Abstract
Two unlinked genes FER1 and FER2 encoding ferritin subunits were identified in the Chlamydomonas genome. An improved FER2 gene model, built on the basis of manual sequencing and incorporation of unplaced reads, indicated 49% identity between the ferritin subunits. Both FER1 and FER2 transcripts are increased in abundance as iron nutrition is decreased but the pattern for each gene is distinct. Using subunit-specific antibodies, we monitored expression at the protein level. In response to low iron, ferritin1 subunits and the ferritin1 complex are increased in parallel to the increase in FER1 mRNA. Nevertheless, the iron content of the ferritin1 complex is decreased. This suggests that increased expression results in increased capacity for iron binding in the chloroplast of iron-limited cells, which supports a role for ferritin1 as an iron buffer. On the other hand, ferritin2 abundance is decreased in iron-deprived cells, indicative of the operation of iron-nutrition-responsive regulation at the translational or post-translational level for FER2. Both ferritin subunits are plastid localized but ferritin1 is quantitatively recovered in soluble extracts of cells while ferritin2 is found in the particulate fraction. Partial purification of the ferritin1 complex indicates that the two ferritins are associated in distinct complexes and do not coassemble. The ratio of ferritin1 to ferritin2 is 70:1 in iron-replete cells, suggestive of a more dominant role of ferritin1 in iron homeostasis. The Volvox genome contains orthologs of each FER gene, indicating that the duplication of FER genes and potential diversification of function occurred prior to the divergence of species in the Volvocales.
ALTHOUGH iron is abundant on earth, its bioavailability is limited in the aerobic world because of the insolubility of ferric salts, and iron can be a limiting nutrient for most forms of life. A third of the agricultural land and the same fraction of the ocean are considered iron deficient (reviewed by Boyd et al. 2007). Therefore, iron nutrition is a key component of global productivity. Organisms have evolved multiple and varied pathways for assimilating iron in its various chemical forms and at a range of concentrations in the nutrient environment (Staiger 2002; reviewed in Curie and Briat 2003; Hentze et al. 2004). Even though iron can be toxic to cells as a consequence of its propensity for participating in redox chemistry, cells do not generally excrete iron because it is a limiting nutrient (Liochev and Fridovich 1999). Instead, cells tend to store intracellular iron in a less reactive and hence nontoxic form. Because of the importance of iron for life, these pathways of uptake and storage are subject to layers of homeostatic regulation.
We have developed Chlamydomonas as a model organism for understanding trace metal nutrition in plants, especially in the context of chloroplast function and photosynthesis (Merchant et al. 2006). As a microorganism, Chlamydomonas lends itself to nutritional studies because of the ease with which the aqueous growth medium can be manipulated. Furthermore, the absence of proteins or amino acid supplements that provide metal-binding ligands simplifies the provision of metal micronutrients. Previously, using genomic and proteomic approaches, we identified a number of proteins that might function in parallel pathways of iron assimilation (La Fontaine et al. 2002; Allen et al. 2007). We proposed that the FRE1 gene encodes a ferrireductase that mobilizes iron by reduction of Fe3+ to Fe2+. Two uptake pathways appear to operate in Chlamydomonas: a high-affinity one involving a ferroxidase coupled to a ferric ion transporter, encoded by FOX1 and FTR1, respectively, and one of likely lower affinity, involving inducible ZIP family transporters that we named IRT1 and IRT2 (Allen et al. 2007; Chen et al. 2008). We also identified extracellular proteins FEA1 and FEA2 that appear to facilitate iron uptake when iron is present at low concentrations in the medium. The increased expression of FOX1, FTR1, and FEA1 is attributed to transcriptional regulation (Allen et al. 2007; Deng and Eriksson 2007).
In most eukaryotic cells, ferritin is the major, and perhaps only, iron storage protein (Theil 2004; Koorts and Viljoen 2007). Although iron can be “scavenged” from abundant iron-containing proteins (such as plant ferredoxins) in a situation of iron deficiency, this serves only as a last resort source of iron. Ferritin is a multimeric complex, consisting of 24 ferritin subunit polypeptides that form a shell around a core that can hold up to 4.5 × 103 iron atoms as an insoluble iron-oxy-hydroxide mineral. In animals, there are two types of chains—the light (L) chain and the heavy (H) chain (Theil 1987). The H chain carries ferroxidase active sites that oxidize ferrous to ferric iron, a prerequisite for mineralization in the core (reviewed by Chasteen 1998). The L chain, present only in vertebrates, does not have a ferroxidase site but is able to form a complex and bind iron at neutral but not acidic pH (Levi et al. 1989). Therefore changes in the ratio of H/L subunits, e.g., in response to iron nutrition or in particular cell types, can affect the iron storage capability of ferritin (reviewed by Koorts and Viljoen 2007). The importance of ferritin in biology is underscored by the lethal phenotype of the knockout in mouse (Ferreira et al. 2000). In animal cells, ferritin is primarily a cytosolic protein, but a mitochondrial ferritin gene has also been identified (Levi et al. 2001).
In vascular plants, ferritin is located in the plastids rather than in the cytosol and is the source of iron for de novo synthesis of the cytochromes and FeS proteins in the photosynthetic apparatus during chloroplast development (reviewed by Lobréaux and Briat 1991; Merchant and Dreyfuss 1998). Accordingly, it is synthesized as an N-terminally extended precursor that is post-translationally targeted to the plastid. Although ferritin is encoded by a multigene family in plants (four FER genes in Arabidopsis; Petit et al. 2001a), the subunits are all of one type with a ferroxidase active site and characteristics of both the H and the L chain (Wade et al. 1993). Plant ferritins also have a distinctive extension peptide (EP) not found in mammalian homologs. Although the function of this EP is not known, an involvement in protein stability has been proposed (Van Wuytswinkel and Briat 1995). The iron mineral in plant ferritin also has a high P content, which affects iron release. Recently, ferritin was also found in plant mitochondria and it was suggested that this might be a consequence of dual targeting of one of the preproteins (Zancani et al. 2004).
In our previous study of iron assimilation components in Chlamydomonas, we described a cDNA sequence encoding preapoferritin and named the locus FER1. The abundance of FER1 mRNAs increased in iron deficiency (La Fontaine et al. 2002)—which is counterintuitive for an iron storage protein—and we accordingly hypothesized a role for plastid ferritin in buffering iron or holding it transiently as it was released from PSI by degradation (Moseley et al. 2002). The version 2.0 draft genome suggested the presence of a second FER gene although there was no EST evidence for its expression and the gene model was incomplete because of a gap in the sequence. By amplification and sequencing of genomic DNA and cDNAs, we have constructed a complete model of the FER2 locus (incomplete also in version 3.0). Since Chlamydomonas cells contain only one plastid, the presence of a second gene opened the door to the possibility that the two gene products might be functionally distinct with respect to subcellular location or that they may coassemble in different proportions to generate biochemically distinct complexes. Monospecific antibodies raised against the products of the FER1 and FER2 genes, referred to as ferritin1 and ferritin2, have allowed us to characterize the proteins with respect to pattern of expression, subcellular location, and association in complexes.
