Abstract
In Chlamydomonas reinhardtii several nucleus-encoded proteins that participate in the mitochondrial oxidative phosphorylation are targeted to the organelle by unusually long mitochondrial targeting sequences. Here, we explored the components of the mitochondrial import machinery of the green alga. We mined the algal genome, searching for yeast and plant homologs, and reconstructed the mitochondrial import machinery. All the main translocation components were identified in Chlamydomonas as well as in Arabidopsis thaliana and in the recently sequenced moss Physcomitrella patens. Some of these components appear to be duplicated, as is the case of Tim22. In contrast, several yeast components that have relatively large hydrophilic regions exposed to the cytosol or to the intermembrane space seem to be absent in land plants and green algae. If present at all, these components of plants and algae may differ significantly from their yeast counterparts. We propose that long mitochondrial targeting sequences in some Chlamydomonas mitochondrial protein precursors are involved in preventing the aggregation of the hydrophobic proteins they carry.
THE endosymbiotic process that originated mitochondria (Poole and Penny 2007) was followed by a massive migration of genes to the nucleus. This gave rise to the highly reduced mitochondrial genomes that exist today (Gray et al. 1999). In many eukaryotes, mitochondrial DNA contains a limited set of genes encoding components of oxidative phosphorylation (OXPHOS): seven genes encoding NADH dehydrogenase subunits (nad1-6 and nad4L), three genes encoding cytochrome c oxidase subunits (cox1-3), two genes encoding ATP synthase polypeptides (atp6 and atp8), and the gene encoding the cytochrome b from the bc1 complex (cob) (Wallace 2007). In contrast, the mitochondrial genomes of chlorophycean algae like Chlamydomonas reinhardtii lack the genes nad3, nad4L, cox2, cox3, atp6, and atp8 (Vahrenholz et al. 1993; Denovan-Wright et al. 1998; Fan and Lee 2002). Many of these genes have migrated to the nucleus and their protein products are imported into the mitochondrion (Pérez-Martínez et al. 2000, 2001; Funes et al. 2002; Cardol et al. 2006). In addition, the cox2 gene was fragmented into the independent genes cox2a and cox2b (Pérez-Martínez et al. 2001). The nucleus-encoded genes nad3, nad4L, cox2a, cox2b, cox3, and atp6 adapted for expression by modifying their codon usage; they also acquired promoters, polyadenylation signals, introns, and mitochondrial targeting sequences (MTSs) (González-Halphen et al. 2004). MTSs are generally small, cleavable N-terminal presequences of 20–40 residues, capable of forming amphiphilic α-helices that are recognized by the import apparatus (Rehling et al. 2004; Neupert and Herrmann 2007). Chlamydomonas MTSs vary in size; the short ones direct the proteins to the mitochondrial matrix, such as the α- and β-subunits of the FoF1-ATP synthase (45 and 26 amino acids, respectively; Franzén and Falk 1992; Nurani and Franzén 1996). The MTSs of intermediate length direct proteins to the inner mitochondrial membrane, such as the Rieske iron–sulfur protein (54 amino acids; Atteia and Franzén 1996) and cytochrome c1 (70 amino acids; Atteia et al.2003). Long MTSs help import of proteins with two or more transmembrane stretches, such as Atp6 (107 amino acids; Funes et al.2002), Cox3 (110 residues; Pérez-Martínez et al. 2000), Cox2A (130 amino acids; Pérez-Martínez et al.2001), Nad3 (160 residues; Cardol et al. 2006), and Nad4L (133 amino acids; Cardol et al. 2006). Although experimental evidence is lacking, they seem to have a bipartite nature that may allow both targeting to the mitochondrial matrix and subsequent insertion into the inner mitochondrial membrane.
Parallel to the migration of mitochondrial genes to the nucleus, mechanisms for the import of cytosol-synthesized proteins into the organelle evolved (Herrmann 2003). The yeast Saccharomyces cerevisiae, has been the system of choice to study the mitochondrial import machinery (Herrmann and Neupert 2003). The four main translocators are the translocase of the outer membrane (TOM) complex, the translocase of the inner membrane (TIM)22 and TIM23 complexes, and the topogenesis of mitochondrial outer membrane β-barrel proteins/sorting and assembly machinery (TOB/SAM) complex.
