Abstract
The ability of Nicotiana tabacum cell cultures to utilize farnesol (F-OH) for sterol and sesquiterpene biosynthesis was investigated. [3H]F-OH was readily incorporated into sterols by rapidly growing cell cultures. However, the incorporation rate into sterols was reduced by greater than 70% in elicitor-treated cell cultures whereas a substantial proportion of the radioactivity was redirected into capsidiol, an extracellular sesquiterpene phytoalexin. The incorporation of [3H]F-OH into sterols was inhibited by squalestatin 1, suggesting that [3H]F-OH was incorporated via farnesyl pyrophosphate (F-P-P). Consistent with this possibility, N. tabacum proteins were metabolically labeled with [3H]F-OH or [3H]geranylgeraniol ([3H]GG-OH). Kinase activities converting F-OH to farnesyl monophosphate (F-P) and, subsequently, F-P-P were demonstrated directly by in vitro enzymatic studies. [3H]F-P and [3H]F-P-P were synthesized when exogenous [3H]F-OH was incubated with microsomal fractions and CTP. The kinetics of formation suggested a precursor–product relationship between [3H]F-P and [3H]F-P-P. In agreement with this kinetic pattern of labeling, [32P]F-P and [32P]F-P-P were synthesized when microsomal fractions were incubated with F-OH and F-P, respectively, with [γ-32P]CTP serving as the phosphoryl donor. Under similar conditions, the microsomal fractions catalyzed the enzymatic conversion of [3H]GG-OH to [3H]geranylgeranyl monophosphate and [3H]geranylgeranyl pyrophosphate ([3H]GG-P-P) in CTP-dependent reactions. A novel biosynthetic mechanism involving two successive monophosphorylation reactions was supported by the observation that [3H]CTP was formed when microsomes were incubated with [3H]CDP and either F-P-P or GG-P-P, but not F-P. These results document the presence of at least two CTP-mediated kinases that provide a mechanism for the utilization of F-OH and GG-OH for the biosynthesis of isoprenoid lipids and protein isoprenylation.
Farnesyl pyrophosphate (F-P-P) is a key intermediate in the isoprenoid biosynthetic pathway positioned at a putative regulatory point capable of diverting isoprene units to a number of branch pathways (1). For example, squalene synthase activity, like HMG-CoA reductase, is down-regulated in mammalian cells supplied with exogenous sterols (2–4). In Solanaceous plant cells responding to pathogen or elicitor challenge, sterol biosynthesis is arrested, and the cells synthesize and secrete antimicrobial sesquiterpenes (5, 6). The reduction in sterol biosynthesis has been correlated with a suppression in squalene synthase activity, whereas the induced accumulation of sesquiterpenes correlates with the activation of unique sesquiterpene biosynthetic enzyme activities (6, 7). Farnesylated and geranylgeranylated proteins also have been documented extensively in both plant and animal cell types, and the requisite farnesyl- and geranylgeranyl-transferase activities have been described (8–11).
Farnesol (F-OH) recently has received considerable attention as another important intracellular metabolite of isoprenoid metabolism. Early work on the feedback regulation of sterol biosynthesis suggested that both a sterol and a nonsterol metabolite derived from mevalonate were necessary for the down-regulation of HMG-CoA reductase activity (2). Subsequent investigations implicated F-OH as the putative nonsterol regulator (12, 13). Exogenous F-OH also has been shown to affect several other physiological processes including: inhibition of phosphatidylcholine biosynthesis (14), progression through the cell cycle (15), and induction of biochemical changes associated with an apoptotic program (16). The recent discovery of a nuclear farnesol receptor, FXR, is consistent with a regulatory role for F-OH in controlling diverse metabolic processes (17).
Work in our laboratories has focused on the metabolic shifts that occur between the central isoprenoid pathway and sterol, sesquiterpene, and dolichol biosynthesis (6, 18, 19). Although we have measured changes in the activities of several branch point enzymes in vitro (6), a limitation of our work has been the absence of experimentation probing the in vivo functioning of the downstream and branch-point portions of the isoprenoid pathway. Work more than 10 years earlier provided an experimental means to overcoming this limitation. In 1988 and 1989, Poulter and coworkers demonstrated the incorporation of radiolabeled prenyl alcohols into the geranylgeranyl lipids of Methanospirillum hungatei (20), as well as into the triterpenes of Botryococcus braunii (21). These investigators suggested that the prenol alcohols could be “activated” via the action of a prenol-kinase. In agreement with these observations, F-OH and GG-OH were shown to be utilized for sterol biosynthesis and protein isoprenylation in mammalian cells (22–24). Related studies have reported preliminary evidence for isoprenol kinases in rat liver (25, 26), a microgreen algae (27), and an archebacterium (28). The current work was undertaken to determine whether plant cells could utilize exogenously supplied prenol alcohols for the synthesis of isoprenoid products and, if so, to determine whether the isoprenols were “activated” by novel pyrophosphorylation or successive monophosphorylation reactions.