MATERIALS AND METHODS
FER2 cDNA assembly:
The FER2 genomic model spans the ends of contigs 97 and 98 on Scaffold 2 in the current version of the Chlamydomonas genome (version 3.0). Standard molecular biology strategies were used to sequence the genomic DNA between contigs and assemble a gene model for FER2 (supplemental Figure 1). The corresponding cDNA sequence has been submitted to GenBank under accession no. EU223296.
Expression of recombinant ferritins and partial purification of recombinant ferritin2:
Plasmid constructs for expressing recombinant ferritin1 and ferritin2 were constructed by standard molecular biology methods using expression vector pET23d and gene-specific primer sets (Table 1). Plasmid constructs (pFer11–249 and pFer278–298 were confirmed by sequencing and transformed into Escherichia coli BL21 (DE3) cells for expression. E. coli cultures transformed with pFer11–249 or pFer278–298 were grown to an OD600 of 0.8 and induced with 1 mm IPTG. After 4 hr cells were collected by centrifugation and resuspended in three-cell pellet volumes of buffer containing 20 mm Tris–HCl, 150 mm NaCl, 1 mm EDTA. The cells were broken by sonication (microtip, 30% intensity, 10 cycles of 30 sec) and the soluble and insoluble protein fractions separated by centrifugation (10,000 × g, 10 min). rFer1 was present in the insoluble pellet and rFer2 was in the soluble extract. To further purify rFer2 the soluble extract was heated at 70° for 10 min and cooled by incubation on ice for 10 min. After removing the resulting denatured proteins by centrifugation (10,000 × g, 10 min), rFer2 remained in the soluble fraction, confirming that the rFer2 polypeptide folds and assembles in vitro into a functional heat-resistant protein shell.
TABLE 1.
List of primer sequences used in this study
| Primer name | Sequence |
|---|---|
| FER1 F | GCTCATGGAGTACCAGAACC |
| FER1 R | CTTCTTCACAGCCTCGACCT |
| FER21–20 | AGTTCTGGCACGCAGAGAAG |
| FER2295–314 | GCTCGTGAAGCAGGTAGTCC |
| FER2374–357 | TCGGGCCTGGTGTTCCAGCC |
| FER2439–420 | CCGTGGTGATGCCGTTACA |
| FER2613–595 | CGCTGGGTCTGGTAGTTCAT |
| FER2657–676 | GACTCCAGGTCAGGCGAATA |
| FER2821–802 | AGTTCTGGCACGCAGAGAAG |
| FER21398–1379 | TGAACCCTGTTCGCTCTTTT |
| C98 F | CTTGGGGTCCATGAGCTG |
| rFer1 F | TTGGATCCTGGCGCTCTGTGCTCG |
| rFer1 R | TTCTCGAGTTACGCGGCGGCACC |
| rFer2 F | TTGGATCCTGGGCGAGGTGCAGCCG |
| rFer2 R | TTCTCGAGGCCAGCCAGCGG |
Primers were designed against the Chlamydomonas genome (version 3.0). Subscript numbers indicate the location of the primer with respect to the FER2 cDNA. F, forward primer; R, reverse primer.
Growth conditions:
Chlamydomonas reinhardtii strains CC1021 (referred to by its more common name 2137 in this article), 17D− (wild type in the 137c background), CC400, and CC425 were used in this study. Starter cultures were maintained in the standard Tris–acetate–phosphate (TAP) medium containing 18 μm Fe at 25° in 50 μmol m−2 sec−1 light with shaking at 175 rpm. For Fe-deficiency experiments, TAP medium was made in acid-washed glassware using trace elements without Fe, which was subsequently added to a final concentration of 0.2–200 μm from a solution of iron–EDTA (Moseley et al. 2000). To avoid carryover of iron, cells were collected by centrifugation (3000 × g, 5 min) and resuspended to a density of 108 cells/ml in TAP containing 0.2 μm Fe. This cell suspension was used to inoculate medium to a final concentration of 105 cells/ml. Cells were collected at midlog phase when the culture reached a density of 5 × 106 cells/ml.
RNA isolation and quantitative real-time PCR:
Total RNA was isolated from Chlamydomonas cells and quantitative real-time PCR was as described (Allen et al. 2007). Gene-specific primers are listed in Table 1.
Extraction of Chlamydomonas protein:
Chlamydomonas proteins were extracted essentially as described previously (Allen et al. 2007). Cells were collected by centrifugation at 1000 × g for 5 min, washed in 10 mm sodium phosphate, pH 7.0, resuspended in the same buffer to a concentration equivalent to 4 × 108 cells/ml, and stored at −80°. For denaturing gel electrophoresis, cells were lysed by freeze/thaw cycling as described (Howe and Merchant 1992). For nondenaturing gels, cells were broken by sonication (microtip, 30% intensity, two cycles of 30 sec). Extracts were separated into soluble and insoluble protein fractions by centrifugation (10,000 × g, 10 min). The pellet was washed once and resuspended to the same volume as that of the soluble fraction. Protein concentration was determined with the BCA assay kit (Pierce, Rockford, IL) or the Lowry method against a bovine serum albumin standard.
Isolation of chloroplasts and mitochondria:
Chloroplasts were isolated from a 4-liter culture of strain CC400. Cells were dark adapted for 2–3 hr prior to harvesting by centrifugation (4000 × g, 10 min). Cells were resuspended in 50 ml buffer A (0.3 m sorbitol, 50 mm Hepes-KOH, 2 mm EDTA, 5 mm MgCl2, pH 7.8) supplemented with 0.1% BSA and broken in a Yeda press (4.5 bar, 30 sec). Crude lysate was centrifuged (1000 × g, 5 min). The pellet fraction was washed twice in buffer A, loaded onto a 45/75% Percoll step gradient in buffer A, and centrifuged (9300 × g, 20 min). Intact chloroplasts were collected at the 45/75% interface and were washed and pelleted twice in buffer A (1000 × g, 5 min). Isolated chloroplasts were lysed in buffer B (10 mm Tricine/NaOH, 2 mm MgCl2, pH 7.8) and broken by two freeze/thaw cycles at −80°. Lysed chloroplasts were loaded onto a discontinuous sucrose gradient (0.4/1.0 m in buffer B) and centrifuged for 1 hr at 80,000 × g. The intact chloroplast fraction floating on top of the 0.4-m sucrose phase was collected. Mitochondria were isolated as described (Eriksson et al. 1995) except that the cells were lysed in a Yeda press (4.5 bar, 30 sec).
Antibody production:
Antibodies that were specific to ferritin1 vs. ferritin2 were supplied by Agrisera (Umeå, Sweden). The synthetic peptides used as immunogen (Figure 1) were designed by Environmental Proteomics (Sackville, NB, Canada).