The entry point for all mitochondria-destined proteins is the TOM complex, which consists of one central channel (formed by Tom40, Tom7, Tom6, and Tom5) and three receptor proteins (Tom70, Tom22, and Tom20) (Rapaport 2005).
Sorting of the incoming proteins occurs in the mitochondrial intermembrane space (IMS). Two hexameric complexes of small Tim proteins (Tim9/Tim10 and Tim8/Tim13) guide the substrate proteins either to the TOB/SAM complex in the outer membrane or to the TIM22 complex in the inner membrane (Neupert and Herrmann 2007).
The TOB/SAM complex, composed of Tob55, Tob38, and Mas37, mediates the topogenesis of outer membrane β-barrel proteins. Tob55 constitutes the translocation channel, and together with Tob38 it conforms the minimal functional unit of the complex. Mas37 might be involved in the release of β-barrel intermediates to the membrane (Paschen et al. 2005).
The IMS proteins are imported by means of a bipartite MTS, they anchor to the TIM23 translocase, they are laterally released into the inner membrane, and finally they are liberated as soluble proteins after a second proteolytic cleavage. Alternatively, soluble proteins that lack a MTS are trapped within the IMS after binding cofactors or by folding via disulfide bridges. This second pathway is mediated by Mia40 and Erv1, which conform the disulfide relay system (Herrmann and Hell 2005).
The proteins that reside within the inner membrane reach their destiny through three different pathways:
Membrane proteins with even-numbered transmembrane segments that expose both N and C termini to the IMS (i.e., the family of carrier proteins) are delivered by the small TIM proteins to the TIM22 complex (composed of Tim12, Tim18, Tim22, Tim54, and a fraction of Tim9 and Tim10; Rehling et al. 2004).
MTS-containing proteins of the inner membrane that have only one transmembrane domain whose N terminus is facing the matrix are imported through TIM23 by a “stop-transfer” mechanism. The transmembrane domain functions as a sorting signal that arrests the import of the precursor and allows its lateral insertion into the inner membrane. The stop-transfer mechanism also works for proteins with the opposite orientation, i.e., N terminus facing the IMS (Van Loon et al. 1986; Glick et al.1992).
MTS-containing proteins with more than one transmembrane domain are imported into the matrix where they bind mtHsp70 and are subsequently integrated into the inner membrane (Hartl et al. 1987; Herrmann et al. 1997; Neupert and Herrmann 2007). This last integration step is mediated by Oxa1 (Hell et al. 1998) and Mba1 (Preuss et al. 2001). These two components are also involved in the insertion of mitochondria-synthesized proteins.
Mitochondrial proteins destined to the matrix are translocated by TIM23 in a process that requires a membrane potential (ΔΨ) and ATP hydrolysis. TIM23 is formed by two sectors that operate sequentially (Kutik et al. 2007; Neupert and Herrmann 2007): the protein-conducting channel (formed by Tim23, Tim17, Tim21, and Tim50) and the import motor (formed by Tim44, Tim16, Tim14, and mtHsp70). The stepwise insertion of preproteins to the matrix is achieved by spontaneous sliding reactions through the pores of TOM and TIM23. The polypeptides are trapped in the matrix by the sequential binding of several molecules of Hsp70 that also prevent possible retrograde movements (Okamoto et al. 2002).
Here, we mined the Chlamydomonas genome (Merchant et al. 2007), looking for yeast and Arabidopsis homologs, to reconstruct the algal mitochondrial import machinery. For broader comparison purposes, we also mined the draft genome of the moss Physcomitrella patens. In this study, we have limited our analysis to those proteins involved directly in the import process; thus, we have omitted several chaperones, as well as nucleotide exchange factors that do not interact directly with the incoming polypeptides or that do not belong to the import complexes described above.
