Abstract
Large, multisubunit Ccr4-Not complexes are evolutionarily conserved global regulators of gene expression. Deletion of CCR4 or several components of Ccr4-Not complexes results in abnormally large cells. Since yeast must attain a critical cell size at Start to commit to division, the large size of ccr4Δ cells implies that they may have a size-specific proliferation defect. Overexpression of CLN1, CLN2, CLN3, and SWI4 reduces the size of ccr4Δ cells, suggesting that ccr4Δ cells have a G1-phase cyclin deficiency. In support of this, we find that CLN1 and CLN2 expression and budding are delayed in ccr4Δ cells. Moreover, overexpression of CCR4 advances the timing of CLN1 expression, promotes premature budding, and reduces cell size. Genetic analyses suggest that Ccr4 functions independently of Cln3 and downstream of Bck2. Thus, like cln3Δbck2Δ double deletions, cln3Δccr4Δ cells are also inviable. However, deletion of Whi5, a transcriptional repressor of CLN1 and CLN2, restores viability. We find that Ccr4 negatively regulates the half-life of WHI5 mRNAs, and we conclude that, by modulating the stability of WHI5 mRNAs, Ccr4 influences the size-dependent timing of G1-phase cyclin transcription.
CCR4-NOT complexes have multiple subunits and diverse functions involved in the global control of mRNA expression (reviewed in Denis and Chen 2003; Collart and Timmers 2004). These large complexes contain at least nine core components: Ccr4, Caf1, Caf40, Caf130, and five Not proteins (reviewed in Denis and Chen 2003; Collart and Timmers 2004). Importantly, genes for all of these components are conserved in humans (Denis and Chen 2003; Collart and Timmers 2004). Ccr4-Not complexes are involved in both gene activation and repression (Collart and Struhl 1994; Liu et al. 1998). Since the yeast CCR4 gene encodes a cytoplasmic deadenylase (Chen et al. 2002; Tucker et al. 2002) whose activity is intimately involved in mRNA degradation, the diverse roles of Ccr4-Not complexes in regulating gene expression may be predominately due to changes in mRNA half-lives.
Components of Ccr4-Not complexes have long been tangentially associated with cell cycle control. For example, two core components, Not1 and Not2, were initially isolated as yeast cell division cycle (cdc) mutants (e.g., cdc39 and cdc36) that arrest cells in G1-phase at the restrictive temperature (Reed 1980). In addition, many Ccr4-Not complex components associate or interact genetically with gene products with known cell cycle roles (e.g., Clb2, Cdc73, Dbf2, Paf1, and Sic1) (Liu et al. 1997; Chang et al. 1999; Gavin et al. 2002; Pan et al. 2006). Also, recent evidence has implicated Ccr4 and Caf1 in the DNA replication checkpoint (Woolstencroft et al. 2006). Moreover, Ccr4-Not complex components appear to be essential for G1-phase cell cycle transition in irradiated cells (Westmoreland et al. 2004). Nevertheless, the mechanistic relationship between Ccr4-Not complexes and cell cycle control remains to be fully elucidated.
In yeast, cell cycle progression is primarily controlled at the G1/S-phase transition at a point called Start (reviewed in Cross 1995; Futcher 1996; Bloom and Cross 2007). Importantly, the basic yeast cell cycle machinery involved in regulating Start, including the G1-phase cyclin-dependent kinases (Cdks) that promote cell cycle progression, is highly conserved in mammalian cells (reviewed in Sherr and Roberts 2004; Cooper 2006). In yeast, cells must attain a minimum cell size to progress past Start (reviewed in Rupes 2002; Jorgensen and Tyers 2004). Two constitutively expressed yeast proteins, Cln3 and Bck2, function equivalently in mammalian cyclin D and couple cell cycle progression to the attainment of a minimum cell size. As cells near the critical cell size requirement for Start, Cln3 and Bck2 activate the transcription of the downstream G1-phase cyclins CLN1 and CLN2 (Tyers et al. 1993; Epstein and Cross 1994; Di Como et al. 1995; Dirick et al. 1995). Of interest, overexpression of CLN3 or BCK2 causes premature CLN1 and CLN2 transcription, advances Start, and produces abnormally small cells (Tyers et al. 1993; Epstein and Cross 1994; Di Como et al. 1995; Dirick et al. 1995). In contrast, loss of Cln3 or Bck2 function delays Start, CLN1, and CLN2 expression and results in unusually large cells (Epstein and Cross 1994; Di Como et al. 1995; Dirick et al. 1995; Wijnen and Futcher 1999). In addition, cln3Δbck2Δ double mutants are inviable and arrest as large unbudded G1-phase cells (Epstein and Cross 1994; Wijnen and Futcher 1999). Despite their essential role in initiating CLN1 and CLN2 transcription, the mechanism whereby Cln3's and Bck2's activity is modulated by cell size is not known.
To elucidate the genetic pathways involved in cell size control, others and we conducted systematic genomewide genetic screens (Jorgensen et al. 2002; Zhang et al. 2002). Notably, the yeast equivalent of the pRB tumor suppressor gene was identified from these screens (Jorgensen et al. 2002; Zhang et al. 2002; Costanzo et al. 2004; De Bruin et al. 2004). This protein, Whi5, functions synonymously with pRb to suppress G1-phase cyclin expression, despite the fact that it is not a sequence homolog (Jorgensen et al. 2002; Zhang et al. 2002; Costanzo et al. 2004; De Bruin et al. 2004). Therefore, loss of Whi5 function results in premature CLN1 and CLN2 expression, which in turn advances cell cycle progression, leading to abnormally small cells. Importantly, loss of Whi5 restores viability to cln3Δbck2Δ double mutants (Jorgensen et al. 2002; Zhang et al. 2002; Costanzo et al. 2004; De Bruin et al. 2004). Analogously, in mammalian cells, loss of pRB restores viability to cells lacking all cyclin D genes (reviewed in Sherr and Roberts 2004).
In addition to gene deletions that make cells small, gene deletions that result in abnormally large cells have also been identified (Jorgensen et al. 2002; Zhang et al. 2002). Interestingly, a large number of these genes encode or associate with components of Ccr4-Not complexes (e.g., CCR4, PAF1, HPR1, CAF1, and others) (Jorgensen et al. 2002; Zhang et al. 2002). While data indicate that loss of function of a number of gene products associated with Ccr4 (e.g., paf1, ctr9, cdc73, and cdc68) alters G1-phase cyclin expression, a similar function for Ccr4 has not yet been identified (Reed et al. 1988; Rowley et al. 1991; Chang et al. 1999; Koch et al. 1999; Porter et al. 2002). On the basis of these observations and the similarity between the large-cell phenotype of ccr4Δ cells and cln3Δ or bck2Δ cells, the goal of this work was to test the hypothesis that Ccr4 modulates cell size by regulating CLN1 and CLN2 expression.
In this study, we report that Ccr4 assists in coordinating cell cycle progression with the attainment of a critical cell size by modulating the timing of G1-phase cyclin gene expression. Here we show that Ccr4 negatively regulates the half-life of WHI5 mRNAs. By a strikingly analogous mechanism, human Ccr4 and the BTG2 tumor suppressor inhibit cyclin D1 transcription via pRb, and mouse Ccr4 regulates the half-life of p27 mRNAs (Tirone 2001; Morita et al. 2007). These mechanistic similarities further solidify a highly conserved cell cycle role for Ccr4.