Materials and Methods
Materials.
t,t-[3H]Farnesol (60 Ci/mmol), t,t-[3H]farnesyl monophosphate (15 Ci/mmol), t,t-[3H]farnesyl pyrophosphate (15 Ci/mmol), t,t,t-[3H]geranylgeraniol (60 Ci/mmol), [γ-32P]GTP (>3,000 Ci/mmol), and [γ-32P]ATP (>6,000 Ci/mmol) were obtained from American Radiolabeled Chemicals (St. Louis). t,t,t-[3H]Geranylgeranyl pyrophosphate (19.3 Ci/mmol) was obtained from DuPont/NEN. Reverse-phase octadecyl (C18) silica gel plates were obtained from J. T. Baker. Benzyl-DEAE cellulose, baker’s yeast nucleoside 5′ diphosphate kinase, bacterial alkaline phosphatase (Type III-S), and Merck precoated silica gel G 60 thin-layer plates were obtained from Sigma. Squalestatin was provided by Glaxo Wellcome. All other solvents and chemicals were reagent grade and purchased from standard commercial sources.
Synthesis of [γ-32P]CTP.
[γ-32P]CTP was synthesized enzymatically from CDP and [γ-32P]ATP by using baker’s yeast nucleoside 5′ diphosphate kinase (N-3380; Sigma) as described previously (19). Enzymatic reactions contained 80 mM CDP, 10 mM MgCl2, 80 mM Tris⋅HCl (pH 7.4), 4 units of yeast nucleoside 5′ diphosphate kinase, and 1 mCi [γ-32P]ATP in a total volume of 0.05 ml. After 1 hr at room temperature, the reaction mixture was chromatographed on a column of Benzyl-DEAE cellulose (1.5 cm × 15 cm) in 10 mM NH4HCO3. [γ-32P]CTP was eluted from the benzyl-DEAE column with a linear gradient (250 ml) of 0–0.5 M NH4HCO3. Fractions containing [γ-32P]CTP were combined, concentrated to a volume of 1 ml by rotary evaporation under reduced pressure at 30°C, and desalted by gel-filtration chromatography on a column (1.5 cm × 30 cm) of Sephadex G-10 eluted with distilled water. Fractions containing [γ-32P]CTP were combined, concentrated again, and stored at −20°C.
Cell Cultures and [3H]Farnesol-Labeling Studies.
Cell suspension cultures of Nicotiana tabacum KY14 were maintained in Murashige and Skoog media, subcultured weekly, and growth-monitored as increases in fresh weight (29). Cultures in the rapid phase of growth (approximately 3 days after subculturing, fresh weight doubling every 24 hr) were used for all the in vivo labeling experiments and microsomal isolation procedures. All additions to the cell cultures including metabolic inhibitors and elicitin were first dissolved in culture media.
For the in vivo metabolic labeling experiments, 10–50 ml of cell culture was incubated with or without squalestatin 1 (SQ) at the designated concentrations, or 100 nM elicitin to induce sesquiterpene metabolism (30), for 5–7 hr before the addition of radiolabeled F-OH. After a further incubation for 4–5 hr with approximately 0.5–1 μCi [3H]F-OH/ml cell culture, cells and media were collected by filtration, frozen in liquid nitrogen, and maintained at −80°C until analyzed. Chloroform extracts of the media samples were examined for the incorporation of radioactivity into the extracellular components including capsidiol by a TLC method (29), and incorporation of radioactivity into cellular components including digitonin precipitable sterols was according to Vögeli and Chappell (6).
For the in vivo protein labeling experiments, 75–100 μl of tobacco cells was treated with 5 μM lovastatin for 16 hr before being incubated with 3 μCi of [3H]F-OH or [3H]GG-OH for an additional 8 hr. Entire cell samples then were homogenized in SDS sample buffer (50 mM Tris⋅HCl, pH 6.8/10 mM DTT/2% SDS/0.01% bromophenol blue/10% glycerol) and centrifuged 10 min at 10,000 × g, which was followed by size separation of proteins on a 12% polyacrylamide-SDS gel (23). After electrophoresis, the gel was infused with Entensify fluorography reagent (Dupont) and exposed to x-ray (Kodak X-Omat-AR) film for 3–6 weeks.
Microsomal Preparations and Squalene Synthase Enzyme Assay.
A crude microsomal fraction necessary for measurements of squalene synthase and prenyl alcohol kinase enzyme activity was prepared as described previously (6). Essentially, 1 g of cells was homogenized in 2 ml of 100 mM potassium phosphate buffer, pH 7.5/250 mM sucrose/4 mM MgCl2/5 mM 2-mercaptoethanol, filtered, and a 10,000 × g supernatant was used to obtain a high-speed pellet (100,000 × g). The microsomal pellets were resuspended in 200 μl of 20 mM Tris⋅HCl (pH 7.5)/10 mM MgCl2/2 mM 2-mercaptoethanol. Squalene synthase enzyme activity, which measures the conversion of [3H]F-P-P into squalene, was determined according Vögeli and Chappell (6).
F-OH Kinase, F-P Kinase, and GG-OH Kinase Assays.