RESULTS AND DISCUSSION
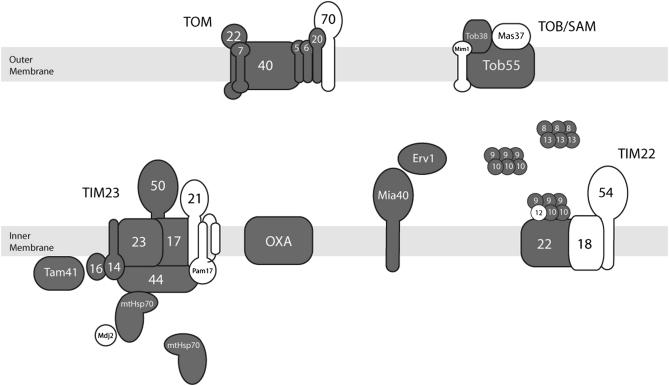
Since atypical MTSs are present in several mitochondrial OXPHOS precursors of Chlamydomonas, it is likely that uncharacterized machineries exist to ensure the import of proteins containing two or more transmembrane stretches into the inner mitochondrial membrane. As a starting point we explored the components of the mitochondrial import machinery that are conserved in the yeast system. Table 1 lists the identified components of the Chlamydomonas mitochondrial import machinery and compares them with its yeast and Arabidopsis counterparts. Figure 1 shows the disposition of the green alga import machinery components in the different mitochondrial compartments. All the main translocation components seem to be present in Chlamydomonas as well as in plants. Indeed, isolated mitochondria from Chlamydomonas are able to import plant mitochondrial precursor proteins, although with much less efficiency than its own (Nurani et al. 1997). Arabidopsis also exhibits duplication of several components, since isoforms of Tom7, Tom20, Tom22, Tom40, Tim14, Tim17, Tim23, Tim44, and Tim22 are present. Chlamydomonas seems to posses at least three isoforms of Tim22. The duplication of Tim22 in land plants and green algae could be related to the absence of Tim18 and Tim54 in the TIM22 complex. The functional significance of multiple Tim22 copies in plants and green algae remains to be ascertained.
TABLE 1.
Genomic analysis of the mitochondrial protein import machinery components from Chlamydomonas
| Name in yeast (accession no.) | Alternative names in yeast | Counterparts in Arabidopsis (accession no.) | Counterpart in Chlamydomonas (accession no.) | Counterpart present in Physcomitrella patens |
|---|---|---|---|---|
| Outer membrane: TOM complex | ||||
| Tom70 | Mas70, Mom72, Omp1 | NI | NI | NI |
| (NP_014278) | ||||
| Tom40 | Isp42, Mom38 | TOM40-1 | Tom40 | (+) |
| (NP_013930) | (NP_188634) | (EDP06354) | ||
| TOM40-2 | ||||
| (NP_175457) | ||||
| Tom22 | Mas17, Mas22, Mom22 | TOM9-1 | Tom22a | (+) |
| (NP_014268) | (NP_563699) | |||
| TOM9-2 | ||||
| (Q9FNC9) | ||||
| Tom20 | Mas20, Mom19 | TOM20-1 | Tom20 | (+) |
| (NP_011596) | (NP_189343) | (EDP03564) | ||
| TOM20-2 (NP_174059) | ||||
| TOM20-3 (NP_189344) | ||||
| TOM20-4 | ||||
| (NP_198909) | ||||
| Tom7 | Mom7, Yok22 | TOM7-1 | Tom7 | (+) |
| (NP_014329) | (NP_568593) | (EDP05834) | ||
| TOM7-2 | ||||
| (Q3ECI7) | ||||
| Tom6 | Isp6, Mom8B | TOM6 | Tom6 | (+) |
| (NP_014688) | (NP_564545) | (EDO98875)b | ||
| Tom5 | Mom8A | TOM5 | Tom5 | N.I. |
| (NP_015459) | (NP_196421) | (EDP08420) | ||
| Outer membrane: TOB/SAM complex | ||||
| Mim1 | Tom13 | NI | NI | (+) |
| (NP_014616) | ||||
| Tob55 | Sam50, Tom50, Omp85 | TOB55 | Tob55 | (+) |
| (NP_014372) | (NP_568157) | (EDP05088) | ||
| Tob38 | Sam35, Tom38 | METAXIN | Tob38 | (+) |
| (NP_011951) | (NP_565446) | (EDP05944) | ||
| Mas37 | Sam37, Tom37 | NI | NI | NI |
| (NP_013776) | ||||
| Intermembrane space | ||||
| Mia40 | Tim40 | Mia40 | Mia40 | NI |
| (NP_012726) | (NP_200377) | (EDO96731) | ||
| Erv1 | Erv1 | Erv1 | (+) | |
| (NP_011543) | (NP_564557) | (EDP03768) | ||
| Tim13 | TIM13 | Tim13 | (+) | |