Figure 1.—
Comparison of algal ferritins. (A) Chlamydomonas FER1 (accession no. AAM27205) and FER2 (accession no. EU223296), Volvox FER2 (protein ID 58531) and FEN1 (protein ID 81290), Ostreococcus lucimarinus PFE1 (protein ID 27552) and PFE2 (protein ID 29953), and O. tauri FER1 (protein ID 5942) were aligned with Multalin (http://prodes.toulouse.inra.fr/multalin/multalin.html). Lowercase sequence indicates transit peptides for plastid targeting for Chlamydomonas FER1 based on the N terminus determined in this study. The sequences of peptides used to generate antibodies specific to Chlamydomonas FER1 and FER2 (anti-FER1 and anti-FER2, respectively) are highlighted in yellow. Peptides used to generate antibodies that recognize both ferritins (anti-FERcore, experimentally verified) are underlined and double underlined (Busch et al. 2008). Residues corresponding to the ferroxidase center are shown in red. (B) A phylogenetic tree was generated with MAFFT (http://align.bmr.kyushu-u.ac.jp/mafft/online/server/phylogeny.html).
Immunoblotting:
Proteins were separated on SDS-containing polyacrylamide gels (15% monomer) or nondenaturing gels (6% monomer) and transferred (mini-Trans-Blot cell; Bio-Rad, Hercules, CA) onto PVDF (0.45 μm; Millipore, Bedford, MA) for 90 min under constant voltage (150 V) in transfer buffer [25 mm Tris, 192 mm glycine, 20% (v/v) methanol]. For immunoblot analysis, the rapid immunodetection method was used (Mansfield 1995). Primary antisera were diluted in antibody dilution buffer: 1% BSA in phosphate-buffered saline (10 mm Na-phosphate, 2.7 mm KCl, 137 mm NaCl, pH 7.2) with 0.05% Tween-20. In competition assays (Figure 3B), antisera were preincubated at room temperature for 1 hr at 1:500 in the same buffer containing 0.05 ng/μl of recombinant ferritin and diluted to 1:5000 before use. After a 1-hr incubation with primary antibody, the membranes were washed (phosphate-buffered saline with 0.1% Tween-20) until the membrane was completely wet (typically two to three washes of 15 min each), incubated for 30 min in a 1:5000 dilution of goat anti-rabbit horseradish peroxidase (Pierce Biotechnology) in the dilution buffer, and washed twice with that solution followed by a final wash in phosphate-buffered saline without Tween-20. Bound antibody was detected with the Supersignal West Pico or Supersignal West Femto (ferritin2 only) chemiluminescent substrate (Pierce Biotechnology).
Figure 3.—
Specificity of anti-ferritin1 and anti-ferritin2. (A) Recombinant protein. Extracts from E. coli cells expressing recombinant ferritin 1, rFER11–249 (corresponding to residues 1–249 of the preprotein), or recombinant ferritin 2, rFER278–298 (corresponding to residues 78–298 of the preprotein), were separated by denaturing polyacrylamide gel electrophoresis (15% monomer). The lane on the left, containing 3 μg of extract, was stained with Coomassie Blue. The specificity of the antibodies was analyzed by immunoblotting after transfer to PVDF membranes. (B) Endogenous protein. Total protein extracts from Chlamydomonas strain CC400, corresponding to 35 μg protein were analyzed by immunoblotting as in A. Ferritin antibodies were incubated for 60 min with 0.01 μg of E. coli extract from cells expressing recombinant ferritin 1 (+rFER1), ferritin 2 (+rFER2), or no protein (none) prior to immunoblotting (see materials and methods).
For chloroplast keto-acid reductase isomerase (KARI) and COX2b immunoblot analysis, proteins were separated on an SDS-containing polyacrylamide gel (10% monomer for KARI or 15% monomer for COX2b) and transferred in a semidry blotter onto PVDF (0.45 μm, Millipore) for 1.5 hr under constant current (400 mA) in 25 mm Tris, 192 mm glycine, 0.01% SDS, 20% methanol. Membranes were blocked with 5% dry milk in TBS (10 mm Tris–HCl, 150 mm NaCl, pH 7.5) with 0.05% (w/v) Tween-20. Primary antibodies were used at 1:10,000 (KARI) or 1:25,000 (COX2b) and a 1:5000 dilution of goat anti-rabbit horseradish peroxidase was used as the secondary antibody. Signals were detected using SuperSignal West Pico chemiluminescent substrate (Pierce).
Iron staining:
A total of 20 μg of soluble extracts from cells grown in TAP medium containing 0.2–200 μm iron–EDTA were separated on a nondenaturing polyacrylamide gel. Horse spleen ferritin (Sigma, St. Louis) was used as a control. Iron staining was essentially as described in Leong et al. (1992).
Immunopurification of ferritin1:
An affinity column was prepared as follows: 200 μl of polyclonal anti-ferritin1 (anti-FER1) serum (Agrisera) was coupled to 0.5 g of lyophilized CNBr-activated Sepharose 4B (GE Healthcare) in a total volume of 5 ml according to the manufacturer's instructions. Uncoupled antibody was washed out and the remaining active groups were blocked by incubation with 0.1 m Tris–HCl, pH 8, for 4 hr at room temperature. The material was packed in a column and washed with 10 column volumes binding buffer (50 mm sodium phosphate, 0.15 m NaCl, 5 mm EDTA, 1% Triton-X-100, pH 7.5), and nonspecific binding sites were blocked by washing with 0.2% BSA in binding buffer. Soluble cell extracts from Chlamydomonas strain 2137 were prepared as described above. One milliliter of the cell extract was diluted in 10 ml binding buffer and incubated for 2 hr with the column material by repeatedly loading on the column. The column was washed with 20 ml of binding buffer, and then the buffer was exchanged to 10 mm sodium phosphate, pH 6.8, and the bound protein was eluted with 100 mm glycine-Cl, pH 2.8. The eluate was collected in 0.5-ml fractions and immediately neutralized using Na2CO3 solution. Fractions 2–5 contained the major amount of purified ferritin1 and were pooled and concentrated to ∼1 ml. Thirty-microliter aliquots of the crude cell extract, the flow through from the binding step, the last wash step, and the pooled ferritin1 fractions were used for analysis.
Sequence analysis of ferritin polypeptides:
The affinity-purified ferritin1 complex sample was analyzed by separation on SDS-containing gels. For mass spectrometry, the proteins were visualized by Coomassie staining. Two major bands in the region of 25 kDa were cut out separately, subjected to in-gel tryptic digest as described previously (Naumann et al. 2005), and analyzed by nanoLC-MS/MS as described (Ramachandran et al. 2006). The resulting data were searched against a Chlamydomonas protein database consisting of the version 3.1 protein models (http://genome.jgi-psf.org/Chlre3/Chlre3.download.ftp.html), using the Mascot database search engine (Matrix Science, London). In all searches, one missed tryptic cleavage was tolerated, and a mass tolerance of 0.3 Da was set for the precursor and product ions. In a more focused analysis, the mass spectrometry data were searched against a database consisting of only the two Chlamydomonas ferritins. In this case, the search did not specify a protease so that nontryptic peptides could be found. The results were examined manually to identify N-terminal nontryptic peptides only. For Edman sequencing, the proteins were transferred to a PVDF membrane and visualized by colloidal Coomassie staining. The doublet in the region of 25 kDa was cut out and subjected to Edman sequencing for five cycles by Jack Presley at the University of California (Davis, CA) Molecular Structure Facility (ABI 494-HT Procise; Applied Biosystems, Foster City, CA).