Enzymatic assay mixtures for F-OH, F-P, and GG-OH kinase activities contained tobacco cell membranes (10–250 μg protein), 5 mM Tris⋅HCl (pH 7.4), 50–200 μM CTP, 2–5 mM MgCl2, 0.05% CHAPS, 10 mM sodium orthovanadate, and 0.01–0.1 μCi of either t,t-[3H]F-OH (60 Ci/mmol), t,t-[3H]F-P (15 Ci/mmol), or t,t,t-[3H]GG-OH (60 Ci/mmol) in a total volume of 20 μl. The kinase reactions were terminated after 10 min at 37° by the addition of 80 μl of CH3OH. After centrifugation for 1 min in a microcentrifuge, the supernatant was removed and the pellet was resuspended in 50 μl CH3OH/H2O (4:1). After a second centrifugation, the supernatants were combined and dried under N2 gas. The labeled products were dissolved in a small volume of water-saturated butanol and analyzed chromatographically by applying aliquots of each sample to Silica G 60 thin-layer plates and developing with isopropanol/NH4OH/H2O (6:3:1). Radioactive products were detected by using a Bioscan Imaging System (Washington, DC), and the level of kinase activity was calculated as a percentage of radiolabeled substrate (F-OH, F-P, or GG-OH) converted to product (F-P, F-P-P, GG-P, GG-P-P). Enzymatic reactions containing [32P]NTPs as the isotopic substrate contained unlabeled isoprenyl alcohols (20 μM) and 0.1–0.5 μCi of either [γ-32P]ATP, [γ-32P]GTP, or [γ-32P]CTP. The formation of isoprenyl [32P]phosphate products was determined as described above.
Identification of F-OH and F-P Kinase Reaction Products.
The reaction products from the F-OH and F-P kinase assays had the chromatographic mobility of authentic F-P and F-P-P when analyzed on Silica gel 60 TLC plates developed with isopropanol/NH4OH/water (6:3:1). The enzymatically labeled products were located by scanning with a Bioscan Imaging System, and the standards were detected by an anisaldehyde spray reagent (31). For further characterization, the radioactive zones corresponding to F-P and F-P-P were scraped from the TLC plates and these zones were eluted with CHCl3/CH3OH/H2O (10:10:3). The purified compounds were incubated for 60 min at 37°C with 0.1% CHAPS/10 mM MgCl2/50 mM Tris⋅HCl, pH 8.5/0.05 unit of bacterial alkaline phosphatase in a total volume of 40 μl. After 60 min, the dephosphorylation reactions were terminated by the addition of 40 μl of ethanol. The samples then were dried under N2 and dissolved in 20 μl of water-saturated butanol. These samples were analyzed chromatographically on TLC plates as described above. Quantitative conversion of authentic [3H]F-P and [3H]F-P-P to [3H]F-OH by treatment with bacterial alkaline phosphatase served as a control for this experimental procedure.
Results
[3H]Farnesol Incorporation into Sterols by Cell Suspension Cultures of Nicotiana tabacum Is SQ-Sensitive.
Similar to reports on the incorporation of radiolabeled F-OH into triterpenoids and prenylated proteins in archebacteria (20), algae (21), and animal cells (23), preliminary experiments with the tobacco cell suspension cultures suggested substantial incorporation of radiolabeled farnesol into cellular isoprenoids. These results, however, did not distinguish between a possible direct incorporation, perhaps involving a novel farnesyl donor, or incorporation via the conversion to F-P-P. Because there is no precedent for farnesyl intermediates other than F-P-P, conversion of F-OH to the allylic pyrophosphate form before being utilized for isoprenoid biosynthesis was considered likely. If incorporation into sterols were to occur via F-P-P, then sensitivity to specific inhibitors of squalene synthase like SQ would be expected.
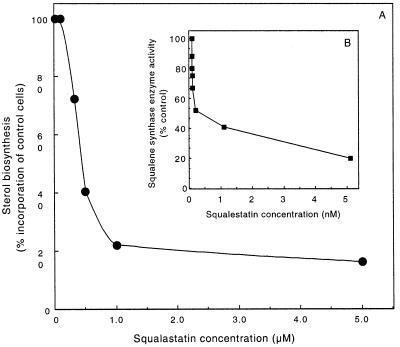
SQ is a well known inhibitor of squalene synthase activity in mammalian cells (32–36), and preliminary reports indicate that it is an effective inhibitor of this enzyme in plants (37). Tobacco squalene synthase activity was found to be strongly inhibited at subnanomolar concentrations of SQ (Fig. 1B). Inhibition of squalene synthase activity was evident at 10 pM SQ, and 1 nM SQ was sufficient to inhibit tobacco squalene synthase activity in vitro greater than 50% in the presence of 20 μM F-P-P.
Figure 1.
SQ inhibits the incorporation of [3H]F-OH into sterols by N. tabacum cell cultures (A) and microsomal squalene synthase activity measured in vitro (B). Cell cultures were incubated with the indicated concentrations of SQ for 6.5 hr before adding 0.5 μCi [3H]F-OH for an additional 4.5 hr. The incorporation of radiolabel into digitonin-precipitable sterols by control cells (3,000 dpm) is compared with that for the SQ-treated cells (A). The effect of SQ on the in vitro squalene synthase activity (100% = 40 nmol/hr⋅mg protein) was determined after preincubating microsomes for 15 min with the indicated concentrations of SQ (B).
F-OH incorporation into sterols by tobacco cell suspension cultures in their rapid growth phase (fresh weight doubling every 18–24 h) was also sensitive to SQ (Fig. 1A). It is important to note that the amounts of SQ required to inhibit F-OH incorporation in the in vivo studies were in the micromolar range instead of the nanomolar concentrations needed in the in vitro studies (Fig. 1B). This difference presumably is due to limitations on the rate of entry of the drug into intact cells. To facilitate uptake of SQ, cultures were pretreated with the inhibitor at the indicated concentrations for 6.5 hr before the addition of [3H]F-OH. After a further incubation for 4.5 hr, uptake of radioactivity into the cells and incorporation into sterols were determined. [3H]F-OH uptake was inhibited slightly by the SQ treatments (2–15%) and was 85% of the control level at the highest SQ concentrations tested. Therefore, incorporation rates into sterols first were normalized to the percentage of the total [3H]F-OH taken up, and then incorporation into sterols by control cells was compared with the SQ-treated cells. Similar to the in vitro inhibition studies, inhibition of F-OH incorporation into sterols by cell cultures showed a biphasic curve with respect to SQ concentrations, with a greater than 80% reduction at 1 μM SQ.
