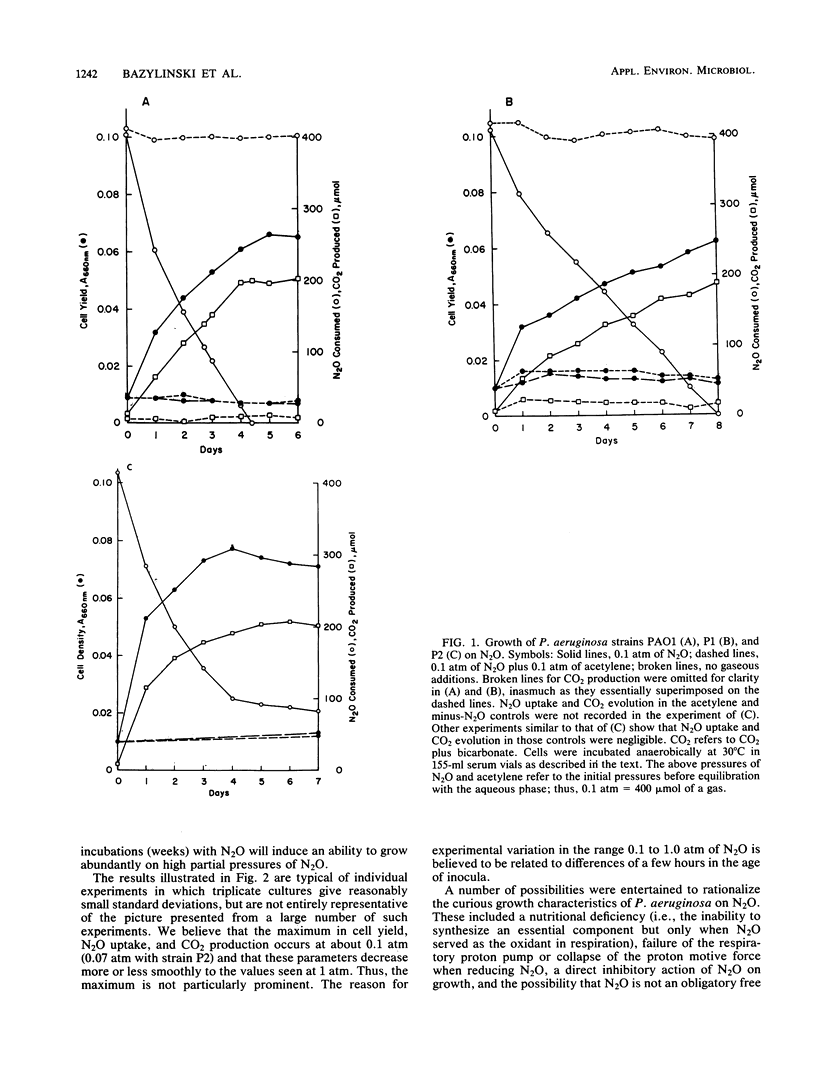
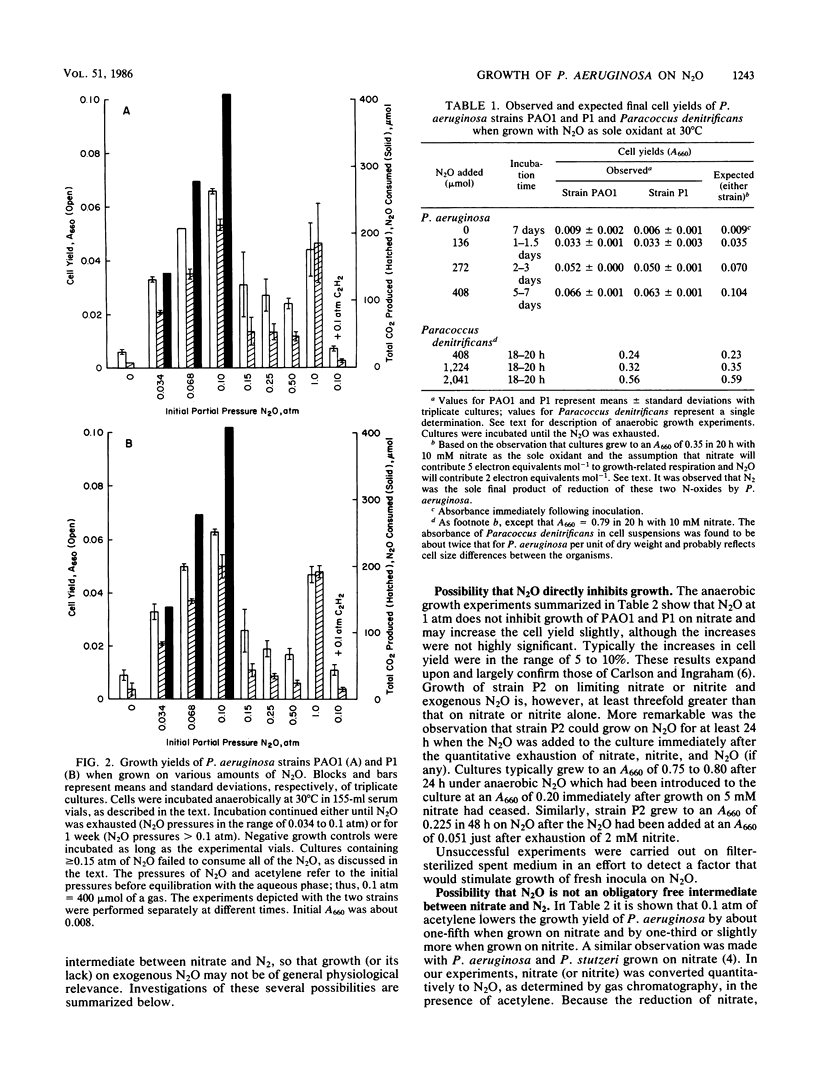
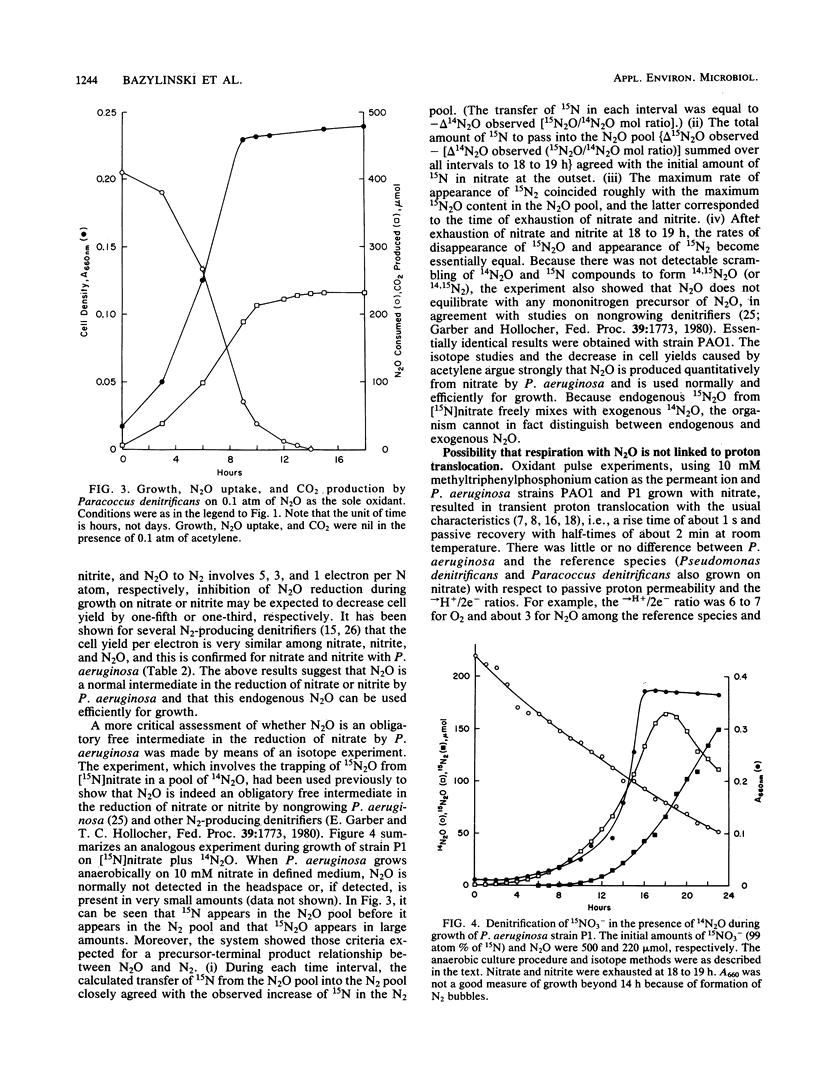
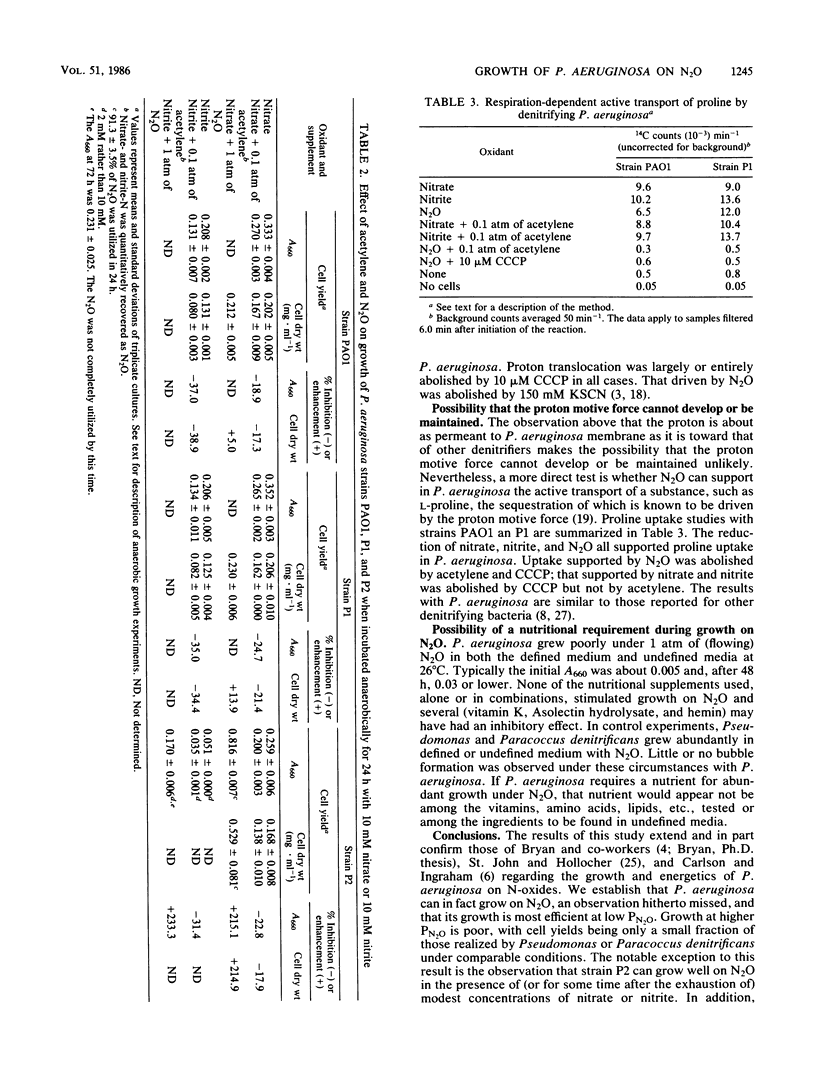
Abstract
Three strains of Pseudomonas aeruginosa were grown anaerobically on exogenous N2O in a defined medium under conditions that assured the maintenance of highly anaerobic conditions for periods of 1 week or more. The bacteria were observed reproducibly to increase their cell density by factors of 3 to 9, but not more, depending on the initial amount of N2O. Growth on N2O was cleanly blocked by acetylene. Cell yields, CO2 production, and N2O uptake all increased with initial PN2O at PN2O less than or equal to 0.1 atm. Growth curves were atypical in the sense that growth rates decreased with time. This is the first observation of growth of P. aeruginosa on N2O as the sole oxidant. N2O was shown to be an obligatory, freely diffusible intermediate during growth of strains PAO1 and P1 on nitrate. All three strains used this endogenous N2O efficiently for growth. For strains PAO1 and P1, it was confirmed that exogenous N2O had little effect on the cell yields of cultures growing with nitrate; thus, for these strains exogenous N2O neither directly inhibited growth nor was used significantly for growth. On the other hand, strain P2 grew abundantly on exogenous N2O when small and growth-limiting concentrations of nitrate