MATERIALS AND METHODS
Growth of yeast strains and media:
All strains used in this work are diploids derived from S288c (Table 1). All deletions are full open reading frame (ORF) deletions (Winzeler et al. 1999). Yeast cultures were grown in YEP-based media prepared as previously described (Schneider et al. 2004). After autoclaving, sterile filtered carbon sources were added to a final concentration of 2% (e.g., YEP + 2% glucose aka YPD). To induce ectopic overexpression, strains transformed with galactose-inducible contructs were grown in YEP supplemented with 1% raffinose and 1% galactose (YEPRG). For time-course experiments, yeast cultures were inoculated at ∼1 × 106 cells/ml in the appropriate media and grown in a shaking incubator at 30° for 12–24 hr. Samples were taken at regular intervals to determine cell concentration and cell size distributions as described below. Mean cell size was determined by averaging the size of cells from four to six Coulter Channelyzer samples taken from cultures in mid-log phase.
TABLE 1.
Yeast strains used in the study
| Straina | Relevant genotype | Reference |
|---|---|---|
| MT2643 #49 | MATa/MATα trp1Δ/leu2Δ cln3Δ∷LEU2 whi5Δ∷NAT bck2Δ/kanmx∷URA3 | Costanzo et al. (2004) |
| Wild type (BY4743) | MATa/MATα his3Δ/his3Δ leu2Δ/leu2Δ met15Δ/met15Δ ura3Δ/ura3Δ | Winzeler et al. (1999) |
| bck2Δ | Isogenic to BY4743, except for bck2∷KanMX | Winzeler et al. (1999) |
| cln1Δ | Isogenic to BY4743, except for cln1∷KanMX | Winzeler et al. (1999) |
| cln2Δ | Isogenic to BY4743, except for cln2∷KanMX | Winzeler et al. (1999) |
| cln3Δ | Isogenic to BY4743, except for cln3∷LEU2 | Winzeler et al. (1999) |
| ccr4Δ | Isogenic to BY4743, except for ccr4∷KanMX | Winzeler et al. (1999) |
| mbp1Δ | Isogenic to BY4743, except for mbp1∷KanMX | Winzeler et al. (1999) |
| swi4Δ | Isogenic to BY4743, except for swi4∷KanMX | Winzeler et al. (1999) |
| swi6Δ | Isogenic to BY4743, except for swi6∷KanMX | Winzeler et al. (1999) |
| ccr4Δ | Isogenic to BY4743, except for ccr4∷KanMX trp1Δ/trp1Δ | This work |
| cln1Δ cln2Δ | Isogenic to BY4743, except for cln1∷kanmx∷LEU2 cln2∷KanMX | This work |
| bck2Δ ccr4Δ | Isogenic to BY4743, except for bck2∷KanMX ccr4∷KanMX | This work |
| cln1Δ ccr4Δ | Isogenic to BY4743, except for cln1∷kanmx∷LEU2 ccr4∷KanMX | This work |
| cln2Δ ccr4Δ | Isogenic to BY4743, except for cln2∷KanMX ccr4∷KanMX | This work |
| mbp1Δ ccr4Δ | Isogenic to BY4743, except for mbp1∷KanMX ccr4∷KanMX | This work |
| swi4Δ ccr4Δ | Isogenic to BY4743, except for swi4∷KanMX ccr4∷KanMX | This work |
| whi5Δ | Isogenic to BY4743, except for whi5∷NAT | This work |
| whi5Δ ccr4Δ | Isogenic to BY4743, except for whi5∷NAT ccr4∷KanMX | This work |
| whi54Δ ccr4Δ cln3Δ | Isogenic to BY4743, except for whi5∷NAT ccr4∷KanMX cln3∷LEU2 | This work |
All strains are homozygous gene deletions and are abbreviated in the text such that bck2Δ/bck2Δ is labeled either bck2−/− or bck2Δ. Strains where more than one ORF was deleted with KanMX were confirmed by PCR.
Cell cycle synchronizations and cell size analyses:
Centrifugal elutriation was used for cell cycle synchronizations as previously described (Day et al. 2004; Schneider et al. 2004). Fractions containing the smallest unbudded cells were collected, pelleted, and resuspended in fresh medium. Samples were taken at regular intervals during the course of the experiment, and cell size and cell cycle progression was measured as a function of the percentage of budded cells. To determine budding indices, a minimum of 200 cells were counted on a Zeiss Axiolab microscope, and the average percentage of budded cells was confirmed in at least two independent experiments. Flow cytometry was used to assess cell cycle distributions as previously described (Day et al. 2004; Schneider et al. 2004).
To determine cell concentrations and cell size distributions, 0.1-ml culture samples were resuspended in 10 ml of Isoton buffer, briefly sonicated, and immediately analyzed using a Coulter Counter Channelyzer (model Z2). Coulter Accucomp Z2 (version 3.01a) was used to analyze cell size and cell concentration data. Average cell sizes are given as the geometric mean and data were exported into 86 bins for analysis and plotting in Microsoft Excel. In figures illustrating Coulter Counter Channelyzer cell size data, normalized cell numbers are plotted as a function of mean cell size. Paired, two-tailed t-test analyses were used to determine statistical significance.
Yeast transformation, sporulation, and tetrad dissection:
Standard yeast genetic techniques were used to transform cells or to combine genetic deletions (Ausubel 1987). Zygotes were sporulated in 3 ml of sporulation medium (1% potassium acetate, 0.005% zinc acetate) for 3–7 days at 30°. Tetrads were dissected with a Singer MSM Series 300 micromanipulator, and spores were placed on YPD plates and incubated at 30° for 3–5 days. Spore phenotypes were determined by their ability to grow on selective plates and deduced genotypes were confirmed by PCR. Yeast genomic DNA for PCR reactions was prepared as previously described (Ausubel 1987).
Recombinant DNA techniques:
Escherichia coli transformations, plasmid extractions, restriction digestions, and molecular cloning techniques were conducted using standard protocols as previously described (Ausubel 1987). Plasmids used in this work are listed in Table 2. To make a GAL-CCR4 construct, oligonucleotides were designed for the amplification of the complete open reading frame. Specific sequences and plasmid maps are available upon request. Oligonucleotides for primers were synthesized by Sigma-Genosys and reconstituted in distilled water to a final concentration of 100 μm. A typical 100-μl PCR reaction contained 10 ng of DNA template, 2–5 units of Pfu polymerase (Stratagene, La Jolla, CA), 100 pmol of each primer, 1 μl of 25 mm dNTPs, and 10 μl 10× Pfu buffer. Typical reactions had 30 cycles consisting of denaturation for 1 min at 95°, annealing at 50°–58° for 1 min, and a 1- to 3-min extension at 72° (1 min for a 1-kb fragment) performed with an Eppendorf Mastercycler. For cloning or DNA labeling purposes, DNA fragments from endonuclease digestions were separated with 1% agarose gels with 0.5 μg/ml ethidium bromide. Gels were run in Tris–acetate EDTA buffer, and DNA fragments of expected sizes were extracted from the gel with QIAEX kits (QIAGEN, Valencia, CA) following the manufacturer's instructions. Molecular cloning was accomplished using the pYES2.1 TOPO TA expression kit (Invitrogen, San Diego) following manufacturer's instructions. Appropriate expression from GAL-CCR4 constructs was confirmed by Northern analysis and by their ability to rescue cell size defects of ccr4Δ cells.
TABLE 2.