| (NP_011697) | (NP_564780) | (EDO99447) | ||
| Tim8 | TIM8 | Tim8 | (+) | |
| (NP_058168) | (NP_199894) | (EDP04523) | ||
| Tim10 | Mrs11 | TIM10 | Tim10 | (+) |
| (NP_011869) | (NP_565682) | (EDO96752) | ||
| Tim9 | TIM9 | Tim9 | (+) | |
| (NP_010894) | (NP_190240) | (EDP09353) | ||
| Tim12 | Mrs5 | NI | NI | NI |
| (NP_009649) | ||||
| Inner membrane: TIM23 complex | ||||
| Tim50 | TIM50 | Tim50 | (+) | |
| (NP_015262) | (NP_175986) | (EDP05270) | ||
| Tim44 | TIM44-1 | Tim44 | (+) | |
| (NP_012242) | (NP_565473) | (EDO99746) | ||
| TIM44-2 | ||||
| (NP_181151) | ||||
| Tim23 | Mim23, Mpi3, Mas6 | NP_564028 | Tim23 | (+) |
| (NP_014414) | (TIM23-1) | (EDP09567) | ||
| NP_177419 | ||||
| (TIM23-2) | ||||
| (TIM23-3) | ||||
| NP_187131 | ||||
| Tim21 | NI | NI | NI | |
| (NP_011547) | ||||
| Tim17 | Mim17, Mpi2, Sms1 | NP_173460 | Tim17 | (+) |
| (NP_012392) | (TIM17-1) | (EDP07835) | ||
| NP_973621 | ||||
| (TIM17-2) | ||||
| NP_196730 | ||||
| (TIM17-3) | ||||
| Pam17 | NI | NI | NI | |
| (NP_012991) | ||||
| Tim16 | Pam16, Tim16, Mia1 | NP_851243 | Tim16 | (+) |
| (NP_012431) | (Tim16) | (EDP00908) | ||
| Tim14 | Pam18, Tim14 | NP_565824 | Tim14 | (+) |
| (NP_013108) | (TIM14-1) | (EDP02285) | ||
| NP_566352 | ||||
| (TIM14-2) | ||||
| Inner membrane: TIM22 complex | ||||
| Tim54 | NI | NI | NI | |
| (NP_012481) | ||||
| Tim22 | NP_566368 | Tim22A | (+) | |
| (NP_010064) | (TIM22-1) | (EDP09249) | ||
| NP_567754 | Tim22B | |||
| (TIM22-2) | (EDO96777) | |||
| NP_200362 | Tim22C | |||
| (TIM22-3) | (EDP09499) | |||
| Tim18 | NI | NI | NI | |
| (NP_014940) | ||||
| Matrix | ||||
| Tam41 | Mmp37 | NP_190347 | Tam41 | (+) |
| (NP_011560) | (Tam41) | (EDP05657) | ||
| Hsp70 | Ssc1 | NP_196521 | Hsp70 | (+) |
| (NP_012579) | (Hsp70) | (EDP02463) | ||
| Protein export | ||||
| Oxa1 | Alb3, YidC | NP_201011 | Oxa1 | (+) |
| (NP_011081) | (OXA1P) | (EDO98825) | ||
Homologous sequences in yeast and Arabidopsis were identified in Chlamydomonas. Accession numbers of protein sequences were obtained from Entrez at the National Center for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov/). Homologous sequences usually exhibited e-values <1 × 10−5. NI (not identified) indicates that no sequence of significant similarity was found. (+) indicates that a counterpart is present in the draft version of the genome of Physcomitrella patens (http://genome.jgi-psf.org/Phypa1_1/Phypa1_1.home.html).
Not identified in the protein database, but present in the Chlamydomonas genome [scaffold_38 (1,040,279 bp): nucleotides 212,622–212,814 (192 bp)].
The protein model is probably wrong. Tim6 would correspond only to the N-terminal region of a protein EDO98875. A stop codon was probably overlooked in the original ORF. Also present in the Chlamydomonas genome [scaffold_45 (891,468 bp): nucleotides 206,948–207,055 (108 bp)].
Figure 1.—
The mitochondrial protein import machinery of yeast and the corresponding homologs identified in Chlamydomonas. Shaded components are yeast components that have counterparts in plants and green algae. Open components are proteins present in the yeast mitochondrial import machinery that were not present or could not be identified in plants and green algae.