RESULTS
Two FER genes in Chlamydomonas:
Previous work identified a ferritin, encoded by FER1, that was upregulated by Fe deficiency. In the version 2.0 draft, and subsequently in version 3.1, a second gene, named FER2, was identified on the basis of homology. The occurrence of multiple genes in Chlamydomonas is consistent with the situation in vascular plants as well as other organisms where ferritin is also encoded as multigene families. The gene model in version 2.0 was not supported by EST data and a considerable sequence gap prevented further analysis. The gap in the model was therefore closed by manual sequencing of cDNA and genomic DNA PCR products and assembly of sequence data from unplaced reads (see supporting supplemental online material). An EST was mapped to the FER2 gene model in the version 3.1 genome sequence and the corresponding cDNA was also sequenced. The version 4.0 finished genome sequence confirms the one submitted under accession no. EU223296.
A multiple-sequence alignment of Chlamydomonas FER gene products with those of other algae suggests that both ferritins have N-terminal extensions and are therefore likely to be plastid localized (see below). The ferroxidase active site is highly conserved in all algal ferritins (Figure 1A), suggesting that they do indeed function as subunits of an iron-binding complex (see below). Sequence analysis of a partially purified ferritin1 complex (see below) by mass spectrometry and Edman degradation provided excellent support for the derived sequence (see supplemental material). Analysis of the draft Volvox genome revealed the presence of two genes, annotated as FEN1 and FER2 (March 2008), orthologous to the Chlamydomonas FER1 and FER2, respectively (Figure 1B). The FER2 sequence has a small C-terminal extension that we refer to as a Gly-rich sequence, but since this is not conserved in Volvox, it may not be functionally significant.
Unique but overlapping pattern of expression of FER mRNAs:
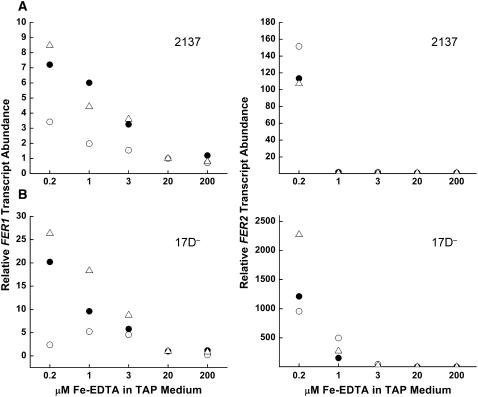
When we analyzed the pattern of expression of FER mRNAs as a function of iron nutrition, we noted that both FER1 and FER2 mRNAs were increased in abundance in iron-limited (0.2 μm) vs. iron-replete (20 μm) cells (Figure 2). Nevertheless, while FER1 mRNA abundance increases in proportion to the degree of iron depletion, FER2 mRNA is increased only when medium iron content drops below a threshold value. Since individual “wild-type” strains of Chlamydomonas respond slightly differently to external iron supply (see Allen et al. 2007, for example) we tested multiple strains and noted the same general pattern. Specifically, in iron-replete or iron-excess situations, FER2 mRNA was in low abundance and increased dramatically only when the iron-deficiency status was severe enough to result in chlorosis (Figure 2). There is also some experiment-to-experiment variation and therefore the results from three separate experiments are shown. Since the ferritin subunits associate to assemble into complexes, this result allows the possibility of distinct ferritin complexes in cells experiencing different degrees of iron nutrition, assuming that both proteins are in the same compartment. By comparing cycle threshold values for FER mRNAs, we noted that FER1 constitutes a larger fraction (∼102- to 103-fold relative to FER2) of the ferritin-encoding mRNAs in all strains tested (data not shown) and this is validated by Solexa tag sequencing of cDNAs derived from iron-replete cultures (M. Castruita, D. Casero, J. Kropat, S. Karpowicz, M. Pellegrini and S. S. Merchant, unpublished results).
Figure 2.—
Transcripts encoding both ferritin subunits are increased in iron-limited cells. The abundance of FER1 and FER2 mRNAs was analyzed by quantitative real-time PCR. Strains 17D− and 2137 were cultured in TAP medium supplemented with the indicated amounts of iron. cDNA corresponding to RNA isolated from Chlamydomonas strains (A) 2137 (top) or (B) 17D− (bottom) was used as a template with primers specific to FER1 or FER2. The fold change in abundance of FER1 (left) or FER2 (right) after normalization to CβLP and relative to the abundance in a 20-μm grown culture was calculated according to the 2−ΔΔCT method (Livak and Schmittgen 2001). Each data point is the average of technical triplicates and represents a separate experiment.
Accumulation of ferritin subunits:
To distinguish between the gene products of FER1 and FER2, antibodies were generated by Agrisera against antigenic peptides designed by Environmental Proteomics to correspond to unique regions of the predicted protein sequences (Figure 1). The antiserum preparations were tested against recombinant FER1 and FER2 produced in E. coli (see materials and methods). Immunoblot analyses confirmed high selectivity of each antiserum to its respective antigen (Figure 3A).
When extracts of Chlamydomonas were tested for the presence of ferritin1 and ferritin2, we noted that each antibody recognized a distinct polypeptide. Anti-FER1 recognized a protein with a mobility corresponding to 25 kDa while anti-FER2 recognized a protein that migrated at ∼60 kDa (Figure 3B). It is not unusual for ferritin and other iron storage proteins such as Dps to migrate as multimeric species during gel electrophoresis even under denaturing conditions (Clegg et al. 1980; Gupta and Chatterji 2003). Because of the unexpected size of ferritin2, the identity of the signal was confirmed by competition with rFER1 or rFER2. Only rFER2, but not rFER1 competed with the 60-kDa species, suggesting that the 60-kDa species corresponds to an aggregate of ferritin 2 subunits, possibly a trimer. The ratio of monomer to trimer is modified by heating the samples prior to electrophoresis but only rarely is the conversion to monomer complete (data not shown). Quantitation of ferritin subunit abundance indicated a ratio of ferritin1:ferritin2 of ∼70:1 (data not shown).
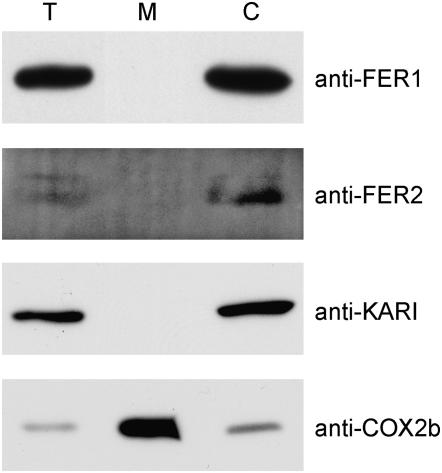
The availability of specific antibodies allowed us to test the localization of each protein by analyzing purified chloroplasts and mitochondria vs. total cell extract for ferritin content. Chloroplasts and mitochondria were purified from Chlamydomonas and analyzed for ferritin content. Both ferritins were detected exclusively in the chloroplast fraction (Figure 4).