Plasmids used in the study
| Construct | Name | Vector | Insert | Marker | Reference |
|---|---|---|---|---|---|
| pGAL-BCK2 | CB2232 | YCp50 | BCK2 | URA3 | Di Como et al. (1995) |
| pGAL-CLN1 | YCpG2 | YCpG1 | CLN1 | URA3 | Wijnen and Futcher (1999) |
| pGAL-CLN3 | pS395 | YCp50 | CLN3 | URA3 | Fred Cross |
| pGAL-SWI4 | CB1491 | YCplac33 | SWI4 | URA3 | Wijnen and Futcher (1999) |
| pGAL-CCR4 | pB1304 | pYES2.1 | CCR4 | URA3 | This work |
| pGPD-CCR4 | pRP1045 | pG1 | CCR4 | TRP1 | Tucker et al. 2002 |
| pGPD-CCR4 (D713A) | pRP1046 | pG1 | Mutant CCR4 | TRP1 | Tucker et al. 2002 |
| pGPD-CCR4 (D715A) | pRP1047 | pG1 | Mutant CCR4 | TRP1 | Tucker et al. 2002 |
| pGPD-CCR4 (D817A) | pRP1048 | pG1 | Mutant CCR4 | TRP1 | Tucker et al. 2002 |
| pGPD-CCR4 (H818A) | pRP1049 | pG1 | Mutant CCR4 | TRP1 | Tucker et al. 2002 |
| pGAL-WHI5 | pB1401 | pGreg576 | WHI5-GFP | URA3 | This work |
Northern analysis of yeast RNAs:
To isolate total RNA, log-phase yeast cells were quickly chilled on ice and washed once with ice-cold water before being frozen at −80° for at least 1 hr. Pellets were resuspended in 600 μl of TES buffer (100 mm Tris, 10 mm EDTA, 0.2% SDS, pH 7.5) and 400 μl of water-equilibrated phenol. The mixture was heated to 65°, vortexed vigorously for 10 sec, and then incubated at 65° for 45 min with an occasional inversion. Subsequently, tubes were chilled on ice for 1 min and centrifuged at 4° at 14,000 × g for 5 min. The aqueous phase was transferred to microcentrifuge tubes and vortexed with another 400 μl of water-equilibrated phenol for 10 sec. This was repeated twice before the aqueous phase was precipitated with 2 vol of ice-cold ethanol and 0.1 vol of sodium acetate. Following incubation at −20° for at least 30 min, tubes were centrifuged at 4° at 14,000 × g for 10 min. Pellets were dissolved in 50 μl DEPC H2O, washed with 70% ethanol, and air dried on ice. RNA samples were diluted ∼500-fold and quantitated by UV absorbance at 260 nm with a SmartSpec 3000 (Bio-Rad, Hercules, CA). An OD of 1.0 at 260 nm was equivalent to 40 mg/ml RNA.
For Northern analysis, 7.5 μg of RNA was added to 17.5 μl of RNA loading buffer (12.5 mm MOPS, pH 7.1, 2.5 mm sodium acetate, 0.25 mm EDTA, 3.1% formaldehyde, 25% formamide, 2% glycerol dye, 4 mg/ml bromphenol blue, 4 mg/ml xylene blue, and 50 μg/ml ethidium bromide) and heated to 60° for 10 min. Samples were loaded onto a 1% agarose gel containing 6.7% formaldehyde and run at 100 V for several hours at 4°. Gels were soaked in distilled H2O for 30 min to remove formaldehyde, and RNAs were transferred onto nylon membranes (Hybond N+, Amersham, Piscataway, NJ) with the VacuGene XL vacuum blotting system (Pharmacia, Piscataway, NJ) according to the manufacturer's instructions. Wet membranes were then UV-crosslinked with 150 mJ UV exposure in a GS Gene Linker UV chamber (Bio-Rad) before prehybridization with 25 ml of Church buffer (0.3 m Na2HPO4, pH 7.2, and 6.25% SDS) at 65° for at least 1 hr in a hybridization oven (Amersham Pharmacia).
Probes for Northern analysis were obtained by PCR and by restriction digestion of plasmids containing the open reading frame of the CLN1, CLN2, CCR4, or ACT1 genes. Specific sequences and plasmid maps are available upon request. To radiolabel probes, 100 ng of the probe were boiled and labeled with [α-32P]dCTP (deoxycytosine 5′-[α-32P] triphosphate 3000 Ci/mmol, MP Biomedicals), following the protocol of the Prime-A-Gene kit (Promega, Madison, WI). Subsequently, labeled probes were boiled for 5 min to denature the DNA and chilled on ice immediately before being added to 10 ml of prewarmed Church buffer [7% w/v SDS, 0.1% w/v BSA (fraction V, Sigma), 0.1 mm EDTA, and 0.25 m Na2HPO4, pH 7.2] at 65°. Probes were incubated with membranes overnight at 65°. Membranes were washed twice with 1× SSC plus 0.1% SDS before being exposed to a blank phosphorimager screen (Molecular Probes, Eugene, OR) that was scanned with the 9410 Typhoon Scanner (Amersham Phamacia). Images were digitally stored and quantitated with the FluorChem 2.0 spot densitometry analysis program (Alpha Innotech). To control for loading, CLN mRNA signals were normalized to the ACT1 mRNA.
RESULTS
Ccr4 is involved in cell size homeostasis:
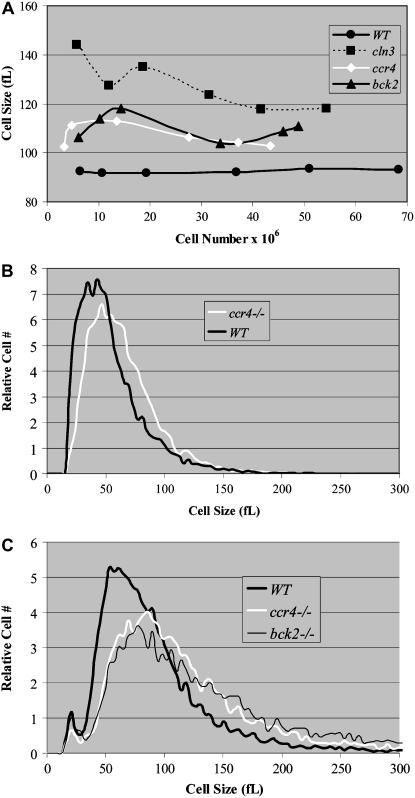
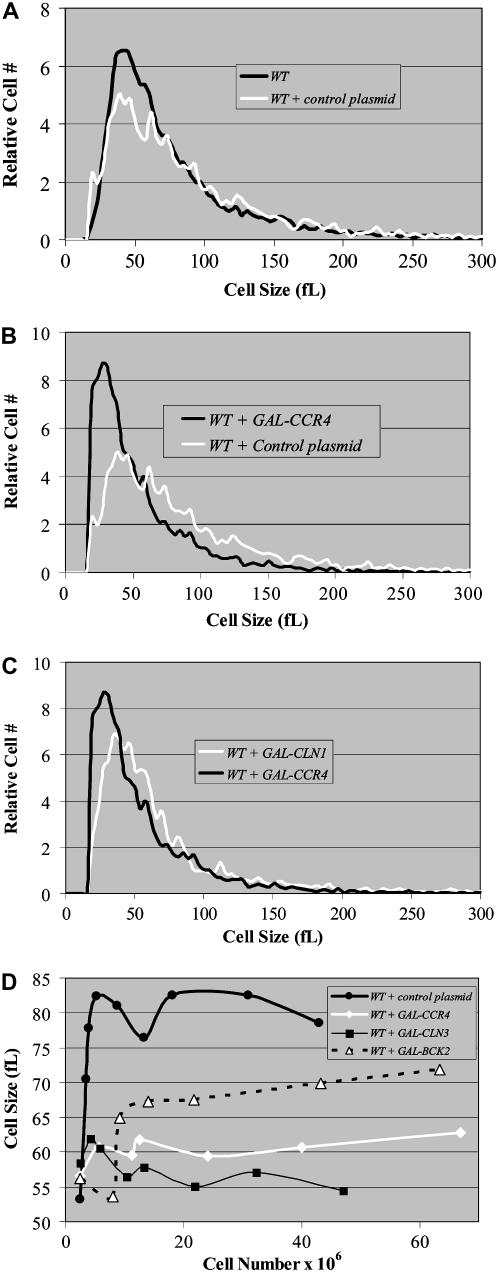
Log-phase diploid wild-type cells (BY4743) from the S288c background display an average cell size of 92 ± 1 fl in rich medium containing glucose when measured over a time course (Figure 1A). Under the same conditions, the size of diploid ccr4Δ cells is significantly larger (106 ± 4 fl; P = 0.0005), similar in size to diploid bck2Δ cells (110 ± 5 fl) but not as large as diploid cln3Δ cells (128 ± 10 fl) (Figure 1A). As cells exit log phase and enter a nonproliferative phase in saturated cultures, the average size of cells decreases. Nonetheless, saturated cultures of ccr4Δ diploid cells are considerably larger than wild-type cells (62 ± 3 fl as compared to 50 ± 4 fl) (Figure 1B). The striking size similarities (P = 0.2) between ccr4Δ and bck2Δ cells suggest that Ccr4 may modulate cell size via a mechanism related to Bck2 or Cln3 (Figure 1C).