Notably, some components that have relatively large hydrophilic regions exposed to the cytosol (Tom70 in the case of TOM and Mim1 and Mas37 in the case of TOB/SAM) or to the IMS (Tim 21 in the case of TIM23 and Tim54 and Tim18 in the case of TIM22) seem to be absent in land plants and green algae. These proteins, which are present but are not essential in the yeast import system, may have originated later in evolution. This hypothesis is also supported by the fact that in human mitochondria, and presumably in other animals as well, Mas37, Tim21, Tim54, and Tim18 seem to be also absent (MacKenzie and Payne 2007). Components with large regions protruding out of the lipid bilayer suggest an important function in the recognition of precursor proteins and might reflect the uniqueness of each system. In contrast with what we observed in land plants and green algae, human mitochondria seem to lack receptors like Tom6 and Tom5 but contain Tom70, which appears to be missing in plants and green algae (MacKenzie and Payne 2007). The plant and algal receptors may have a structure that differs from the one of yeast; this may allow them to recognize specific MTSs, such as the long ones described above. These novel receptors have escaped detection by the in silico approach used in this work, but they may be identified by future biochemical and proteomic studies. Efforts in this direction have already been undertaken with the Arabidopsis mitochondrial proteome (Heazlewood and Millar 2005; Millar et al. 2005), and initial novel components as well as minimal units of import complexes have also been described in this land plant (Lister et al. 2007).
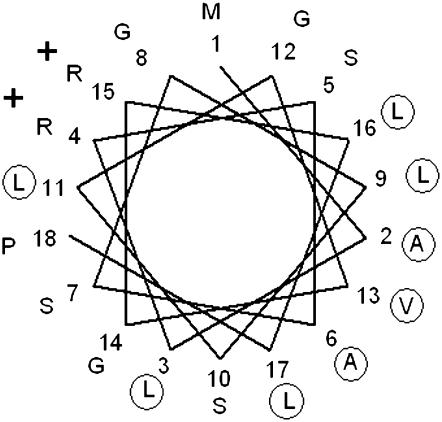
As the receptor components that have relatively large hydrophilic regions are absent in both Chlamydomonas and Arabidopsis, we looked for other ways to explain the presence of atypical MTSs. Nucleus-encoded proteins with two or more transmembrane stretches containing exceedingly long MTSs are difficult to import into organelles due to their high hydrophobicity (Popot and de Vitry 1990; Claros et al. 1995). To date, Chlamydomonas seems to be unique in the sense that it is able to import into the matrix several cytosol-synthesized proteins with two or more transmembrane stretches and to insert them in the mitochondrial inner membrane. When the nucleus-encoded Atp6, Cox3, Cox2A, Nad3, and Nad4L proteins from Chlamydomonas were compared with their mitochondrion-encoded counterparts from other organisms, it was found that the algal ones are less hydrophobic (Pérez-Martínez et al. 2000, 2001; Funes et al. 2002; Cardol et al. 2006). This reduction in hydrophobicity may increase the importability of the proteins. However, as the proteins contain transmembrane helices, they are still much more hydrophobic than most other proteins that are imported into the mitochondrion. Therefore, it is probable that the proteins have large hydrophobic regions exposed at the surface. To prevent aggregation, it is necessary to mask these hydrophobic surfaces. To some extent, chaperones may help proteins to stay in solution, but for very hydrophobic proteins additional mechanisms may be needed. We now propose that the long MTSs of hydrophobic mitochondrial proteins form amphipatic structures that cover the hydrophobic surfaces of the proteins, increasing their hydrophilicity. Figure 2 shows the helical wheel projection of the first 18 residues of the MTS of the Nad3 preprotein. As shown for other mitochondrial MTS sequences of Chlamydomonas (Atteia and Franzén 1996), the amphiphilic helices exhibit a large hydrophobic sector. Possibly the long MTSs of hydrophobic preproteins are indeed bipartite: one first segment can direct the protein to the matrix, leaving the second segment available to be recognized by inner membrane components that would guide the proteins to the site where insertion can occur. This processing step linked to insertion could be regulated by inner membrane proteases as it happens in yeast (Koppen and Langer 2007).