[3H]F-OH Is Incorporated into Sesquiterpenes in Elicitor-Treated Cells.
Tobacco cell suspension cultures respond to attenuated pathogens and pathogen-derived elicitors by the de novo synthesis and secretion of capsidiol, an extracellular sesquiterpenoid possessing antimicrobial properties (38). This induction occurred simultaneously with a repression of sterol biosynthesis mediated by suppression of squalene synthase enzyme activity and the induction of 5-epi-aristolochene synthase (EAS), a sesquiterpene cyclase (6). The EAS enzyme, like squalene synthase, can utilize F-P-P but not F-OH as a substrate (J.C., unpublished observation).
Cell cultures in rapid growth phase were left untreated or a fungal elicitor was added to the cultures for 6.5 hr before the addition of [3H]F-OH. After a further incubation for 4.5 hr, the incorporation of [3H]F-OH into sterols and extracellular capsidiol was determined. In Table 1, the amount of [3H]F-OH incorporated into sterols or capsidiol was calculated as a percentage of that taken up by the cells. This was done to account for a 7% reduction in F-OH taken up by the elicitor-treated cells. Consistent with previous labeling studies with acetate and mevalonate (6), the incorporation rate of F-OH into sterols was reduced approximately 70% by the elicitor-treatment whereas a dramatic increase in the incorporation rate into capsidiol was observed.
Table 1.
Incorporation of [3H]F-OH into sterols and capsidiol by control and elicitor-treated cell cultures
| Treatment | Incorporation of [3H]F-OH into
|
|
|---|---|---|
| Sterols | Capsidiol | |
| Control | 26.5 | 0.6 |
| Elicitor | 7.7 | 4.3 |
Cells were incubated without or with 100 nM elicitin for 6.5 hr before adding 0.5 μCi [3H]F-OH for an additional 4.5 hr. Incorporation of radioactivity into cellular sterols and extracellular capsidiol is expressed as a percentage of the total [3H]F-OH incorporation.
Metabolic Labeling of Proteins by [3H]F-OH and [3H]GG-OH.
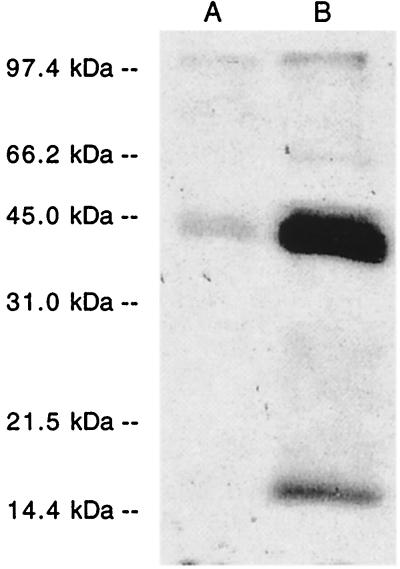
To determine whether [3H]F-OH and [3H]GG-OH were incorporated into tobacco cell proteins as reported for animal cells (22, 23), cells first were treated with lovastatin, a competitive inhibitor of HMG-CoA reductase to deplete the internal pools of isoprenoids, then were incubated with [3H]F-OH or [3H]GG-OH. After an 8-hr incubation, cells were homogenized in an SDS extraction buffer and the proteins were size-separated by SDS/PAGE. The autoradiogram of the gel in Fig. 2 shows that radiolabel was incorporated into several molecular species of proteins, many of which correlate with the sizes of prenylated proteins previously reported by Randall et al. (8).
Figure 2.
[3H]F-OH and [3H]GG-OH are incorporated into N. tabacum cell proteins. Cell cultures were pretreated with 5 μM lovastatin for 16 hr before incubating with either [3H]F-OH (A) or [3H]GG-OH (B) for 8 hr. Total, radiolabeled proteins were extracted in SDS sample buffer, size-separated by SDS/PAGE, and visualized by fluorography.
Characterization of F-OH and F-P Kinase Activities.
The metabolic labeling studies with [3H]F-OH indicated that the free isoprenols might be incorporated into the two lipid products and proteins via F-P-P. To determine whether kinase activities capable of converting F-OH to F-P/F-P-P could be detected, various cellular fractions including a 10,000 × g supernatant and pellet and a 100,000 × g microsomal preparation were incubated with [3H]F-OH and various nucleoside triphosphates. The formation of anionic products (F-P and F-P-P) was assessed initially by the retention of radioactivity to an ion-exchange resin. In these pilot experiments, the formation of anionic products was detected when incubations included 100 μM ATP, CTP, GTP, or UTP, and greater than 90% of the kinase activity was found in association with the microsomal fraction. The observation that the formation of the anionic products was nucleotide-dependent strongly suggested that they were phosphorylated intermediates and not simply oxidation products. More extensive in vitro assays using a direct evaluation of the reaction products by TLC revealed that some [3H]F-P was formed in the presence of ATP, GTP, or UTP (Table 2). However, much more significant levels of [3H]F-P and [3H]F-P-P were observed with incubations containing 100 μM CTP. In addition, when [3H]F-P was incubated with microsomes and unlabeled ATP, CTP, UTP, or GTP, [3H]F-P-P was observed only in the presence of CTP (data not shown).