Figure 1.—
Loss of Ccr4 function results in abnormally large cells. The mean cell sizes of diploid wild-type, ccr4Δ, cln3Δ, or bck2Δ cells grown in YPD as described were plotted as a function of cell number (A). Average mean cell sizes were 92 ± 1 fl (WT), 106 ± 4 fl (ccr4Δ), 110 ± 5 fl (bck2Δ), and 128 ± 10 fl (cln3Δ). Coulter counter plots of quiescent wild-type cells from YPD cultures grown continuously in a shaking 30° incubator for 5 days demonstrate that cells from saturated cultures are smaller (50 ± 4 fl) than proliferating log-phase cells (92 ± 1 fl). However, similarly derived ccr4Δ cells were considerably larger (62 ± 3 fl) (B). Coulter counter plots of mid-log-phase cells from YPD grown cultures indicate that ccr4Δ cells have cell size plots that resemble bck2Δ cells (C).
Induced expression of CLN1 or CLN2 reduces the size of ccr4Δ cells:
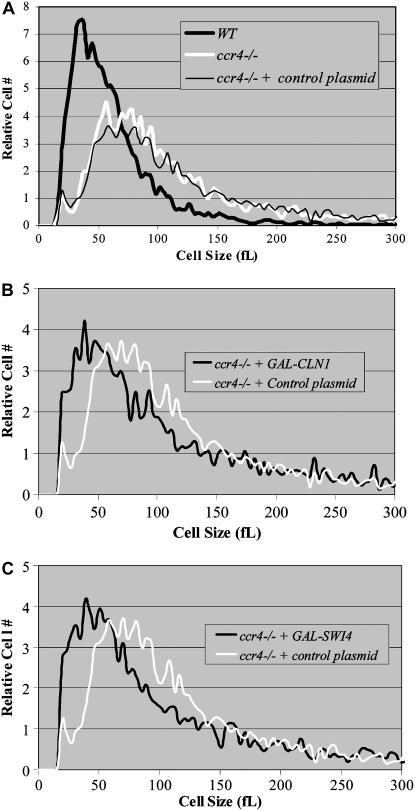
Because delayed or reduced CLN1 and CLN2 expression produces abnormally large cells (Tyers et al. 1993; Epstein and Cross 1994; Di Como et al. 1995; Dirick et al. 1995), we examined the ability of induced CLN1 or CLN2 expression (e.g., using a galactose-inducible vector) to rescue the ccr4Δ large-cell phenotype. First, we confirmed that ccr4Δ cells are significantly larger than wild-type cells in rich medium containing galactose (Figure 2A). Next, we found that overexpression of CLN1 or CLN2 reduced the cell size of ccr4Δ cells (Figure 2B) (e.g., 85 ± 3 or 83 ± 4 fl for GAL-CLN1 and GAL-CLN2, respectively, as compared to 94 ± 6 fl for control cells). The upstream transcription factor SWI4 is intimately involved in CLN1 and CLN2 transcription (Nasmyth and Dirick 1991; Di Como et al. 1995). Indicating that Swi4 is still functional in ccr4Δ cells, GAL-SWI4 decreased the size of ccr4Δ cells to 83 ± 2fl (Figure 2C). Because ectopic expression of the other two major G1-phase transcription factors, Mbp1 and Swi6, does not reduce cell size, they were not tested in this manner. These observations suggest that ccr4Δ cells are abnormally large due to a Cln1/Cln2 deficiency.
Figure 2.—
Ectopic overexpression of CLN1 and SWI4 reduces the size of ccr4Δ cells. Coulter counter plots of mid-log-phase cells from YEPRG grown cultures indicate that ccr4Δ cells (94 ± 6 fl) are considerably larger than wild-type cells (76 ± 7 fl). However, there is no discernible size difference between ccr4Δ cells and ccr4Δ cells transformed with control plasmid (e.g., an empty GAL-promoter construct) (A). In contrast, overexpression of CLN1 (B) and SWI4 (C) reduces the size of ccr4Δ cells. The mean cell sizes of diploid wild-type cells transformed with control plasmid, GAL-CLN1, or GAL-SWI4 grown in YEPRG as described were 94 ± 6, 85 ± 3, and 83 ± 2 fl, respectively. Similar data (83 ± 4 fl) were obtained with overexpression of CLN2 (not shown). Statistical analysis indicated that all conditions were significantly different: ccr4Δ + GAL-SWI4 (P = 0.05), ccr4Δ + GAL-CLN1 (P = 0.04), and ccr4Δ + GAL-CLN2 (P = 0.02).
Start and CLN transcription is delayed in ccr4Δ cells:
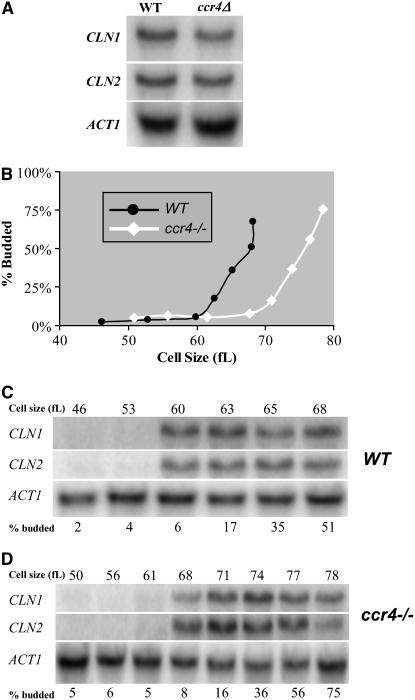
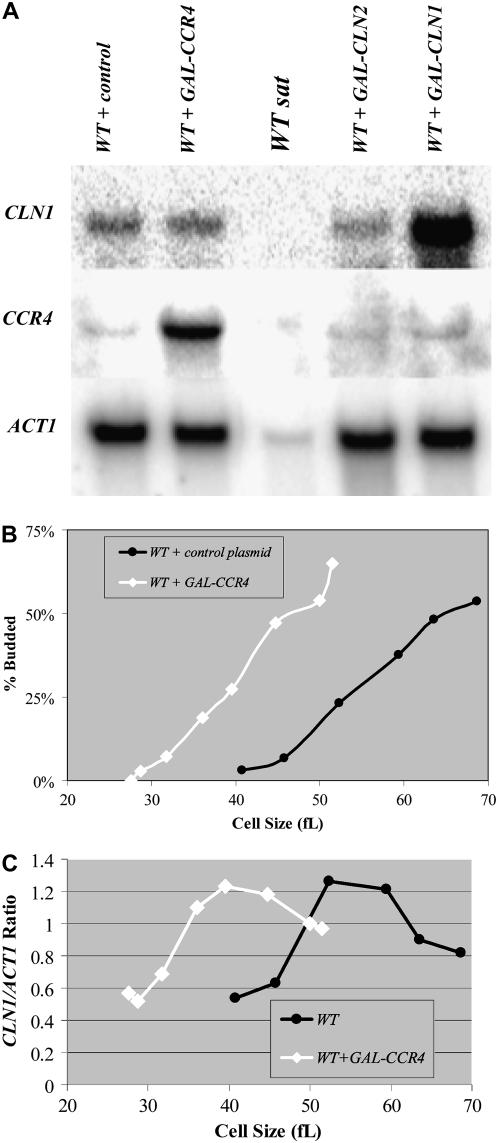
Because Bck2 induces CLN1 and CLN2 transcription (Di Como et al. 1995), and on the basis of the similar size phenotypes between ccr4Δ and bck2Δ cells, the relationship between Ccr4 and CLN1 and CLN2 transcription was investigated. Northern analysis revealed that the levels of CLN1 and CLN2 mRNAs were only modestly reduced in ccr4Δ cells (Figure 3A), suggesting that decreased CLN1 and CLN2 mRNA expression levels were unlikely to be the reason that ccr4Δ cells are unusually large.