Figure 2.—
Helical-wheel projection of the first 18 residues of the MTS of the nucleus-encoded mitochondrial Nad3 protein of Chlamydomonas. Hydrophobic amino acids are circled and charged residues are indicated by their charge. The amphipatic character of the helix, exposing a large hydrophobic sector, is evident.
There are few other examples of nuclear genes encoding highly hydrophobic mitochondrial proteins that migrated to the nuclear genome; such is the case of the cox2 gene of some legumes (Adams et al. 1999). The mitochondrial import of the Cox2 protein has been studied in soybean (Daley et al. 2002a,b). As in Chlamydomonas, the soybean Cox2 protein is less hydrophobic than the mitochondria-encoded Cox2 proteins, and it also has a very long tripartite MTS of 136 amino acids. This MTS cannot be replaced by other MTSs (Daley et al. 2002a). The first 20 amino acids are required for mitochondrial targeting and may be replaced by another MTS, the central portion is required for efficient import, and the last 12 amino acids are required for correct maturation. When this MTS was fused to a mitochondrion-encoded Cox2 protein, the protein could not be imported into soybean mitochondria unless the hydrophobicity of the first transmembrane helix of Cox2 was decreased (Daley et al. 2002b). Thus, the successful import of Cox2 requires two adaptations: a decreased hydrophobicity of the first transmembrane helix and a very long MTS. The central portion of the MTS contains both hydrophobic and hydrophilic amino acids (36% hydrophobic amino acids, 16% charged amino acids). This amino acid composition is compatible with our proposal that the MTS forms an amphipatic structure that masks hydrophobic surfaces of the protein during import.
In summary, the recent completion of the Chlamydomonas genome (Merchant et al. 2007) allowed the partial reconstruction of the mitochondrial protein import machinery of the green alga. A future proteomic survey of Chlamydomonas mitochondria should focus on identifying probable novel components that function as receptors of protein precursors with a specific plant/algal MTS, in particular, the putative substitutes of the yeast Tom70, Mim1, Mas37, Tim54, Tim21, and Tim18. We expect that this partial reconstruction of the Chlamydomonas mitochondrial import machinery may stimulate experimental proteomic approaches that will establish the identity of novel components. Of particular interest is to acquire knowledge of the pathway through which preproteins with two or more transmembrane segments that participate in OXPHOS are imported and inserted into the mitochondrial inner membrane of Chlamydomonas.
Acknowledgments
The technical expertise of Miriam Vázquez-Acevedo (D.G.-H. laboratory) is gratefuly acknowledged. Work in our laboratories is supported by grants 56619 from Consejo Nacional de Ciencia y Tecnología (Mexico) and IN217108 from Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México. L.-G.F. was supported by a grant from the Carl Trygger Foundation.
References
- Adams, K. L., K. Song, P. G. Roessler, J. M. Nugent, J. L. Doyle et al., 1999. Intracellular gene transfer in action: dual transcription and multiple silencings of nuclear and mitochondrial cox2 genes in legumes. Proc. Natl. Acad. Sci. USA 96 13863–13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atteia, A., and L.-G. Franzén, 1996. Identification, cDNA sequence and deduced amino acid sequence of the mitochondrial Rieske iron-sulfur protein from the green alga Chlamydomonas reinhardtii. Implications for protein targeting and subunit interaction. Eur. J. Biochem. 237 792–799. [DOI] [PubMed] [Google Scholar]
- Atteia, A., R. van Lis, D. Wetterskog, E. B. Gutiérrez-Cirlos, L. Ongay-Larios et al., 2003. Structure, organization and expression of the genes encoding mitochondrial cytochrome c(1) and the Rieske iron-sulfur protein in Chlamydomonas reinhardtii. Mol. Genet. Genomics 268 637–644. [DOI] [PubMed] [Google Scholar]