or nitrate (2 to 10 mM) were included in the medium. The dramatic effect of these N-anions was realized in large part even when the exogenous N2O was introduced immediately after the quantitative conversion of anion-nitrogen to N2. No evidence was found for a factor in filter-sterilized spent medium that stimulated fresh inocula to grow abundantly on N2O.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazylinski D. A., Blakemore R. P. Denitrification and Assimilatory Nitrate Reduction in Aquaspirillum magnetotacticum. Appl Environ Microbiol. 1983 Nov;46(5):1118–1124. doi: 10.1128/aem.46.5.1118-1124.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogerd F. C., Van Verseveld H. W., Stouthamer A. H. Respiration-driven proton translocation with nitrite and nitrous oxide in Paracoccus denitrificans. Biochim Biophys Acta. 1981 Dec 14;638(2):181–191. doi: 10.1016/0005-2728(81)90226-7. [DOI] [PubMed] [Google Scholar]
- Bryan B. A., Jeter R. M., Carlson C. A. Inability of Pseudomonas stutzeri denitrification mutants with the phenotype of Pseudomonas aeruginosa to grow in nitrous oxide. Appl Environ Microbiol. 1985 Nov;50(5):1301–1303. doi: 10.1128/aem.50.5.1301-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. A., Ingraham J. L. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl Environ Microbiol. 1983 Apr;45(4):1247–1253. doi: 10.1128/aem.45.4.1247-1253.