Figure 4.—
Ferritin1 and ferritin2 are located in the chloroplast. Proteins (20 μg) from total cellular protein extracts of Chlamydomonas strain CC425 (T), isolated mitochondria (M), and chloroplasts (C) were separated on a denaturing polyacrylamide gel containing SDS and transferred to PVDF membranes for immunoblot analysis with anti-FER1, anti-FER2, anti-KARI (marker for chloroplast), and COX2b (marker for mitochondria).
Fractionation of cell extracts after sonication showed that ferritin1 is predominantly a soluble protein while ferritin2 is predominantly found in the particulate fraction, where it could be associated with membranes and this difference in localization is independent of iron nutrition (Figure 5A). The abundance of ferritin1 follows the pattern of FER1 mRNA; i.e., it increases with progressively increasing iron deficiency. However, the abundance of ferritin2 decreases as the iron content of the medium (and cells) is decreased, which is more typical of ferritin behavior in animal cells.
Figure 5.—
Differential accumulation of ferritin1 as a function of iron nutrition. (A) Chlamydomonas strain 17D− was grown in TAP medium containing the indicated iron concentration to midlog cell density. Soluble (S) and insoluble (P) fractions were prepared from the cells (see materials and methods). Five micrograms of protein from each growth condition were separated on a denaturing SDS–PAGE gel (15% monomer) and transferred to PVDF for immunoblot analysis with anti-FER1 and anti-FER2. Five micrograms of soluble protein (17D−) from each growth condition were separated on a nondenaturing gel (6% monomer) and analyzed with subunit-specific anti-FER1 and anti-FER2 (B) or stained for iron content with ferricyanide (C). Horse spleen ferritin (5 μg) was used as a marker (M).
Fe content of the ferritin complex:
When we analyzed Chlamydomonas extracts by native gel electrophoresis, we noted that both ferritin subunits were associated in high-molecular-weight complexes as is typical for ferritin (Figure 5B). Furthermore, the pattern of accumulation of the ferritin complexes followed the pattern of individual subunit accumulation; complexes containing ferritin1 increase in response to iron deficiency while those containing ferritin2 decrease. However, when we assessed the iron content of the complex using an iron stain procedure applied to the nondenaturing gels, we found that the iron content of the ferritin complex was decreased in iron-deficient and iron-limited cells (Figure 5C). Interestingly, we did not see an increase either in the abundance of ferritin subunits or in the iron content of the complex in cells grown in excess iron (compare 200 μm to 20 μm). Yet, we do know that cells grown in 200 μm iron do overaccumulate the metal (two to five times the amount found in iron-replete cultures grown at 20 μm iron). Taken together, this suggests that Chlamydomonas ferritin likely does not have a predominant function in iron storage.
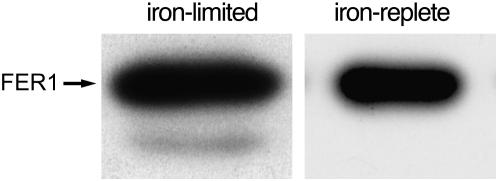
When we used an antibody that recognized a core sequence of ferritin (Figure 1A), we invariably noted a protein of higher mobility in extracts from iron-limited cells (Figure 6). This higher-mobility form was not recognized by either of the subunit-specific antibodies (Figure 3), suggesting that this form results from a modification of ferritin that involves the antigen site for anti-FER1 and anti-FER2. Peptide sequencing and Edman degradation (see below and supplemental online material) indicate that the faster-migrating species represents an N-terminal cleavage product.
Figure 6.—
Modification of ferritin1 in iron-limited cells. Protein (5 μg) from iron-replete (18 μm) vs. iron-limited (0.5 μm) cells was separated on a denaturing gel and analyzed for the abundance of ferritin by immunoblotting with anti-FERcore, which recognizes the internal segment of both ferritins on the basis of reactivity with the recombinant proteins (see Figure 1 legend).
Two separate ferritin1 and ferritin2 complexes:
In animals, various isoforms of ferritin consisting of different proportions of L and H chains are found (Koorts and Viljoen 2007). These molecules have different biochemical properties with respect to the kinetics of iron mineralization and release. Although plants have only one type of chain, sequence variants occur because the subunit is encoded by a multigene family. A recent study showed that recombinant soybean ferritin subunits can associate to form complexes consisting of a single subunit type or combinations of different subunits (Masuda et al. 2007). Therefore, given that both Chlamydomonas ferritin polypeptides are localized to the chloroplast, we were prompted to test whether they might be found in the same or different complexes.
Two approaches were used. First, we tested fractions of Chlamydomonas cell extracts separated by anion exchange chromatography for the presence of ferritin1 and ferritin2. If the two proteins coassemble in a heteropolymer, we would expect them to cofractionate. However, the proteins eluted as different peaks (data not shown). In a second approach, we immunopurified ferritin1 on an anti-ferritin1 affinity column (Figure 7). Although both ferritin1 and -2 are present in the starting material (load lane), when we analyzed the complex bound to the column, we did not find any ferritin2 (eluate lane). Although these results do not rule out the possibility that a small proportion of ferritin1 and ferritin2 might coassemble, they do indicate that the proteins are assembled largely into separate complexes.
Figure 7.—
Ferritin2 is not associated with ferritin1. The ferritin1 complex was purified on an affinity column. Equivalent volumes of material from various steps in the immunopurification procedure were analyzed for the presence of ferritin1 (top) or ferritin2 (bottom) by immunodetection after separation on SDS-containing gels. Lane 1, total soluble cell extract; lane 2, flow through; lane 3, final wash; lane 4, eluate.
N-terminal degradation of ferritin1:
The affinity-purified ferritin that eluted from the column was separated by denaturing gel electrophoresis to reveal two polypeptides ∼25 and 23 kDa (supplemental Figure 2). Edman sequencing of the mixture (supplemental Table 1) indicated two different N-terminal sequences, one at position 42GIVV and the other either at position 59ATVDK or at position 35ATVDK of preapoferritin1, respectively. On the basis of the size of the 23-kDa band and the fact that it did react with anti-FERcore but not with the subunit-specific anti-FER1 (see supplemental Figure 2), we suggest that the N terminus of ferritin1 corresponds to 59ATVDK. The two forms were also analyzed by mass spectrometry after trypsin digestion (see supplemental Table 2). The identified peptides corresponded to ferritin1 and support the two proposed N termini (supplemental Table 3). Since peptides corresponding to the C terminus could be detected in both bands, we conclude that the mobility difference corresponds to N-terminal degradation that is stimulated in the iron-deficient ferritin complex. This is consistent with the structure of the subunit within the crystallized bullfrog ferritin complex (pdb no. 1MFR; Ha et al. 1999): the N terminus is exposed on the surface of the complex and thus accessible to proteases, while the C terminus is buried inside the complex.