Table 2.
Nucleoside triphosphate specificity of phosphoryl donors for the phosphorylation of F-OH, F-P, GG-OH, and GG-P by tobacco microsomes
| Nucleotide added, 100 μM | % [3H]F-OH converted to
|
% [3H]GG-OH converted to
|
||
|---|---|---|---|---|
| [3H]F-P | [3H]F-P-P | [3H]GG-P | [3H]GG-P-P | |
| None | <0.3 | <0.3 | <0.3 | <0.3 |
| CTP | 18.2 | 22.1 | 37.4 | 20 |
| UTP | 13.8 | <0.3 | 66.0 | 2.3 |
| GTP | 13.2 | <0.3 | 50.1 | <0.3 |
| ATP | <0.3 | <0.3 | 12.8 | <0.3 |
The percent conversion of [3H]F-OH or [3H]GG-OH to the corresponding monophosphorylated and pyrophosphorylated forms was determined as described in Materials and Methods.
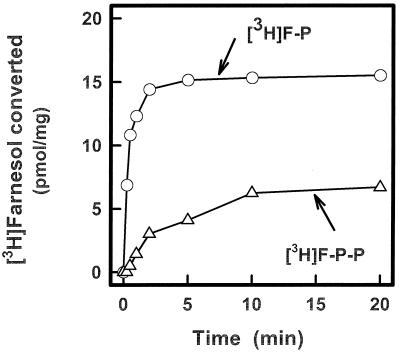
The phosphorylation reactions were characterized further for their divalent cation requirement, optimal substrate concentrations, and for addition of antagonists to competing reactions. The kinase activity was optimal at 2–5 mM MgCl2, whereas the generation of phosphorylated products was maximal at 500 μM CTP and 20 μM F-OH. The addition of sodium orthovanadate (5 mM) enhanced the recovery of radioactive F-P and F-P-P, presumably by inhibiting nonspecific phosphatases that might degrade CTP and the isoprenyl phosphate products (18) generated in the reactions. The time course illustrated in Fig. 3 shows that [3H]F-OH was converted to [3H]F-P and [3H]F-P-P in the presence of CTP and that the initial rate of F-P formation was higher than F-P-P.
Figure 3.
Time course for the CTP-mediated phosphorylation of [3H]F-OH catalyzed by membrane fractions from N. tabacum cells. The reaction mixtures and the procedure for assaying the formation of [3H]F-P and [3H]F-P-P are described in Materials and Methods.
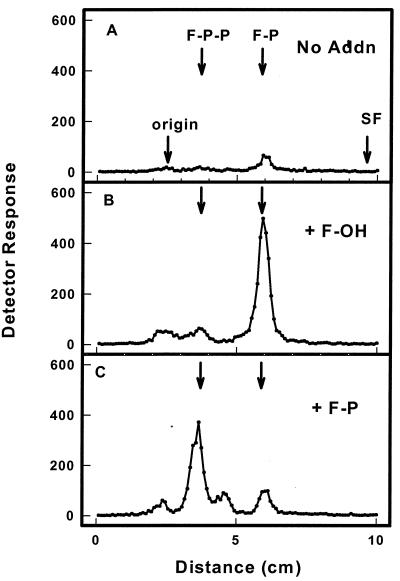
Because [3H]F-OH was the isotopic precursor used in the experiment depicted in Fig. 3, it was possible that [3H]F-P-P was synthesized by a pyrophosphoryl transfer and [3H]F-P was a degradation product generated by endogenous phosphatases. Alternatively, the microsomal kinase(s) could catalyze successive transfers of monophosphoryl groups from CTP to F-OH and F-P. To distinguish between these two possibilities, [γ-32P]CTP was tested as a substrate. From Fig. 4 it can be seen that 32P was transferred from [γ-32P]CTP to exogenous F-OH, forming [32P]F-P and a small amount of radiolabeled F-P-P (Fig. 4B). When exogenous F-P was added to the reaction mixture, [32P]F-P-P was formed (Fig. 4C). Only a minor peak of radiolabel comigrated with the mobility of F-P in the later incubation mix, possibly reflecting the phosphorylation of a small amount of endogenous F-OH in the microsomal preparation. Consistent with this notion, similar amounts of this labeled product were observed in control incubations that contained only [γ-32P]CTP but no exogenous F-OH (Fig. 4A).
Figure 4.
Enzymatic transfer of [32P]phosphoryl groups from [γ-32P]CTP to exogenous F-OH (B) and F-P (C). Reaction mixtures contained 5 mM Tris⋅HCl, pH 7.4, tobacco microsomes (30 μg protein), 0.05% CHAPS, 5 mM MgCl2, 1 mM EDTA, 10 mM sodium orthovanadate, 1 μM [γ-32P]CTP (20,000 cpm/pmol), and either no exogenous substrate (A), 2 μM F-OH (B), or 2 μM F-P (C) in a total volume of 20 μl. After incubation at 37°C for 10 min, the reaction was stopped by the addition of 0.5 ml of H2O-saturated n-butanol. The butanol extract was washed three times with 0.5 ml of n-butanol-saturated H2O to remove unreacted [γ-32P]CTP and dried under a stream of N2. The enzymatically labeled products were identified by chromatography on Silica gel G plates developed with isopropanol/NH4OH/H2O (6:3:1). The chromatographic positions of authentic F-P and F-P-P are indicated by arrows.