- Cardol, P., M. Lapaille, P. Minet, F. Franck, R. F. Matagne et al., 2006. ND3 and ND4L subunits of mitochondrial complex I, both nucleus encoded in Chlamydomonas reinhardtii, are required for activity and assembly of the enzyme. Eukaryot. Cell 5 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claros, M. G., J. Perea, Y. Shu, F. A. Samatey, J. L. Popot et al., 1995. Limitations to in vivo import of hydrophobic proteins into yeast mitochondria. The case of a cytoplasmically synthesized apocytochrome b. Eur. J. Biochem. 228 762–771. [PubMed] [Google Scholar]
- Daley, D. O., K. L. Adams, R. Clifton, S. Qualmann, A. H. Millar et al., 2002. a Gene transfer from mitochondrion to nucleus: novel mechanisms for gene activation from Cox2. Plant J. 30 11–21. [DOI] [PubMed] [Google Scholar]
- Daley, D. O., R. Clifton and J. Whelan, 2002. b Intracellular gene transfer: reduced hydrophobicity facilitates gene transfer for subunit 2 of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 99 10510–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denovan-Wright, E. M., A. M. Nedelcu and R. W. Lee, 1998. Complete sequence of the mitochondrial DNA of Chlamydomonas eugametos. Plant Mol. Biol. 36 285–295. [DOI] [PubMed] [Google Scholar]
- Fan, J., and R. W. Lee, 2002. Mitochondrial genome of the colorless green alga Polytomella parva: two linear DNA molecules with homologous inverted repeat termini. Mol. Biol. Evol. 19 999–1007. [DOI] [PubMed] [Google Scholar]
- Franzén, L. G., and G. Falk, 1992. Nucleotide sequence of cDNA clones encoding the beta subunit of mitochondrial ATP synthase from the green alga Chlamydomonas reinhardtii: the precursor protein encoded by the cDNA contains both an N-terminal presequence and a C-terminal extension. Plant Mol. Biol. 19 771–780. [DOI] [PubMed] [Google Scholar]
- Funes, S., E. Davidson, M. G. Claros, R. van Lis, X. Pérez-Martínez et al., 2002. The typically mitochondrial DNA-encoded ATP6 subunit of the F1F0-ATPase is encoded by a nuclear gene in Chlamydomonas reinhardtii. J. Biol. Chem. 277 6051–6058. [DOI] [PubMed] [Google Scholar]
- Glick, B. S., A. Brandt, K. Cunningham, S. Müller, R. L. Hallberg et al., 1992. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell 69 809–822. [DOI] [PubMed] [Google Scholar]
- González-Halphen, D., S. Funes, X. Pérez-Martínez, A. Reyes-Prieto, M. G. Claros et al., 2004. Genetic correction of mitochondrial diseases: using the natural migration of mitochondrial genes to the nucleus in chlorophyte algae as a model system. Ann. NY Acad. Sci. 1019 232–239. [DOI] [PubMed] [Google Scholar]
- Gray, M. W., G. Burger and B. F. Lang, 1999. Mitochondrial evolution. Science 283 1476–1481. [DOI] [PubMed] [Google Scholar]
- Hartl, F. U., J. Ostermann, B. Guiard and W. Neupert, 1987. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell 51 1027–1037. [DOI] [PubMed] [Google Scholar]
- Heazlewood, J. L., and A. H. Millar, 2005. AMPDB: the Arabidopsis mitochondrial protein database. Nucleic Acids Res. 33 D605–D610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell, K., J. M. Herrmann, E. Pratje, W. Neupert and R. A. Stuart, 1998. Oxa1p, an essential component of the N-tail protein export machinery in mitochondria. Proc. Natl. Acad. Sci. USA 95 2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, J. M., 2003. Converting bacteria to organelles: evolution of mitochondrial protein sorting. Trends Microbiol. 11 74–79. [DOI] [PubMed] [Google Scholar]
- Herrmann, J. M., and K. Hell, 2005. Chopped, trapped or tacked–protein translocation into the IMS of mitochondria. Trends Biochem. Sci. 30 205–211. [DOI] [PubMed] [Google Scholar]
- Herrmann, J. M., and W. Neupert, 2003. Protein insertion into the inner membrane of mitochondria. IUBMB Life 55 219–225. [DOI] [PubMed] [Google Scholar]
- Herrmann, J. M., W. Neupert and R. A. Stuart, 1997. Insertion into the mitochondrial inner membrane of a polytopic protein, the nuclear-encoded Oxa1p. EMBO J. 16 2217–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutik, S., B. Guiard, H. E. Meyer, N. Wiedemann and N. Pfanner, 2007. Cooperation of translocase complexes in mitochondrial protein import. J. Cell Biol. 179 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppen, M., and T. Langer, 2007. Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit. Rev. Biochem. Mol. Biol. 42 221–242. [DOI] [PubMed] [Google Scholar]