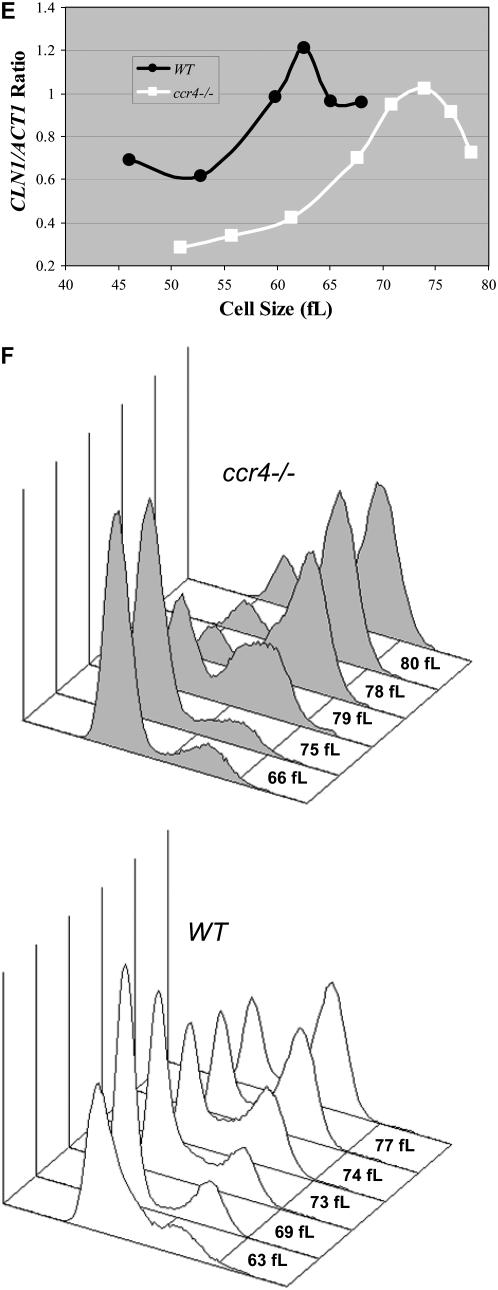
Figure 3.—
CLN1 and CLN2 mRNA expression is delayed in cells lacking CCR4. Total RNA isolated from mid-log-phase wild-type and ccr4Δ YPD cultures was analyzed by Northern analysis. Blots were hybridized with CLN1 and CLN2 probes. ACT1 probes were used as loading controls. Quantitation revealed that CLN1 and CLN2 levels were reduced only 22 and 15%, respectively, in ccr4Δ cells compared to wild type. (A). Diploid wild-type and ccr4Δ cells were grown to mid-log phase in YPD at 25°, and centrifugal elutriation was used to isolate small, unbudded G1-phase cells. Following elutriation, cells were resuspended in fresh medium at 25° and time points were taken every 15 min. The percentage of budded cells is plotted as a function of cell size (B). Total RNA was isolated from the elutriation fractions. Blots from wild-type cells (C) and ccr4Δ cells (D) were hybridized with CLN1 and CLN2 probes. ACT1 probes were used as loading controls. Cell size and the percentage of budded cells are given for comparison. Northerns were quantitated and CLN1/ACT1 ratios were plotted as a function of cell size (E). Flow cytometry was used to monitor cell cycle progression from a similar experiment. Analysis of data reveals that, in ccr4Δ cells, ∼50% of cells have progressed past G1-phase when cells are 75–79 fl, while in wild-type cells this happens at ∼73 fl (F).
Since CLN1 and CLN2 transcription is considerably delayed in bck2Δ or cln3Δ cells (Tyers et al. 1993; Epstein and Cross 1994; Di Como et al. 1995; Dirick et al. 1995), we tested the hypothesis that delayed CLN1 and CLN2 transcription could account for the increased cell size of ccr4Δ cells. Using centrifugal elutriation to obtain similar-sized synchronized cells, we found that initiation of budding was delayed 8–10 fl in ccr4Δ cells compared to wild-type cells (Figure 3B). Moreover, initiation of CLN1 and CLN2 transcription was comparably delayed in ccr4Δ cells compared to wild-type diploid cells (Figures 3, C and D). As seen in log-phase cultures, maximal CLN1 and CLN2 mRNA levels were only modestly reduced in ccr4Δ cells compared to wild-type cells (Figures 3, A and C–E). S-phase entry and progression was somewhat delayed in ccr4Δ cells but budding was not delayed as much (Figure 3, B and F).
Ectopic overexpression of CCR4 makes cells small:
To rule out that an indirect mechanism was delaying G1-phase cyclin expression to produce large cells, we investigated whether overexpression of CCR4 could reduce cell size. Importantly, we found that overexpression of CCR4 significantly (P = 0.002) decreased the size of wild-type cells compared to controls (Figures 4, A and B). Moreover, overexpression of CCR4 and CLN1 similarly decreased the size of wild-type cells (Figure 4C). In fact, time-course experiments revealed that induced GAL-CCR4 expression reduced cell size more than GAL-BCK2 and nearly as much as GAL-CLN3 constructs compared to controls (Figure 4D). However, mutations that disrupted Ccr4's deadenylase activity compromised the ability of ectopically overexpressed CCR4 to decrease cell size (data not shown). As a whole, these results suggest that Ccr4 appears to have an active role in cell size control.
Figure 4.—
Ectopic overexpression of CCR4 reduces cell size. Coulter counter plots of mid-log-phase cells from YEPRG grown cultures indicate that there is no size difference between untransformed wild-type cells and wild-type cells transformed with control plasmid (e.g., an empty GAL-promoter construct) (A). In contrast, overexpression of CCR4 results in small cells (B). Northern analysis was used to confirm CCR4 overexpression (see Figure 5A). Similar results were obtained with several independent galactose-inducible CCR4 constructs and found to be comparable to effects seen with GAL-CLN1 constructs (C). The mean cell sizes of diploid wild-type cells transformed with control plasmid, GAL-CCR4, GAL-CLN3, or GAL-BCK2 grown in YEPRG as described were plotted as a function of cell number (D).