DISCUSSION
Two ferritins in Chlamydomonas are differently regulated and may have different functions:
In this work, we have shown that both FER genes in Chlamydomonas are expressed (Figure 2). The corresponding proteins are both localized to the chloroplast (Figure 4) but it is likely that they assemble into individual complexes (Figure 7) and that they function in different subcompartments of the plastid (Figures 4 and 5A). The major difference between the two ferritins is a predicted longer extension peptide in ferritin2 and a C-terminal gly-rich sequence. One or both of these domains might contribute to the membrane association of ferritin2 (Figure 5A) and to the stability of oligomers (dimers/trimers) of ferritin2 under denaturing conditions (Figure 3B). If the two proteins are compartmentalized within the chloroplast, this might explain why they do not coassemble in a single complex (Figure 7).
For ferritin1, the pattern of protein accumulation parallels the pattern of RNA increase while for ferritin2, the dramatic increase in RNA abundance is not recapitulated at the level of protein accumulation (Figures 2 and 5, A and B). This suggests that mechanisms that control translation or ferritin2 half-life may be in operation. For instance, the apoform of ferritin2 might be highly protease susceptible. In the case of ferritin1, we did note that N-terminal cleavage was significantly more pronounced in iron-limited cells (Figure 6) where the protein occurs in the apoform (Figure 5C) relative to iron-replete cells that contain iron in the ferritin complex. This may result from a structural difference between the apo- and the holoform of ferritin and hence increased protease susceptibility of the N terminus because of its accessibility in the ferritin shell or perhaps simply from increased expression of proteases in iron-deficient chloroplasts as part of an iron-scavenging program. The question of regulation of ferritin abundance in chloroplasts by mechanisms that act at the translational or post-translational level has not been addressed yet in plants.
Because of its abundance, ferritin1 is likely to be the dominant player in chloroplast iron homeostasis, but given the occurrence of a ferritin2 ortholog in Volvox, it may well have a more specific function. One possibility is that ferritin2 may provide iron for membrane protein biogenesis. The different physical properties of the two proteins and the different pattern of expression as a function of iron nutrition (Figures 2 and 5) argue in favor of a distinct function for ferritin2. In other work, we noted that FER1 and FER2 also have a different pattern of expression in response to high light stress (J. C. Long and S. S. Merchant, unpublished results). There may well be specific modifications of chloroplast iron homeostasis as part of the high light acclimation response.
The distinct pattern of expression of the homologs, the difference in their physical properties, and the relative abundance of each isoform underscore the importance of functional analysis of each member of a multigene family. The FEA genes in Chlamydomonas, also involved in iron homeostasis, also show distinct patterns of expression (Allen et al. 2007). Interestingly, like the FER genes, the FEA genes also respond differently to high light stress (J. C. Long and S. S. Merchant, unpublished results), and they also respond differently to CO2 (Allen et al. 2007). A relationship between carbon and iron metabolism is evident from measurements of iron content in Chlamydomonas cells grown under various types of carbon nutrition but the molecular basis of the relationship has not been determined (Semin et al. 2003).
Function of ferritin1 in the iron-deficiency response:
Surprisingly, and in contrast to the situation in animal cells, FER gene expression is increased in response to iron deficiency rather than iron excess. There is no significant difference in the abundance of FER RNA and FER protein in cells acclimated to 200 μm vs. 20 μm iron nor in the iron content of ferritin1 (Figures 2 and 5). Measurement of iron content of cells nevertheless indicates that these cells do have increased iron content, up to two to five times the iron maintained in an iron-replete culture grown with 20 μm Fe–EDTA (J. C. Long and S. S. Merchant, unpublished results). This raises the question of whether there is another, ferritin-independent, mechanism for sequestering iron in Chlamydomonas. In Arabidopsis, a homolog of Saccharomyces cerevisiae Ccc1p called VIT1 functions to transport iron to the vacuole (Kim et al. 2006). While a protein with some similarity to a Schizosaccharomyces pombe Ccc1 is found in the Chlamydomonas genome, whether it is a VIT1 homolog is not evident.
In cells acclimated to iron-poor growth conditions, the iron content of the ferritin complex is greatly reduced even though the abundance of the complex is increased (Figure 5, B and C). This is an important distinction. We conclude that the increase in ferritin1 in iron deficiency increases the capacity for iron binding in the chloroplast. This is consistent with a role for ferritin1 as a potential buffer for iron that is released from degradation of abundant iron–sulfur proteins in the chloroplast, like ferredoxins and photosystem I (Moseley et al. 2002). This iron may be then redistributed to other chloroplast iron proteins or to other organelles like the mitochondrion (Naumann et al. 2007). Interestingly, the iron content of ferritin1 is already decreased in cells grown in medium containing 3 μm iron–EDTA and is greatly reduced when the iron content of the medium is decreased to 1 μm, suggesting that it is mobilized, perhaps for use in maintenance of plastid iron proteins. This change is exactly paralleled by an increase in the expression of assimilatory components and precedes the appearance of chlorosis and the degradation of the photosynthetic apparatus, which confirms our description of cells grown in 1–3 μm iron–EDTA as being marginally iron deficient (Moseley et al. 2002; Allen et al. 2007).
In previous work, we compared the ferritin content of cells grown in 0.1 μm iron–EDTA vs. 1 μm iron–EDTA and concluded that the abundance of the protein did not change as a function of iron nutrition (La Fontaine et al. 2002). However, the fact that cells grown in 1 μm iron–EDTA were already iron deficient was not appreciated at that time, and indeed the abundance of ferritin1 in cells grown in 1 μm vs. 0.2 μm iron–EDTA is not significantly different (Figure 5).
In vascular plants, the iron-responsive element/iron-regulatory protein system is not present (Arnaud et al. 2007). Instead, mechanisms that act at the transcriptional level regulate FER gene expression in the iron overload situation (Wei and Theil 2000; Petit et al. 2001b; Tarantino et al. 2003). While a role in iron deficiency has not been described, it is possible that this might be a cell- or organ-specific phenomenon that is restricted to chloroplast-containing cells that are rich in iron proteins.
The upregulation of FER genes in senescent plants may be related to the iron-deficiency response described in this study (Buchanan-Wollaston and Ainsworth 1997; Hörtensteiner et al. 2000; Murgia et al. 2007): specifically, that ferritin accumulation is increased to handle the iron that is released from degradation of the photosynthetic apparatus (Himelblau and Amasino 2000). Individual FER genes respond to various developmental cues, growth factors, pathogen attack, H2O2, and various stress situations that generate reactive oxygen species (Lobréaux and Briat 1991; Lobreaux et al. 1993; Kimata and Theil 1994; Fobis-Loisy et al. 1995; Savino et al. 1997; Petit et al. 2001a; Dellagi et al. 2005; Boughammoura et al. 2007). These studies suggest that cell-specific analyses of the abundance of RNA and protein for individual ferritin isoforms and the iron content of the ferritin complex may reveal the operation of mechanisms that act to buffer iron release from the photosynthetic apparatus as seems to be the situation in Chlamydomonas, degreening Chlorella, and senescent Brassica. Arabidopsis plants carrying a knockout in the AtFER1 gene showed symptoms of accelerated aging in the light that was attributed to an inability to handle iron toxicity in the presence of light-dependent reactive oxygen species, which emphasizes the importance of ferritin as an iron buffer during plastid development (Murgia et al. 2007).