- Lister, R., C. Carrie, O. Duncan, L. H. Ho, K. A. Howell et al., 2007. Functional definition of outer membrane proteins involved in preprotein import into mitochondria. Plant Cell 19 3739–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie, J. A., and R. M. Payne, 2007. Mitochondrial protein import and human health and disease. Biochem. Biophys. Acta 1772 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant, S. S., S. E. Prochnik, O. Vallon, E. H. Harris, S. J. Karpowicz et al., 2007. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, A. H., J. L. Heazlewood, B. K. Kristensen, H. P. Braun and I. M. Møller, 2005. The plant mitochondrial proteome. Trends Plant Sci. 10 36–43. [DOI] [PubMed] [Google Scholar]
- Neupert, W., and J. M. Herrmann, 2007. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76 723–749. [DOI] [PubMed] [Google Scholar]
- Nurani, G., and L.-G. Franzén, 1996. Isolation and characterization of the mitochondrial ATP synthase from Chlamydomonas reinhardtii. cDNA sequence and deduced protein sequence of the alpha subunit. Plant Mol. Biol. 31 1105–1116. [DOI] [PubMed] [Google Scholar]
- Nurani, G., M. Eriksson, C. Knorpp, E. Glaser and L.-G. Franzén, 1997. Homologous and heterologous protein import into mitochondria isolated from the green alga Chlamydomonas reinhardtii. Plant Mol. Biol. 35 973–980. [DOI] [PubMed] [Google Scholar]
- Okamoto, K., A. Brinker, S. A. Paschen, I. Moarefi, M. Hayer-Hartl et al., 2002. The protein import motor of mitochondria: a targeted molecular ratchet driving unfolding and translocation. EMBO J. 21 3659–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen, S. A., W. Neupert and D. Rapaport, 2005. Biogenesis of beta-barrel membrane proteins of mitochondria. Trends Biochem. Sci. 30 575–582. [DOI] [PubMed] [Google Scholar]
- Pérez-Martínez, X., M. Vázquez-Acevedo, E. Tolkunova, S. Funes, M. G. Claros et al., 2000. Unusual location of a mitochondrial gene. Subunit III of cytochrome C oxidase is encoded in the nucleus of Chlamydomonad algae. J. Biol. Chem. 275 30144–30152. [DOI] [PubMed] [Google Scholar]
- Pérez-Martínez, X., A. Antaramian, M. Vázquez-Acevedo, S. Funes, E. Tolkunova et al., 2001. Subunit II of cytochrome c oxidase in Chlamydomonad algae is a heterodimer encoded by two independent nuclear genes. J. Biol. Chem. 276 11302–11309. [DOI] [PubMed] [Google Scholar]
- Poole, A. M., and D. Penny, 2007. Evaluating hypotheses for the origin of eukaryotes. BioEssays 29 74–84. [DOI] [PubMed] [Google Scholar]
- Popot, J.-L., and C. de Vitry, 1990. On the microassembly of integral membrane proteins. Annu. Res. Biophys. Chem. 19 369–403. [DOI] [PubMed] [Google Scholar]
- Preuss, M., K. Leonhard, K. Hell, R. A. Stuart, W. Neupert et al., 2001. Mba1, a novel component of the mitochondrial protein export machinery of the yeast Saccharomyces cerevisiae. J. Cell Biol. 153 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport, D., 2005. How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane? J. Cell Biol. 171 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling, P., K. Brandner and N. Pfanner, 2004. Mitochondrial import and the twin-pore translocase. Nat. Rev. Mol. Cell. Biol. 5 519–530. [DOI] [PubMed] [Google Scholar]
- Vahrenholz, C., G. Riemen, E. Pratje, B. Dujon and G. Michaelis, 1993. Mitochondrial DNA of Chlamydomonas reinhardtii: the structure of the ends of the linear 15.8-kb genome suggests mechanisms for DNA replication. Curr. Genet. 24 241–247. [DOI] [PubMed] [Google Scholar]
- Vanloon, A. P., A. W. Brändli and G. Schatz, 1986. The presequences of two imported mitochondrial proteins contain information for intracellular and intramitochondrial sorting. Cell 44 801–812. [DOI] [PubMed] [Google Scholar]
- Wallace, D. C., 2007. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu. Rev. Biochem. 76 781–821. [DOI] [PubMed] [Google Scholar]