Ectopic overexpression of CCR4 advances the timing of CLN transcription:
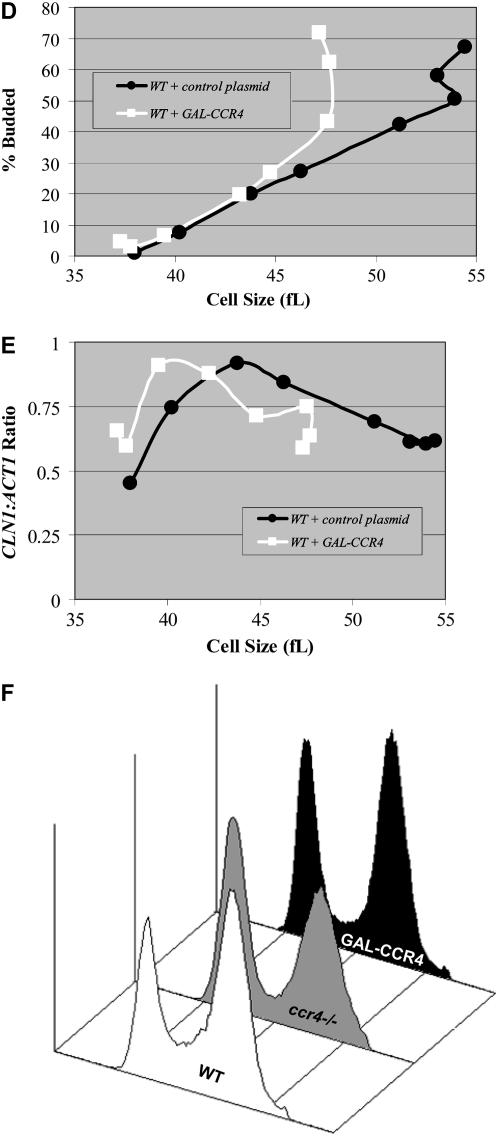
Because overexpression of CCR4 reduces the size of wild-type cells (Figure 4), we investigated whether this was due to the induction of higher-than-normal levels of CLN1 or CLN2 mRNAs. However, we found that constitutive expression of GAL-CCR4 constructs reduced the size of wild-type cells without notably changing the steady-state levels of CLN1 or CLN2 mRNAs in log-phase cells (Figure 5A and data not shown). One possible explanation for this result is that Ccr4 may predominately affect the timing of CLN1 and CLN2 expression. Indeed, elutriation experiments comparing ccr4Δ cells to wild-type cells suggest that Ccr4 is required for timely CLN1 and CLN2 transcription (Figure 3, C–E). To test this possibility directly, we examined how GAL-CCR4 affected the timing of CLN1 transcription in synchronized cultures. When cells were grown under conditions where GAL-CCR4 was induced continuously, elutriation of a GAL-CCR4 strain yielded cells that were 13–14 fL smaller than control cells (Figure 5B). Importantly, in cells constitutively overexpressing CCR4, CLN1 expression was first detected and reached maximal levels in smaller cells compared to wild-type controls (Figure 5C). For example, quantitation of Northern data revealed that, in GAL-CCR4 cells, CLN1 mRNA was first detected in cells 28 fl in volume and reached maximal levels in 40-fl cells. In contrast, in wild-type cells, similar levels of CLN1 expression were first detected in cells 40 fl in volume and reached maximal levels in 52-fl cells (Figure 5C). Premature CLN1, and presumably CLN2, transcription is likely the reason that GAL-CCR4 advanced budding and produced small cells (Figure 5B). Because constitutive GAL-CCR4 expression could produce small cells via cycle effects outside of G1-phase, we examined the effect of induced GAL-CCR4 expression on cell cycle progression. To do so, we used centrifugal elutriation to obtain similarly sized synchronized fractions of cells from strains containing either GAL-CCR4 or a control vector under noninducing conditions. Subsequently, each fraction was transferred to a medium containing galactose. Under these conditions, we found that the induced expression of GAL-CCR4 advanced the initiation of budding only ∼5–7 fl (Figure 5D). Induced expression of CCR4 similarly advanced CLN1 transcription (Figure 5E). Thus, induced CCR4 expression was about half as effective as was constitutive expression. In this respect, induced GAL-CCR4 expression also only modestly advanced S-phase as compared to control (Figure 5F and data not shown). However, in combination with the premature initiation of budding, this is probably sufficient to account for the observed small-cell-size phenotype. The observation that constitutive CCR4 expression has a stronger phenotype than does CCR4 induced in G1-phase suggests a cell size function for Ccr4 outside of G1-phase.
Figure 5.—
Ectopic overexpression of CCR4 modulates the timing of CLN1 and CLN2 expression. Total RNA isolated from the indicated YEPRG grown mid-log-phase cultures was analyzed by Northern analysis. Blots were hybridized with CLN1 and CCR4 probes, and ACT1 probes were used as loading controls (A). RNA from saturated wild-type cultures (WT sat) were used as a negative control because CLN1 is not expressed under these conditions. RNA from cells transformed with GAL-CLN1 constructs (WT + GAL-CLN1) and GAL-CCR4 constructs (WT + GAL-CCR4) were used as positive controls. ACT1 levels are considerably lower in saturated cultures despite even loading. Quantitation revealed that GAL-CCR4 elevated CLN1 and CLN2 (data not shown) levels only very modestly (∼8% higher). Diploid wild-type cells transformed with GAL-CCR4 constructs or control plasmids were grown to mid-log phase in YEPRG, and centrifugal elutriation was used to isolate small, unbudded G1-phase cells. Following elutriation, cells were resuspended in YEPRG fresh medium and regular time points were taken. The percentage of budded cells was plotted as a function of cell size (B). Total RNA was isolated from the elutriation fractions. Blots with RNA from wild-type cells transformed with GAL-CCR4 constructs or control plasmids were hybridized with CLN1 and CCR4 probes. ACT1 probes were used as loading controls. Northerns were quantitated and CLN1/ACT1 ratios were plotted as a function of cell size (C). To determine the effect of induced GAL-CCR4 in similarly sized synchronized cells, cultures were grown in −URA glucose for 12 hr and then transferred to −URA raffinose until mid-log phase. Subsequently, centrifugal elutriation was used to isolate small, unbudded G1-phase cells. Following elutriation, cells were resuspended in YEPRG fresh medium and regular time points were taken. The percentage of budded cells was plotted as a function of cell size (D). Total RNA was isolated from the elutriation fractions. Blots with RNA from wild-type cells transformed with GAL-CCR4 constructs or control plasmids were hybridized with CLN1 and CCR4 probes. CCR4 probes were used to confirm overexpression. ACT1 probes were used as loading controls. CLN1 mRNA expression levels were quantitated and CLN1/ACT1 ratios were plotted as a function of cell size (E). To determine the effect of induced GAL-CCR4, cultures were grown in YEPR and galactose was added to 1% for 3 hr. Flow cytometry analysis revealed that neither induction of GAL-CCR4 nor deletion of CCR4 had strong effects on cell cycle distributions (F). Quantitation revealed that in wild-type cultures 21, 30, and 49% of cells were in G1-, S-, or G2/M-phase. In GAL-CCR4 cultures, 30, 18, and 52% of cells were in G1-, S-, or G2/M-phase while in ccr4Δ cells these numbers were 43, 12, and 45% (F).
Ccr4 functions within cell-size-dependent cell cycle pathways:
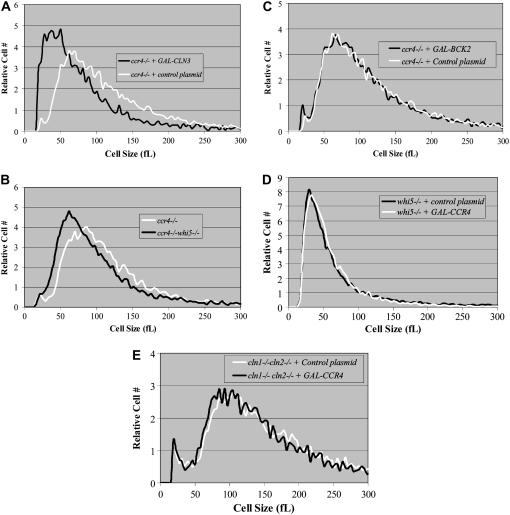
Because Cln3 and Bck2 activate CLN1 and CLN2 transcription in a size-dependent manner, we assessed the ability of ectopically expressed CLN3 or BCK2 to reduce the size of ccr4Δ cells. While the induced expression of GAL-CLN3 (Figure 6A) or the deletion of WHI5 (Figure 6B) reduced the size of ccr4Δ cells, GAL-BCK2 did not (Figure 6C). These data suggest that Ccr4 functions independently of Cln3 and downstream of Bck2.