Enzymatic Synthesis of GG-P-P from GG-OH Catalyzed by Microsomal CTP-Mediated Kinases.
Because the experiment in Fig. 2 demonstrated that [3H]GG-OH can be utilized for geranylgeranylation of tobacco cell proteins, it was of interest to determine whether microsomal fractions could enzymatically convert GG-OH to GG-P-P. Using in vitro conditions identical to the experiments presented in Figs. 3 and 4, [3H]GG-OH apparently was converted to [3H]GG-P when CTP, ATP, GTP, or UTP was included in the incubation mixture (Table 2). However, the conversion of GG-P to GG-PP exhibited a much greater selectivity for CTP.
Biosynthesis of CTP by the Reversibility of the F-P and GG-P Kinase Reactions.
To corroborate the presence of F-P and GG-P kinase activities in the tobacco cell membranes and to examine the reversibility of these kinase reactions, microsomal fractions were incubated with [3H]CDP in the presence and absence of exogenous F-P-P or GG-P-P, and the appearance of [3H]CTP was monitored by TLC analysis (Table 3). When microsomes were incubated with only [3H]CDP, no radiolabeled [3H]CTP was observed. However, when either F-P-P or GG-P-P were included in the reaction mixture, a significant amount of [3H]CDP was converted to [3H]CTP. CTP formation was maximal at 20 μM CDP. CTP was not formed when microsomes were incubated with [3H]CDP and F-P, indicating that the formation of the phosphomonoester linkage in the isoprenyl monophosphate is not freely reversible.
Table 3.
CTP is formed reversibly when microsomes are incubated with [3H]CDP and either F-P-P or GG-P-P
| Addition to incubation mixture, 100 μM | [3H]CDP converted to [3H]CTP, pmol/mg |
|---|---|
| None | <0.1 |
| F-P | <0.1 |
| F-P-P | 580 |
| GG-P-P | 739 |
Reaction mixtures contained 25 mM Tris⋅HCl (pH 7.4), tobacco microsomes (15 μg protein), 5 mM MgCl2, 1 mM EDTA, 10 mM sodium orthovanadate, 20.8 μM [3H]CDP (480 cpm/pmol), and 100 μM F-P, F-P-P, or GG-P-P, where indicated, in a total volume of 0.01 ml. After an incubation for 15 min at 30°C, an aliquot (1 μL) was removed and applied to a polyethyleneimine-cellulose chromatogram. The chromatogram was developed with 0.3 M sodium phosphate (pH 3.5), and the radioactive zones corresponding to [3H]CDP and [3H]CTP were located by scanning with a Bioscan Imaging System. The radioactive zones were scraped from the plate and eluted with 1 ml of 1% SDS, and the amount of [3H]CDP converted to [3H]CTP was determined by scintillation spectrometry.
Discussion
The current work was undertaken as part of a continuing effort to evaluate regulation of metabolic branch points in the isoprenoid biosynthetic pathway in eukaryotes. Earlier work (6) feeding radiolabeled acetate and mevalonate to tobacco cell suspension cultures suggested a diversion of F-P-P from sterol biosynthesis to sesquiterpene biosynthesis in response to elicitor or pathogen challenge. This was supported by experiments demonstrating a suppression of squalene synthase enzyme activity and a coordinate induction of a sesquiterpene cyclase activity (6). Although consistent with the idea of a metabolic switch occurring at the position of F-P-P, more direct in vivo evidence was sought. Although the initial labeling studies with radiolabeled F-OH provided encouraging results as a means for probing this branch point of the pathway, the interpretation of these results was complicated. For example, it was possible that radiolabeled F-OH could be incorporated into isoprenoid end products by a direct or unconventional reaction mechanism or by being converted to its pyrophosphorylated form before being incorporated into isoprenoid end products. The block of [3H]F-OH incorporation into sterols by SQ, an inhibitor of squalene synthase (32–36), strongly suggested that F-OH was utilized via F-P-P in tobacco cells.
The in vitro studies described here have demonstrated the presence of kinase activities capable of converting F-OH to F-P-P by successive monophosphorylation reactions. The evidence for an F-P kinase is based on showing that exogenous F-P is converted to F-P-P by a CTP-dependent reaction. This reaction is corroborated by the observation that CTP could be reversibly formed when microsomes were incubated with CDP and F-P-P or GG-P-P. We are unaware of any previous descriptions of this enzymatic mechanism for the synthesis of CTP. The latter observation also argues against the direct formation of the allylic-P-P forms by a pyrophosphorylation of F-OH or GG-OH. The rapid formation of F-P followed by the somewhat slower accumulation rate of F-P-P is also consistent with the sequential monophosphorylation mechanism.
