Abstract
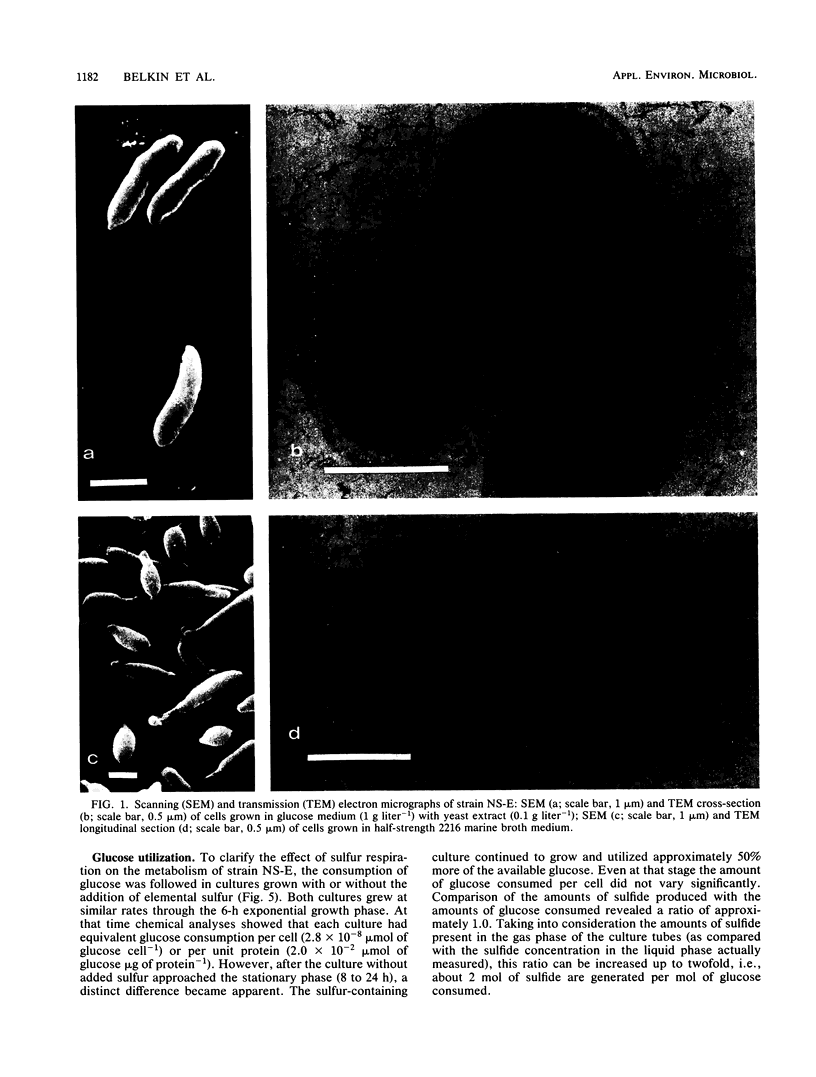
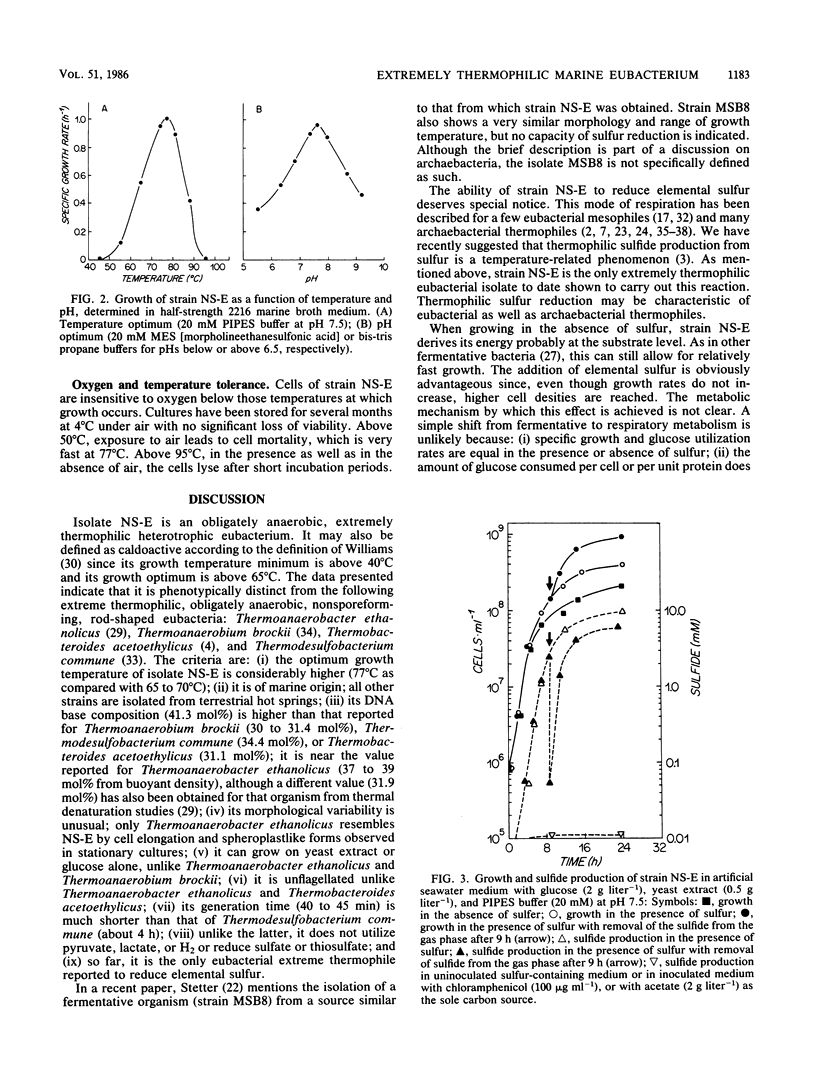
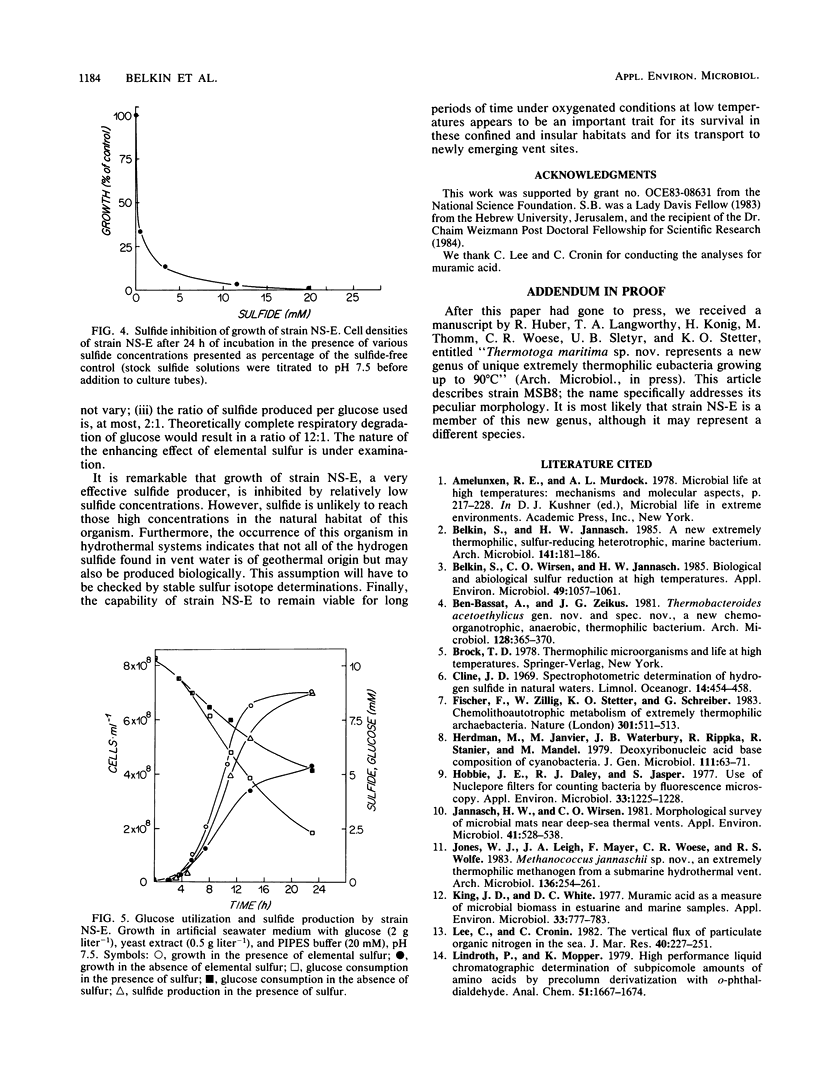
A newly described bacterial isolate, designated strain NS-E, differs from presently known extremely thermophilic bacteria in various characteristics. It is a strictly heterotrophic eubacterium of marine origin and has a temperature range for growth of 50 to 95°C with an optimum at 77°C and a pH of 7.5. Its DNA base composition is 41.3 mol% guanine + cytosine. It is obligately anaerobic, utilizes various sugars as well as yeast extract, and reduces elemental sulfur facultatively to hydrogen sulfide. In 24-h cultures cell densities are up to fourfold higher in the presence than in the absence of elemental sulfur. Sulfide concentrations of 1.0 and 10.0 mM limit growth by 65 and 95%, respectively. Oxygen sensitivity is apparent only at or above that range of temperature at which growth occurs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belkin S., Wirsen C. O., Jannasch H. W. Biological and abiological sulfur reduction at high temperatures. Appl Environ Microbiol. 1985 May;49(5):1057–1061. doi: 10.1128/aem.49.5.1057-1061.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer F., Zillig W., Stetter K. O., Schreiber G. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature. 1983 Feb 10;301(5900):511–513. doi: 10.1038/301511a0. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O. Morphological survey of microbial mats near deep-sea thermal vents. Appl Environ Microbiol. 1981 Feb;41(2):528–538. doi: 10.1128/aem.41.2.528-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. D., White D. C. Muramic acid as a measure of microbial biomass in estuarine and marine samples. Appl Environ Microbiol. 1977 Apr;33(4):777–783. doi: 10.1128/aem.33.4.777-783.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. Physiology of thermophilic bacteria. Adv Microb Physiol. 1979;19:149–243. doi: 10.1016/s0065-2911(08)60199-x. [DOI] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Wirsen C. O., Jannasch H. W. Chemolithotrophic sulfur-oxidizing bacteria from the galapagos rift hydrothermal vents. Appl Environ Microbiol. 1981 Aug;42(2):317–324. doi: 10.1128/aem.42.2.317-324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Wagner L. W., Knowlton S., Ng T. K. Thermophilic anaerobic bacteria which ferment hemicellulose: characterization of organisms and identification of plasmids. Arch Microbiol. 1984 May;138(1):31–36. doi: 10.1007/BF00425403. [DOI] [PubMed] [Google Scholar]
- Wolfe R. S., Penning N. Reduction of sulfur by spirillum 5175 and syntrophism with Chlorobium. Appl Environ Microbiol. 1977 Feb;33(2):427–433. doi: 10.1128/aem.33.2.427-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