Figure 6.—
Ccr4 functions upstream of Whi5, Cln1, and Cln2. Coulter counter plots of mid-log-phase cells from YEPRG-grown cultures indicate that overexpression of CLN3 (A) or deletion of WHI5 (B) reduces the size of ccr4Δ cells. In contrast, overexpression of BCK2 fails to reduce the size of ccr4Δ cells (C). Finally, GAL-CCR4 failed to reduce the size of whi5Δ cells (D) or cln1Δcln2Δ cells in log-phase YEPRG-grown cultures (E), indicating that Ccr4 functions upstream of Whi5, Cln1, and Cln2.
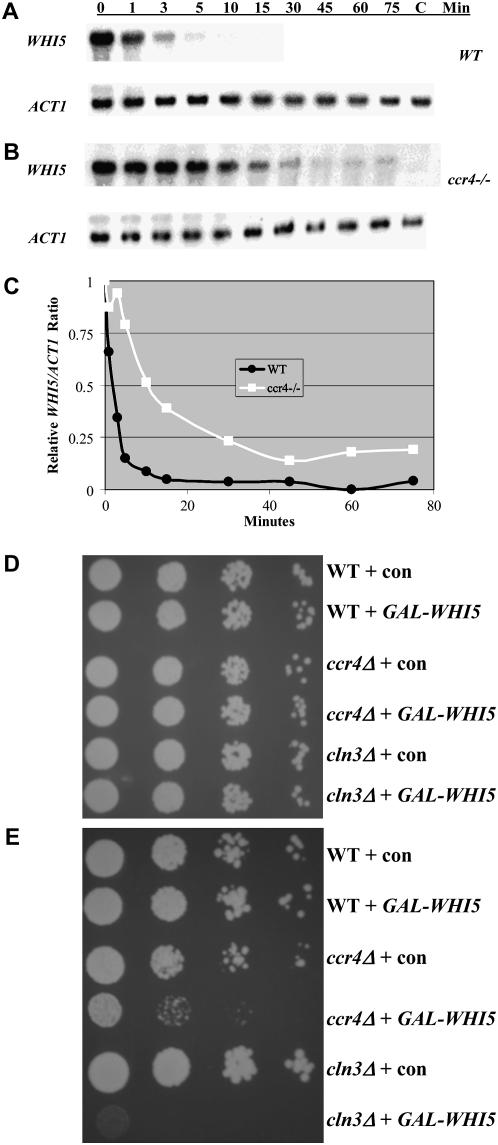
By combining multiple deletions, we examined genetic interactions between CCR4 and genes in the Cln3 and Bck2 genetic pathways. The cell size of ccr4Δbck2Δ, ccr4Δcln1Δ, ccr4Δcln2Δ, and ccr4Δswi4Δ double mutants were similar to single ccr4Δ cells (Table 3). However, ccr4Δcln3Δ, ccr4Δswi6Δ, and ccr4Δcln1Δ cln2Δ combinations were inviable (Table 3), similar to the inviability of bck2Δcln3Δ, bck2Δswi6Δ, and cln1Δcln2Δcln3Δ combinations (Epstein and Cross 1994; Di Como et al. 1995; Wijnen and Futcher 1999). These data further support the hypothesis that Ccr4 functions downstream of Bck2 and independently of Cln3. Moreover, we found that overexpression of CCR4 was unable to reduce the size of whi5Δ or cln1Δcln2Δ cells (Figure 6, D and E). Because bck2Δcln3Δwhi5Δ cells are viable (Costanzo et al. 2004; De Bruin et al. 2004), we examined whether deletion of WHI5 could analogously restore viability to ccr4Δcln3Δ cells. It was found that ccr4Δcln3Δwhi5Δ cells were viable, and while larger than normal, ccr4Δcln3Δwhi5Δ cells have near-normal proliferation rates (data not shown). These data suggest that, like Cln3, Ccr4 might inhibit Whi5 function. Specifically, we hypothesized that the deadenylase activity of Ccr4 may modulate the half-life of WHI5 mRNAs. By examining the half-life of WHI5 mRNAs, we found that, in the absence of Ccr4, WHI5's mRNA half-life increased >10-fold compared to controls (e.g., from ∼1–2 min in the presence of Ccr4 to 10–15 min in the absence of Ccr4) (Figure 7, A–C). Since overexpression of WHI5 is lethal in cln3Δ cells (Costanzo et al. 2004), it might be similarly lethal in ccr4Δ cells. Indeed, using a cell growth assay and time-lapse photography, we found that overexpression of GAL-WHI5-GFP inhibited proliferation (Figure 7D) and led to cell lysis in ccr4Δ but not in wild-type cells (data not shown). Taken together, data presented here suggest that Ccr4 regulates the size-dependent timing of CLN1 and CLN2 mRNA expression by modulating the half-life of WHI5 mRNAs.
TABLE 3.
Genetic interactions assessed by impact on mean cell size
| Cell size (fl) ±SD
|
||
|---|---|---|
| Gene deletions | Single deletion | +ccr4Δ |
| ccr4Δ/ccr4Δ | 106 ± 4 | |
| bck2Δ/bck2Δ | 110 ± 5 | 107 ± 5 |
| cln1Δ/cln1Δ | 95 ± 7 | 101 ± 8 |
| cln2Δ/cln2Δ | 90 ± 4 | 112 ± 8 |
| cln3Δ/cln3Δ | 128 ± 10 | Inviablea |
| mbp1Δ/mbp1Δ | 96 ± 3 | 93 ± 3 |
| swi4Δ/swi4Δ | 96 ± 6 | 111 ± 2 |
| swi6Δ/swi6Δ | 99 ± 5 | Inviableb |
| cln1Δ cln2Δ/cln1Δ cln2Δ | 135 ± 15 | Inviablec |
To examine genetic interactions, diploid cells with the indicated gene deletions were created and mid-log-phase YPD-grown cells were sized. Averages are the result of multiple independent measurements. SD, standard deviation.
From the dissection of 126 tetrads (100 from +/cln3Δ∷LEU2+/whi5Δ∷NAT +/bck2Δ∷kanmx∷URA3 +/ccr4Δ:KanMX heterozygotes and 26 from +/ccr4Δ:KanMX +/cln3Δ:KanMX heterozygotes), no viable ccr4Δcln3Δ spores were obtained.
A total of 49 tetrads were dissected and no viable ccr4Δswi6Δ spores were obtained.
A total of 40 tetrads were dissected and no viable ccr4Δcln1Δcln2Δ spores were obtained.
Figure 7.—
Ccr4 modulates the half-life of WHI5 mRNAs. GAL-WHI5 constructs were transformed in whi5Δ and whi5Δccr4Δ cells. Subsequently, cultures were grown to mid-log phase in −URA medium containing 1% raffinose and 1% galactose. Cells were rapidly centrifuged and resuspended in −URA medium containing 2% glucose to repress the GAL-WHI5 construct. Subsequently, cells were harvested over a time course. Total RNA was isolated from each time point. Blots with RNA from cells with (A) or without CCR4 (B) were hybridized with a WHI5 probe. ACT1 probes were used as loading controls. WHI5 mRNA expression levels were quantitated, and the relative WHI5/ACT1 ratios were plotted as a function of time to determine the effects of CCR4 on WHI5 mRNA half-life (C). Analyses revealed that the half-life of WHI5 mRNA was ∼1 min in the presence of CCR4 compared to ∼10 min in ccr4Δ cells. To assess how overexpression of CCR4 affects proliferation, wild type, ccr4Δ, and cln3Δ cells were transformed with either GAL-WHI5 or a control vector. Subsequently, cells were serially diluted 10-fold onto either −URA glucose (D) or −URA RG (E) plates and grown at 30° for 4 days. Analyses revealed that overexpression of WHI5 inhibited proliferation in ccr4Δ cells almost as much as in cln3Δ cells as previously reported (Costanzo et al. 2004).