There is now good evidence for enzymes capable of converting F-OH and GG-OH to the respective allylic pyrophosphates from a variety of organisms. Five years after Huang and Poulter (21) reported that botryococcenes could be metabolically labeled in Botryococcus braunii by [3H]F-OH, Ogura and coworkers (27) demonstrated that 100,000 × g pellets from the microalga catalyzed the conversion of F-OH to F-P, with CTP acting as the phosphoryl donor. Under these in vitro conditions a small amount of F-P-P was also detected. Cell extracts from a halophilic archeon have been shown to convert F-OH to F-P and F-P-P in the presence of ATP (39). After the reports that F-OH and GG-OH could be utilized for sterol biosynthesis and protein isoprenylation in mammalian cells (reviewed in ref. 40), Westfall et al. (25) reported preliminary evidence for the CTP-dependent conversion of F-OH to F-P-P by microsomal and peroxisomal fractions from rat liver. However, neither the nucleotide specificity nor the direct phosphorylation of F-P was described in this study. More recently, Bentinger et al. (26) found that microsomes, but not peroxisomes, from rat liver catalyzed the phosphorylation of F-OH with ATP, CTP, or GTP serving as the phosphoryl donor. Based on a coupled assay monitoring subsequent GG-P-P biosynthesis, liver microsomes synthesized F-P-P from F-P and CTP. In a related study, membranous and soluble fractions from Sulfolobus acidocaldarus were shown to catalyze the phosphorylation of GG-OH and GG-P (28). The microsomal activity was similar to the tobacco enzyme(s) in that GG-P was formed from GG-OH and ATP, GTP, UTP, or CTP. In contrast to the CTP-dependent GG-P kinase in tobacco cells, the archebacterial enzyme apparently uses ATP as its sole phosphoryl donor.
The metabolic labeling experiments presented here also demonstrate that F-OH and GG-OH can be utilized by tobacco cells for protein prenylation as observed in mammalian cells (40). The presence of protein/prenyltransferases that utilize F-P-P and GG-P-P as their isoprenyl donors is well established in plants (8, 9, 41–44). The current in vitro studies now present evidence for enzymes capable of converting F-OH and GG-OH to F-P-P and GG-P-P, respectively, providing an alternative mechanism to the conventional mevalonate pathway for the synthesis of these isoprenyl donors. Although considerably more work will be required to establish whether F-OH and GG-OH are converted to the respective allylic pyrophosphate intermediates by separate isoprenol and isoprenyl monophosphate kinases, equally important will be investigations to discern the relative contribution of this “salvage pathway” (40) to the recycling of prenyl alcohols to the F-P-P and GG-P-P pools. It also will be interesting to learn if the kinase phosphorylating F-OH and GG-OH is structurally related to the CTP-mediated polyisoprenol kinase that phosphorylates dolichol in mammalian cells (45–47).
Acknowledgments
This work was supported by a National Institutes of Health Grant GM36065 awarded to C.J.W. and a National Science Foundation Grant IBN-9729590 to J.C.
Abbreviations
- F-P-P
farnesyl pyrophosphate
- F-OH
farnesol
- SQ
squalestatin 1
- GG-OH
geranylgeraniol
- GG-P-P
geranylgeranyl pyrophosphate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Grunler J, Ericsson J, Dallner G. Biochim Biophys Acta. 1994;1212:259–277. doi: 10.1016/0005-2760(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein J L, Brown M S. Nature (London) 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 3.Faust J R, Goldstein J L, Brown M S. Proc Natl Acad Sci USA. 1979;76:5018–5022. doi: 10.1073/pnas.76.10.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang G, McKenzie T L, Conrad D G, Shechter I. J Biol Chem. 1993;268:12818–12824. [PubMed] [Google Scholar]
- 5.Bostock R M, Kuc J, Laine R A. Science. 1981;212:67–69. doi: 10.1126/science.212.4490.67. [DOI] [PubMed] [Google Scholar]
- 6.Vögeli U, Chappell J. Plant Physiol. 1988;88:1291–1296. doi: 10.1104/pp.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zook M N, Kuc J A. Physiol Mol Plant Pathol. 1991;39:377–390. [Google Scholar]
- 8.Randall S K, Marshall M S, Crowell D N. Plant Cell. 1993;5:433–442. doi: 10.1105/tpc.5.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian D, Zhou D, Ju R, Cramer C L, Yang Z. Plant Cell. 1996;8:2381–2394. doi: 10.1105/tpc.8.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maltese W A. FASEB J. 1990;4:3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F L, Casey P J. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 12.Correll C C, Ng L, Edwards P A. J Biol Chem. 1994;269:17390–17393. [PubMed] [Google Scholar]
- 13.Meigs T E, Simoni R D. Arch Biochem Biophys. 1997;345:1–9. doi: 10.1006/abbi.1997.0200. [DOI] [PubMed] [Google Scholar]
- 14.Miquel K, Pradines A, Terce F, Selmi S, Favre G. J Biol Chem. 1998;273:26179–26186. doi: 10.1074/jbc.273.40.26179. [DOI] [PubMed] [Google Scholar]
