Abstract
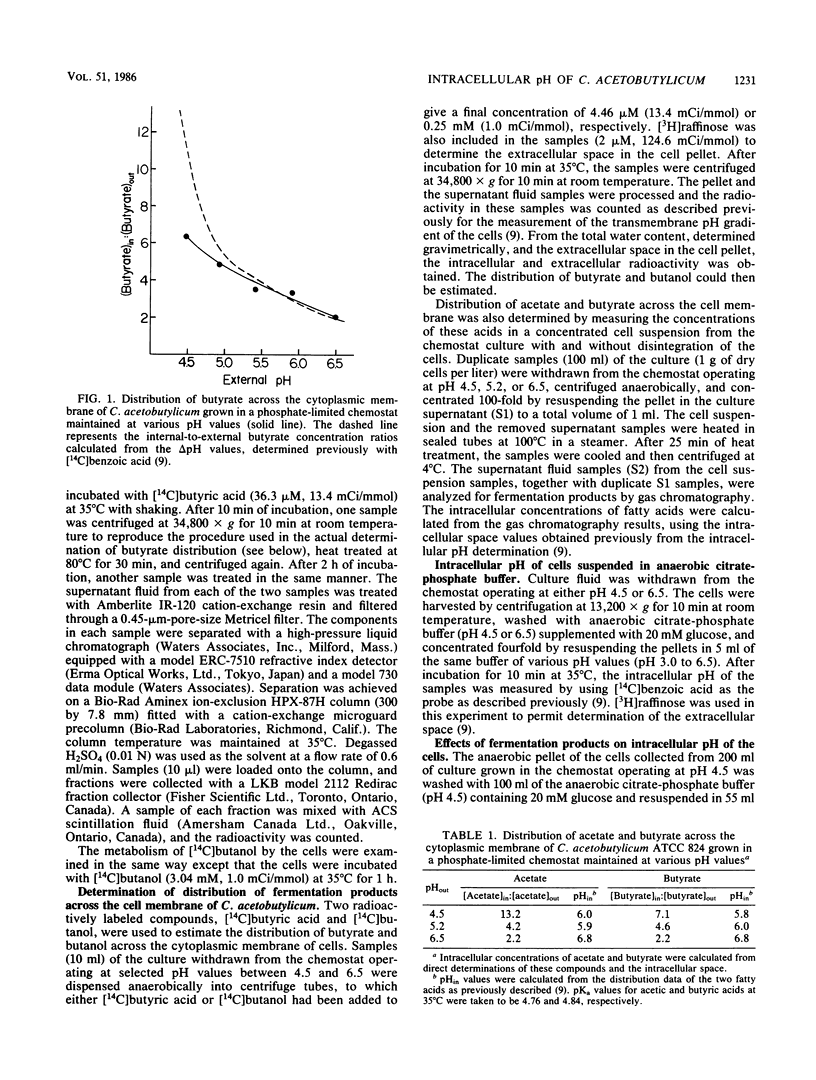
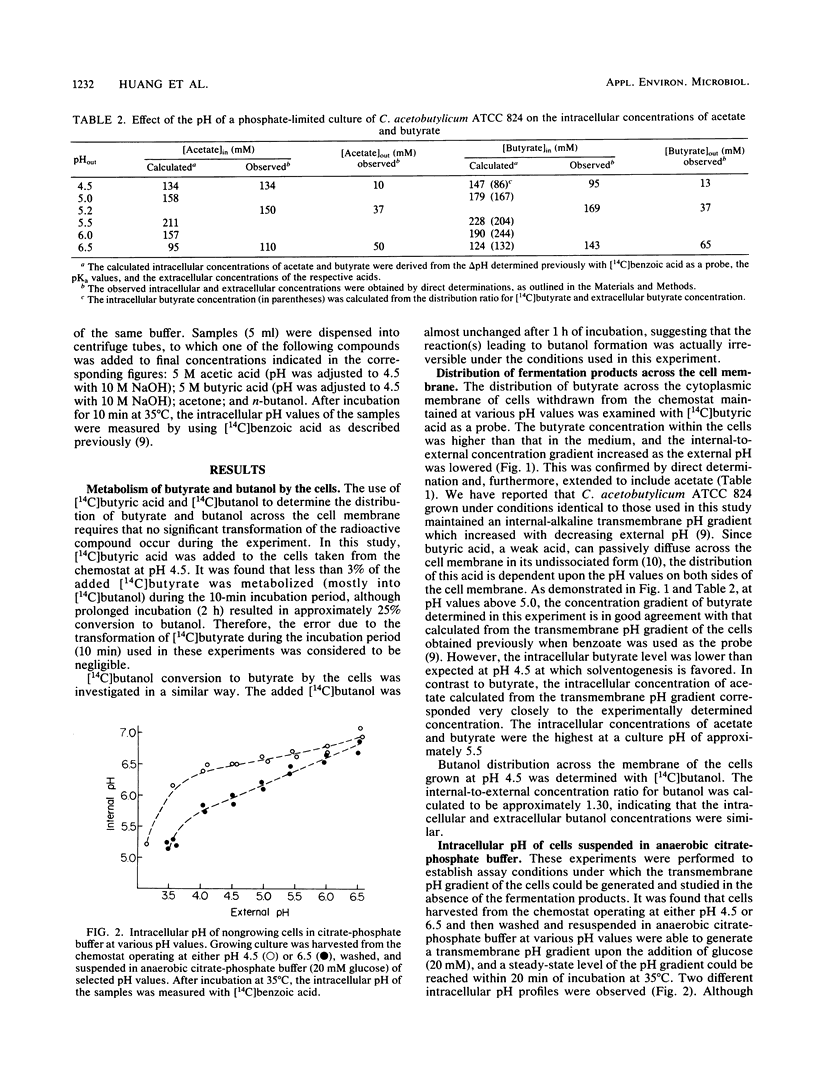
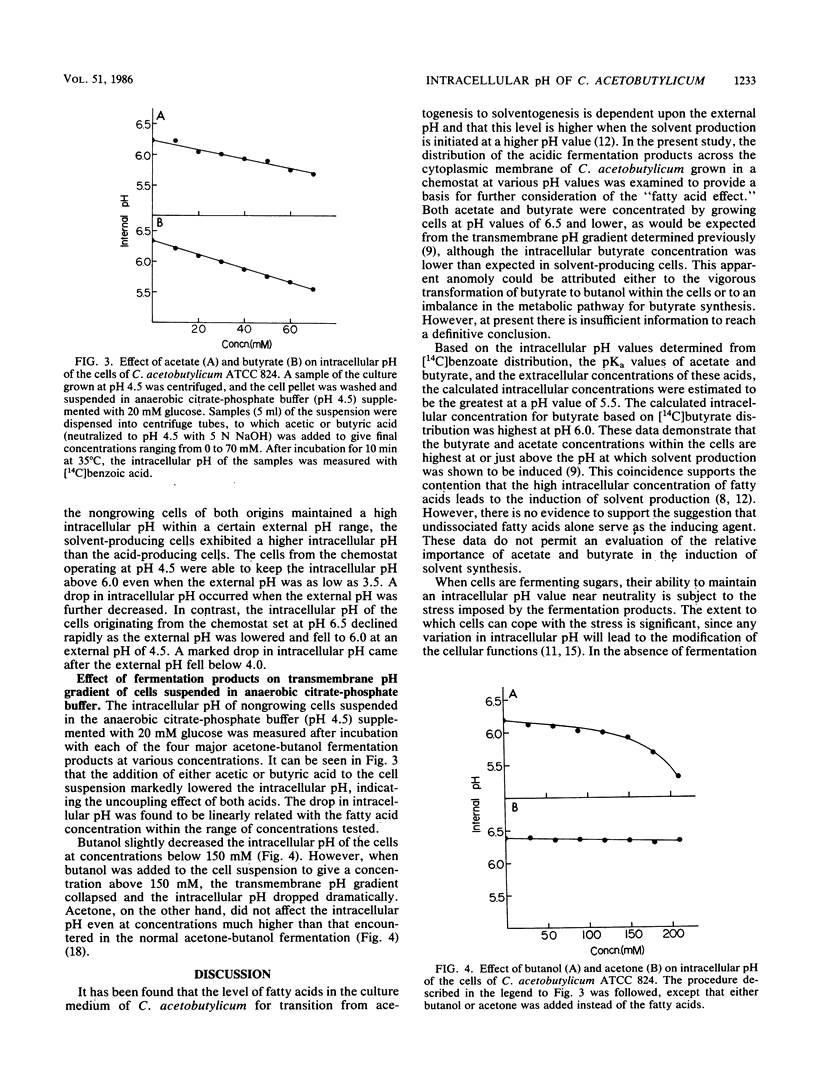
Clostridium acetobutylicum ATCC 824 cells harvested from a phosphate-limited chemostat culture maintained at pH 4.5 had intracellular concentrations of acetate, butyrate, and butanol which were 13-, 7-, and 1.3-fold higher, respectively, than the corresponding extracellular concentrations. Cells from a culture grown at pH 6.5 had intracellular concentrations of acetate and butyrate which were only 2.2-fold higher than the respective external concentrations. The highest intracellular concentrations of these acids were attained at ca. pH 5.5. When cells were suspended in anaerobic citrate-phosphate buffer at pH 4.5, exogenous acetate and butyrate caused a concentration-dependent decrease in the intracellular pH, while butanol had relatively little effect until the external concentration reached 150 mM. Acetone had no effect at concentrations up to 200 mM. These data demonstrate that acetate and butyrate are concentrated within the cell under acidic conditions and thus tend to lower the intracellular pH. The high intracellular butyrate concentration presumably leads to induction of solvent production, thereby circumventing a decrease in the intracellular pH great enough to be deleterious to the cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles L. K., Ellefson W. L. Effects of butanol on Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Nov;50(5):1165–1170. doi: 10.1128/aem.50.5.1165-1170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. A., Stephens G. M., Morris J. G. Production of Solvents by Clostridium acetobutylicum Cultures Maintained at Neutral pH. Appl Environ Microbiol. 1984 Dec;48(6):1166–1170. doi: 10.1128/aem.48.6.1166-1170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Gibbins L. N., Forsberg C. W. Transmembrane pH gradient and membrane potential in Clostridium acetobutylicum during growth under acetogenic and solventogenic conditions. Appl Environ Microbiol. 1985 Oct;50(4):1043–1047. doi: 10.1128/aem.50.4.1043-1047.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D. B., Peck M. W., Rodger G., Morris J. G. On the permeability to weak acids and bases of the cytoplasmic membrane of Clostridium pasteurianum. Biochem Biophys Res Commun. 1981 Mar 16;99(1):81–88. doi: 10.1016/0006-291x(81)91715-0. [DOI] [PubMed] [Google Scholar]
- ROSS D. The acetone-butanol fermentation. Prog Ind Microbiol. 1961;3:71–90. [PubMed] [Google Scholar]
- Vollherbst-Schneck K., Sands J. A., Montenecourt B. S. Effect of butanol on lipid composition and fluidity of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1984 Jan;47(1):193–194. doi: 10.1128/aem.47.1.193-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