Acknowledgments
We are grateful to Steven Karpowicz for assistance with the FER1 gene model, to Scott Hsieh and Joseph Loo's laboratory for help with mass spectrometry, and to Krishna Niyogi for providing strain 17D−. We thank Michael Hippler, Elizabeth C. Theil, and Stephane Lobréaux for their gifts of antibodies to Chlamydomonas and plant ferritins and Maryse Block for the KARI antibody. We are grateful to the Joint Genome Institute for the draft sequences of the Volvox carteri genome. This work is supported by a grant from the Department of Energy (DE-FG02-04ER15529 to S.S.M.). F.S. was supported in part by the Deutsche Forschungsgemeinschaft (DFG SO706/1-1 and SO706/1-2) and M.D.A. in part by Institutional and Individual Ruth L. Kirschstein National Research Service Awards GM07185 and GM077066.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession no. EU223296.
References
- Allen, M. D., J. A. Del Campo, J. Kropat and S. S. Merchant, 2007. FEA1, FEA2, and FRE1, encoding two homologous secreted proteins and a candidate ferrireductase, are expressed coordinately with FOX1 and FTR1 in iron-deficient Chlamydomonas reinhardtii. Eukaryot. Cell 6 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud, N., K. Ravet, A. Borlotti, B. Touraine, J. Boucherez et al., 2007. The iron-responsive element (IRE)/iron-regulatory protein 1 (IRP1)-cytosolic aconitase iron-regulatory switch does not operate in plants. Biochem. J. 405 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughammoura, A., T. Franza, A. Dellagi, C. Roux, B. Matzanke-Markstein et al., 2007. Ferritins, bacterial virulence and plant defence. Biometals 20 347–353. [DOI] [PubMed] [Google Scholar]
- Boyd, P. W., T. Jickells, C. S. Law, S. Blain, E. A. Boyle et al., 2007. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science 315 612–617. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston, V., and C. Ainsworth, 1997. Leaf senescence in Brassica napus: cloning of senescence related genes by subtractive hybridisation. Plant Mol. Biol. 33 821–834. [DOI] [PubMed] [Google Scholar]
- Busch, A., R. Rimbauld, S. Rensch and M. Hippler, 2008. Ferritin is required to permit rapid remodeling of the photosynthetic apparatus and minimize photo-oxidative stress in response to iron-availability in Chlamydomonas reinhardtii. Plant J. (in press). [DOI] [PubMed]
- Chasteen, N. D., 1998. Ferritin. Uptake, storage, and release of iron. Metal Ions Biol. Syst. 35 479–514. [PubMed] [Google Scholar]
- Chen, J. C., S. I. Hsieh, J. Kropat and S. S. Merchant, 2008. A ferroxidase encoded by FOX1 contributes to iron assimilation under conditions of poor iron nutrition in Chlamydomonas. Eukaryot. Cell 7 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg, G. A., J. E. Fitton, P. M. Harrison and A. Treffry, 1980. Ferritin: molecular structure and iron-storage mechanisms. Prog. Biophys. Mol. Biol. 36 56–86. [PubMed] [Google Scholar]
- Curie, C., and J. F. Briat, 2003. Iron transport and signaling in plants. Annu. Rev. Plant Biol. 54 183–206. [DOI] [PubMed] [Google Scholar]
- Dellagi, A., M. Rigault, D. Segond, C. Roux, Y. Kraepiel et al., 2005. Siderophore-mediated upregulation of Arabidopsis ferritin expression in response to Erwinia chrysanthemi infection. Plant J. 43 262–272. [DOI] [PubMed] [Google Scholar]
- Deng, X., and M. Eriksson, 2007. Two iron-responsive promoter elements control the expression of FOX1 in Chlamydomonas reinhardtii. Eukaryot. Cell 6 2163–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, M., P. Gardestrom and G. Samuelsson, 1995. Isolation, purification and characterization of mitochondria from Chlamydomonas reinhardtii. Plant Physiol. 107 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, C., D. Bucchini, M. E. Martin, S. Levi, P. Arosio et al., 2000. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 275 3021–3024. [DOI] [PubMed] [Google Scholar]
- Fobis-Loisy, I., K. Loridon, S. Lobréaux, M. Lebrun and J. F. Briat, 1995. Structure and differential expression of two maize ferritin genes in response to iron and abscisic acid. Eur. J. Biochem. 231 609–619. [DOI] [PubMed] [Google Scholar]
- Gupta, S., and D. Chatterji, 2003. Bimodal protection of DNA by Mycobacterium smegmatis DNA-binding protein from stationary phase cells. J. Biol. Chem. 278 5235–5241. [DOI] [PubMed] [Google Scholar]
- Ha, Y., D. Shi, G. W. Small, E. C. Theil and N. M. Allewell, 1999. Crystal structure of bullfrog M ferritin at 2.8 Å resolution: analysis of subunit interactions and the binuclear metal center. J. Biol. Inorg. Chem. 4 243–256. [DOI] [PubMed] [Google Scholar]
- Hentze, M. W., M. U. Muckenthaler and N. C. Andrews, 2004. Balancing acts: molecular control of mammalian iron metabolism. Cell 117 285–297. [DOI] [PubMed] [Google Scholar]
- Himelblau, E., and R. M. Amasino, 2000. Delivering copper within plant cells. Curr. Opin. Plant Biol. 3 205–210. [PubMed] [Google Scholar]
- Hörtensteiner, S., J. Chinner, P. Matile, H. Thomas and I. S. Donnison, 2000. Chlorophyll breakdown in Chlorella protothecoides: characterization of degreening and cloning of degreening-related genes. Plant Mol. Biol. 42 439–450. [DOI] [PubMed] [Google Scholar]
- Howe, G., and S. Merchant, 1992. The biosynthesis of membrane and soluble plastidic c-type cytochromes of Chlamydomonas reinhardtii is dependent on multiple common gene products. EMBO J. 11 2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. A., T. Punshon, A. Lanzirotti, L. Li, J. M. Alonso et al., 2006. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314 1295–1298. [DOI] [PubMed] [Google Scholar]
- Kimata, Y., and E. C. Theil, 1994. Posttranscriptional regulation of ferritin during nodule development in soybean. Plant Physiol. 104 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koorts, A. M., and M. Viljoen, 2007. Ferritin and ferritin isoforms I: structure-function relationships, synthesis, degradation and secretion. Arch. Physiol. Biochem. 113 30–54. [DOI] [PubMed] [Google Scholar]