- 15.Machida K, Tanaka T, Yano Y, Otani S, Taniguci M. Microbiol. 1999;145:293–299. doi: 10.1099/13500872-145-2-293. [DOI] [PubMed] [Google Scholar]
- 16.Huag J S, Goldner C M, Melnykovych G. Biochim Biophys Acta. 1994;1223:133–140. doi: 10.1016/0167-4889(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 17.Froman B M, Goode E, Chen J, Oro A E, Bradley D J, Perlmann T, Noonan D J, Burka L T, McMorris T, Lamph W W, et al. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 18.Crick D C, Waechter C J. J Neurochem. 1994;62:247–256. doi: 10.1046/j.1471-4159.1994.62010247.x. [DOI] [PubMed] [Google Scholar]
- 19.Crick D C, Scocca J R, Rush J S, Frank D W, Krag S S, Waechter C J. J Biol Chem. 1994;269:10559–10565. [PubMed] [Google Scholar]
- 20.Poulter C D, Aoki T, Daniels L. J Am Chem Soc. 1988;110:2620–2624. [Google Scholar]
- 21.Huang Z, Poulter C D. J Am Chem Soc. 1989;111:2713–2715. [Google Scholar]
- 22.Crick D C, Waechter C J, Andres D A. Biochem Biophys Res Commun. 1994;205:955–961. doi: 10.1006/bbrc.1994.2758. [DOI] [PubMed] [Google Scholar]
- 23.Crick D C, Andres D A, Waechter C J. Biochem Biophys Res Commun. 1995;211:590–599. doi: 10.1006/bbrc.1995.1854. [DOI] [PubMed] [Google Scholar]
- 24.Fliesler S J, Keller R K. Biochem Biophys Res Comm. 1995;210:695–702. doi: 10.1006/bbrc.1995.1715. [DOI] [PubMed] [Google Scholar]
- 25.Westfall D, Aboushadi N, Shackelford J E, Krisans S K. Biochem Biophys Res Commun. 1997;230:562–568. doi: 10.1006/bbrc.1996.6014. [DOI] [PubMed] [Google Scholar]
- 26.Bentinger M, Grünler J, Peterson E, Swiezewska E, Dallner G. Arch Biochem Biophys. 1998;353:191–198. doi: 10.1006/abbi.1998.0611. [DOI] [PubMed] [Google Scholar]
- 27.Inoue H, Korenaga T, Sagami H, Koyama T, Ogura K. Biochem Biophys Res Commun. 1994;200:1036–1041. doi: 10.1006/bbrc.1994.1554. [DOI] [PubMed] [Google Scholar]
- 28.Ohnuma S-I, Watanabe M, Nishino T. J Biochem (Tokyo) 1996;119:541–547. doi: 10.1093/oxfordjournals.jbchem.a021275. [DOI] [PubMed] [Google Scholar]
- 29.Chappell J, Nable R. Plant Physiol. 1987;85:469–473. doi: 10.1104/pp.85.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chappell J, Levine A, Tenhaken R, Lusso M, Lamb C. Plant Physiol. 1997;113:621–629. doi: 10.1104/pp.113.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunphy P J, Kerr J D, Pennock J F, Whittle K J, Feeney J. Biochim Biophys Acta. 1967;13:136–147. doi: 10.1016/0304-4165(67)90329-7. [DOI] [PubMed] [Google Scholar]
- 32.Baxter A, Fitzgerald B J, Hutson J L, McCarthy A D, Motteram J M, Ross B C, Sapra M, Snowden M A, Watson N S, Williams R J, et al. J Biol Chem. 1992;267:11705–11708. [PubMed] [Google Scholar]
- 33.Bergstrom J D, Kurtz M M, Rew D J, Amend A M, Karkas J D, Bostedor R G, Bansal V S, Dufresne C, VanMiddlesworth F L, Hensens O D, et al. Proc Natl Acad Sci USA. 1993;90:80–84. doi: 10.1073/pnas.90.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasumi K, Tachikawa K, Sakai K, Murakawa S, Yoshikawa N, Kumizawa S, Endo A. J Antibiot. 1993;46:689–691. doi: 10.7164/antibiotics.46.689. [DOI] [PubMed] [Google Scholar]
- 35.Thelin A, Peterson E, Hutson J L, McCarthy A D, Ericcson J, Dallner G. Biochim Biophys Acta. 1994;1215:245–249. doi: 10.1016/0005-2760(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 36.Crick D A, Suders J, Kluthe C M, Andres D A, Waechter C J. J Neurochem. 1995;65:1365–1373. doi: 10.1046/j.1471-4159.1995.65031365.x. [DOI] [PubMed] [Google Scholar]
- 37.Fulton D C, Tait M, Threlfall D R. Phytochemistry. 1995;38:1137–1141. doi: 10.1016/0031-9422(94)00798-x. [DOI] [PubMed] [Google Scholar]
- 38.Stoessl A, Unwin C H, Ward E W B. Phytopathology Z. 1972;74:141–152. [Google Scholar]
- 39.Tachibana A, Tanaka T, Taniguchi M, Oi S. FEBS Lett. 1996;379:43–46. doi: 10.1016/0014-5793(95)01479-9. [DOI] [PubMed] [Google Scholar]
- 40.Crick D C, Andres D A, Waechter C J. Biochem Biophys Res Commun. 1997;237:483–487. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- 41.Loraine A E, Yalovsky S, Fabry S, Gruissem W. Plant Physiol. 1996;110:1337–1347. doi: 10.1104/pp.110.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt D, Callan K, Gruissem W. Plant Physiol. 1996;112:767–777. doi: 10.1104/pp.112.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cutler S, Bhassemian M, Bonetta D, Cooney S, McCourt P. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- 44.Pei Z M, Ghassemian M, Kwak C M, McCourt P, Schroeder J I. Science. 1998;282:287–290. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- 45.Allen C M, Kalin J R, Sack J, Verizzo D. Biochemistry. 1978;17:5020–5026. doi: 10.1021/bi00616a025. [DOI] [PubMed] [Google Scholar]
- 46.Burton W A, Scher M G, Waechter C J. J Biol Chem. 1979;254:7129–7136. [PubMed] [Google Scholar]
- 47.Sumbilla C, Waechter C J. Methods Enzymol. 1985;111:471–482. doi: 10.1016/s0076-6879(85)11032-3. [DOI] [PubMed] [Google Scholar]