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castignetti D., Hollocher T. C. Proton translocation during denitrification by a nitrifying--denitrifying Alcaligenes sp. Antonie Van Leeuwenhoek. 1983 Apr;49(1):61–68. doi: 10.1007/BF00457880. [DOI] [PubMed] [Google Scholar]
- Daniels L., Wessels D. A method for the spectrophotometric assay of anaerobic enzymes. Anal Biochem. 1984 Aug 15;141(1):232–237. doi: 10.1016/0003-2697(84)90450-0. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Castignetti D., Hollocher T. C. Proton translocation and proline uptake associated with reduction of nitric oxide by denitrifying Paracoccus denitrificans. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1504–1507. doi: 10.1016/s0006-291x(82)80169-1. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Hollocher T. C. 15N,18O tracer studies on the activation of nitrite by denitrifying bacteria. Nitrite/water-oxygen exchange and nitrosation reactions as indicators of electrophilic catalysis. J Biol Chem. 1982 Jul 25;257(14):8091–8097. [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kim C. H., Hollocher T. C. Catalysis of nitrosyl transfer reactions by a dissimilatory nitrite reductase (cytochrome c,d1). J Biol Chem. 1984 Feb 25;259(4):2092–2099. [PubMed] [Google Scholar]
- Koike I., Hattori A. Energy yield of denitrification: an estimate from growth yield in continuous cultures of Pseudomonas denitrificans under nitrate-, nitrite- and oxide-limited conditions. J Gen Microbiol. 1975 May;88(1):11–19. doi: 10.1099/00221287-88-1-11. [DOI] [PubMed] [Google Scholar]
- Kristjansson J. K., Walter B., Hollocher T. C. Respiration-dependent proton translocation and the transport of nitrate and nitrite in Paracoccus denitrificans and other denitrifying bacteria. Biochemistry. 1978 Nov 14;17(23):5014–5019. doi: 10.1021/bi00616a024. [DOI] [PubMed] [Google Scholar]
- Lieberman M. A., Hong J. S. A mutant of Escherichia coli defective in the coupling of metabolic energy to active transport. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4395–4399. doi: 10.1073/pnas.71.11.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- St John R. T., Hollocher T. C. Nitrogen 15 tracer studies on the pathway of denitrification in Pseudomonas aeruginosa. J Biol Chem. 1977 Jan 10;252(1):212–218. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Walter B., Sidransky E., Kristjansson J. K., Hollocher T. C. Inhibition of denitrification by uncouplers of oxidative phosphorylation. Biochemistry. 1978 Jul 25;17(15):3039–3045. doi: 10.1021/bi00608a015. [DOI] [PubMed] [Google Scholar]
- van Verseveld H. W., Meijer E. M., Stouthamer A. H. Energy conservation during nitrate respiration in Paracoccus denitrificans. Arch Microbiol. 1977 Feb 4;112(1):17–23. doi: 10.1007/BF00446649. [DOI] [PubMed] [Google Scholar]