- La Fontaine, S., J. M. Quinn, S. S. Nakamoto, M. D. Page, V. Göhre et al., 2002. Copper-dependent iron assimilation pathway in the model photosynthetic eukaryote Chlamydomonas reinhardtii. Eukaryot. Cell 1 736–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong, L. M., B. H. Tan and K. K. Ho, 1992. A specific stain for the detection of nonheme iron proteins in polyacrylamide gels. Anal. Biochem. 207 317–320. [DOI] [PubMed] [Google Scholar]
- Levi, S., J. Salfeld, F. Franceschinelli, A. Cozzi, M. H. Dorner et al., 1989. Expression and structural and functional properties of human ferritin L-chain from Escherichia coli. Biochemistry 28 5179–5184. [DOI] [PubMed] [Google Scholar]
- Levi, S., B. Corsi, M. Bosisio, R. Invernizzi, A. Volz et al., 2001. A human mitochondrial ferritin encoded by an intronless gene. J. Biol. Chem. 276 24437–24440. [DOI] [PubMed] [Google Scholar]
- Liochev, S. I., and I. Fridovich, 1999. Superoxide and iron: partners in crime. IUBMB Life 48 157–161. [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and T. D. Schmittgen, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25 402–408. [DOI] [PubMed] [Google Scholar]
- Lobréaux, S., and J. F. Briat, 1991. Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem. J. 274 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobréaux, S., T. Hardy and J. F. Briat, 1993. Abscisic acid is involved in the iron-induced synthesis of maize ferritin. EMBO J. 12 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield, M. A., 1995. Rapid immunodetection on polyvinylidene fluoride membrane blots without blocking. Anal. Biochem. 229 140–143. [DOI] [PubMed] [Google Scholar]
- Masuda, T., F. Goto, T. Yoshihara, T. Ezure, T. Suzuki et al., 2007. Construction of homo- and heteropolymers of plant ferritin subunits using an in vitro protein expression system. Protein Expr. Purif. 56 237–246. [DOI] [PubMed] [Google Scholar]
- Merchant, S., and B. W. Dreyfuss, 1998. Posttranslational assembly of photosynthetic metalloproteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 25–51. [DOI] [PubMed] [Google Scholar]
- Merchant, S. S., M. D. Allen, J. Kropat, J. L. Moseley, J. C. Long et al., 2006. Between a rock and a hard place: trace element nutrition in Chlamydomonas. Biochim. Biophys. Acta 1763 578–594. [DOI] [PubMed] [Google Scholar]
- Moseley, J., J. Quinn, M. Eriksson and S. Merchant, 2000. The Crd1 gene encodes a putative di-iron enzyme required for photosystem I accumulation in copper deficiency and hypoxia in Chlamydomonas reinhardtii. EMBO J. 19 2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley, J. L., T. Allinger, S. Herzog, P. Hoerth, E. Wehinger et al., 2002. Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO J. 21 6709–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia, I., V. Vazzola, D. Tarantino, F. Cellier, K. Ravet et al., 2007. Knock-out of ferritin AtFer1 causes earlier onset of age-dependent leaf senescence in Arabidopsis. Plant Physiol. Biochem. 45 898–907. [DOI] [PubMed] [Google Scholar]
- Naumann, B., E. J. Stauber, A. Busch, F. Sommer and M. Hippler, 2005. N-terminal processing of Lhca3 Is a key step in remodeling of the photosystem I-light-harvesting complex under iron deficiency in Chlamydomonas reinhardtii. J. Biol. Chem. 280 20431–20441. [DOI] [PubMed] [Google Scholar]
- Naumann, B., A. Busch, J. Allmer, E. Ostendorf, M. Zeller et al., 2007. Comparative quantitative proteomics to investigate the remodeling of bioenergetic pathways under iron deficiency in Chlamydomonas reinhardtii. Proteomics 7 3964–3979. [DOI] [PubMed] [Google Scholar]
- Petit, J. M., J. F. Briat and S. Lobréaux, 2001. a Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem. J. 359 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit, J. M., O. van Wuytswinkel, J. F. Briat and S. Lobréaux, 2001. b Characterization of an iron-dependent regulatory sequence involved in the transcriptional control of AtFer1 and ZmFer1 plant ferritin genes by iron. J. Biol. Chem. 276 5584–5590. [DOI] [PubMed] [Google Scholar]
- Ramachandran, P., P. Boontheung, Y. Xie, M. Sondej, D. T. Wong et al., 2006. Identification of N-linked glycoproteins in human saliva by glycoprotein capture and mass spectrometry. J. Proteome Res. 5 1493–1503. [DOI] [PubMed] [Google Scholar]
- Savino, G., J. F. Briat and S. Lobréaux, 1997. Inhibition of the iron-induced ZmFer1 maize ferritin gene expression by antioxidants and serine/threonine phosphatase inhibitors. J. Biol. Chem. 272 33319–33326. [DOI] [PubMed] [Google Scholar]
- Semin, B. K., L. N. Davletshina, A. A. Novakova, T. Y. Kiseleva, V. Y. Lanchinskaya et al., 2003. Accumulation of ferrous iron in Chlamydomonas reinhardtii. Influence of CO2 and anaerobic induction of the reversible hydrogenase. Plant Physiol. 131 1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger, D., 2002. Chemical strategies for iron acquisition in plants. Angew. Chem. Int. Ed. Engl. 41 2259–2264. [DOI] [PubMed] [Google Scholar]
- Tarantino, D., J. M. Petit, S. Lobréaux, J. F. Briat, C. Soave et al., 2003. Differential involvement of the IDRS cis-element in the developmental and environmental regulation of the AtFer1 ferritin gene from Arabidopsis. Planta 217 709–716. [DOI] [PubMed] [Google Scholar]
- Theil, E. C., 1987. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 56 289–315. [DOI] [PubMed] [Google Scholar]
- Theil, E. C., 2004. Iron, ferritin, and nutrition. Annu. Rev. Nutr. 24 327–343. [DOI] [PubMed] [Google Scholar]
- van Wuytswinkel, O., and J. F. Briat, 1995. Conformational changes and in vitro core-formation modifications induced by site-directed mutagenesis of the specific N-terminus of pea seed ferritin. Biochem. J. 305(3): 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, V. J., A. Treffry, J. P. Laulhére, E. R. Bauminger, M. I. Cleton et al., 1993. Structure and composition of ferritin cores from pea seed (Pisum sativum). Biochim. Biophys. Acta 1161 91–96. [DOI] [PubMed] [Google Scholar]
- Wei, J., and E. C. Theil, 2000. Identification and characterization of the iron regulatory element in the ferritin gene of a plant (soybean). J. Biol. Chem. 275 17488–17493. [DOI] [PubMed] [Google Scholar]
- Zancani, M., C. Peresson, A. Biroccio, G. Federici, A. Urbani et al., 2004. Evidence for the presence of ferritin in plant mitochondria. Eur. J. Biochem. 271 3657–3664. [DOI] [PubMed] [Google Scholar]