-Slagt H., de Vos N. M. Composition and ecology of the human intestinal flora. Antonie Van Leeuwenhoek. 1979;45(1):35–40. doi: 10.1007/BF00400776. [DOI] [PubMed] [Google Scholar]
- Hoskins L. C., Agustines M., McKee W. B., Boulding E. T., Kriaris M., Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985 Mar;75(3):944–953. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981 Jan;67(1):163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Zamcheck N. Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology. 1968 Feb;54(2):210–217. [PubMed] [Google Scholar]
- LINDSTEDT G., LINDSTEDT S., GUSTAFSSON B. E. MUCUS IN INTESTINAL CONTENTS OF GERMFREE RATS. J Exp Med. 1965 Feb 1;121:201–213. doi: 10.1084/jem.121.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miller R. S., Hoskins L. C. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a "most probable number" method. Gastroenterology. 1981 Oct;81(4):759–765. [PubMed] [Google Scholar]
- ROY A. B. The sulphatase of ox liver. II. The purification and properties of sulphatase A. Biochem J. 1953 Nov;55(4):653–661. doi: 10.1042/bj0550653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Stanley R. A. In vitro utilization of mucin by Bacteroides fragilis. Appl Environ Microbiol. 1982 Feb;43(2):325–330. doi: 10.1128/aem.43.2.325-330.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. G. Types and distribution of anaerobic bacteria in the large intestine of pigs. Appl Environ Microbiol. 1979 Feb;37(2):187–193. doi: 10.1128/aem.37.2.187-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977 Nov;34(5):529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri L. J., Hurst R. E., Simpson L., Settine J. M. Analysis of sulfate in complex carbohydrates. Anal Biochem. 1982 Jul 1;123(2):303–309. doi: 10.1016/0003-2697(82)90450-x. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Jozwiak Z., Takagi A., Slomiany B. L. The role of covalently bound fatty acids in the degradation of human gastric mucus glycoprotein. Arch Biochem Biophys. 1984 Mar;229(2):560–567. doi: 10.1016/0003-9861(84)90188-7. [DOI] [PubMed] [Google Scholar]
- Stanley R. A., Lee S. P., Roberton A. M. Heterogeneity in gastrointestinal mucins. Biochim Biophys Acta. 1983 Oct 18;760(2):262–269. doi: 10.1016/0304-4165(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Witas H., Sarosiek J., Aono M., Murty V. L., Slomiany A., Slomiany B. L. Lipids associated with rat small-intestinal mucus glycoprotein. Carbohydr Res. 1983 Aug 16;120:67–76. doi: 10.1016/0008-6215(83)88007-0. [DOI] [PubMed] [Google Scholar]
- Zanetta J. P., Breckenridge W. C., Vincendon G. Analysis of monosaccharides by gas-liquid chromatography of the O-methyl glycosides as trifluoroacetate derivatives. Application to glycoproteins and glycolipids. J Chromatogr. 1972 Jul 5;69(2):291–304. doi: 10.1016/s0021-9673(00)92897-8. [DOI] [PubMed] [Google Scholar]
- van der Wiel-Korstanje J. A., Winkler K. C. The faecal flora in ulcerative colitis. J Med Microbiol. 1975 Nov;8(4):491–501. doi: 10.1099/00222615-8-4-491. [DOI] [PubMed] [Google Scholar]