DISCUSSION
A role for Ccr4 in cell size regulation:
Cln3 and Bck2, two constitutively expressed proteins, link cell growth to proliferation by coupling CLN1 and CLN2 transcription to the attainment of a minimum cell size (Tyers et al. 1993; Epstein and Cross 1994; Di Como et al. 1995; Dirick et al. 1995). When cells attain a minimum cell size, Cln3-Cdc28 complexes phosphorylate Whi5, the yeast equivalent of the pRb tumor suppressor gene, to promote CLN1 and CLN2 transcription (Costanzo et al. 2004; De Bruin et al. 2004). In contrast, the mechanistic relationship between Bck2 and Whi5 is not understood nor is it known how Bck2 activates CLN1 and CLN2 transcription. Moreover, the elucidation of Bck2's function is hampered by the lack of a mammalian homolog. However, the conservation of function between basic cell cycle machinery suggests that analyses that shed light on mechanisms of Bck2 and Cln3 action in yeast are likely to also elucidate cell cycle control in mammalian cells. Like bck2Δ or cln3Δ cells, ccr4Δ cells are very large. Thus, the objective of this work was to determine if Ccr4 functions within the Cln3- or Bck2-dependent cell cycle control pathways.
In examining this hypothesis, we demonstrated that ccr4Δ cells have delayed CLN1 and CLN2 expression. The ability of ectopically expressed CLN1, CLN2, or SWI4 to rescue the large-cell phenotype of ccr4Δ cells suggests that a Cln1 and Cln2 deficiency accounts for the cell size defect. In addition, ectopic overexpression of CCR4 reduced cell size by advancing CLN1 and CLN2 mRNA transcription. Moreover, our data suggest that, by modulating the size-dependent timing of CLN1 and CLN2 mRNA transcription, Ccr4 has dramatic effects on the initiation of budding. Importantly, GAL-CCR4 failed to reduce the size of either cln1Δcln2Δ or whi5Δ cells. This infers that Cln1, Cln2, and Whi5 are downstream targets of Ccr4. Our data suggest that Ccr4 modulates cell size by negatively regulating the stability of WHI5 mRNAs. Interestingly, we found that constitutive overexpression of CCR4 has a stronger phenotype than does induced G1-phase expression. Whi5 is also constitutively expressed and localized to the nucleus in late G2-phase (Costanzo et al. 2004; Di Talia et al. 2007). Thus, continuously overexpressed Ccr4 should destabilize WHI5 mRNAs throughout the cell cycle and reduce the levels of Whi5 available to accumulate in nucleus. In this manner, starting with low levels of Whi5 may be more effective in promoting premature G1-phase cyclin expression than destabilizing WHI5 mRNAs in G1-phase.
A new working model:
Prior to this work, it had been shown that loss-of-function mutations in the Ccr4-interacting proteins Paf1 and Ctr9 decrease CLN1 and CLN2 transcription (Chang et al. 1999; Koch et al. 1999; Porter et al. 2002). In addition, recent evidence has implicated Ccr4 in the DNA replication checkpoint where it regulates the mRNA poly(A) tail length of the Crt1 transcriptional repressor (Westmoreland et al. 2004; Woolstencroft et al. 2006). Furthermore, CCR4 has been identified as a gene required for the G1-phase cell cycle transition in irradiated cells (Westmoreland et al. 2004; Woolstencroft et al. 2006). Finally, Ccr4 interacts with Skn7, a protein that can promote CLN1 and CLN2 expression (Morgan et al. 1995; Lenssen et al. 2007). However, to date, no direct interaction between Ccr4 and G1-phase cyclin expression has been demonstrated. On the basis of the data presented here, we propose a new working model incorporating Ccr4 into known cell cycle control pathways (Figure 8). We suggest that Bck2 inhibits Whi5 activity via a mechanism dependent upon Ccr4. This conclusion is supported by the observations that CCR4 is epistatic to BCK2. For example, bck2Δccr4Δ cells are comparable in size to ccr4Δ cells and GAL-BCK2 is unable to rescue the ccr4Δ large-cell phenotype. Further, we find that deletion of CCR4 phenocopies overexpression of WHI5. For example, GAL-WHI5 inhibits the growth of cln3Δ, swi6Δ, and cln1Δcln2Δ cells, but not of bck2Δ cells (Costanzo et al. 2004; De Bruin et al. 2004). We have shown that the very same gene deletions are synthetically lethal with ccr4Δ. These data suggest that Whi5 is a downstream target of Ccr4. In support of this, we have shown that the half-life of the WHI5 mRNAs is 10-fold shorter in the presence of Ccr4. This could provide cells with another size-dependent mechanism, in addition to phosphorylation by Cln3-Cdc28 complexes, for lowering levels of the Whi5 transcriptional repressor.
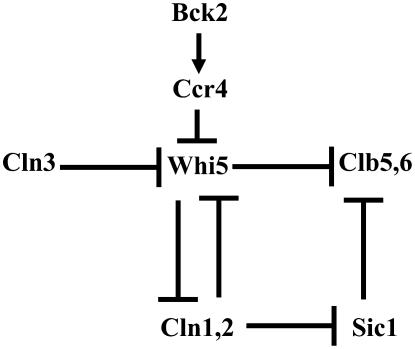
Figure 8.—
A working model. Cln3 and Bck2 promote cell cycle progression via different cell-size-dependent mechanisms. When cells attain the minimum cell size requirement, Cln3-Cdc28 kinase complexes phosphorylate Whi5 to drive it out of the nucleus. In addition, also via a size-dependent mechanism, Bck2 promotes cell cycle progression by stimulating the ability of Ccr4 to destabilize WHI5 mRNAs. This provides cells with a Cln3-independent means for inhibiting Whi5 activity. This model explains how ccr4Δ cells are abnormally large and why Bck2 is not functional in ccr4Δ cells. Moreover, because cln3Δccr4Δ cells are inviable, this model indicates that Bck2 is dependent upon Ccr4 to inhibit Whi5 in the absence of Cln3. This inference is supported by the observations that, like bck2Δswi6Δ cells, ccr4Δswi6Δ cells are inviable. Finally, the finding that cln1Δcln2Δccr4Δ cells, like cln1Δcln2clb5Δclb6Δ cells, are inviable suggests that, in the absence of Cln1 and Cln2, Cln3's ability to induce CLB5 and CLB6 expression is dependent upon Ccr4.
The genetic architecture of our working model (Figure 8) helps explain many of the known synthetic lethal observations. For example, we suggest that bck2Δcln3Δ and ccr4Δcln3Δ double mutants are inviable due to an excess of Whi5. In contrast, bck2Δccr4Δ or GAL-WHI5 bck2Δ are viable because Cln3-Cdc28 can still repress Whi5. This model proposes that deletion of WHI5 would not rescue cln1Δcln2Δcln3Δ cells, as observed by De Bruin et al. (2004), because there is no G1-phase kinase activity to inhibit Sic1, the B-type Cdk inhibitor. Further, the proposal that Whi5 inhibits CLB5 and CLB6 expression explains how bck2Δcln3Δwhi5Δ cells are viable while bck2Δcln3Δsic1Δ cells are not (Wijnen and Futcher 1999; Costanzo et al. 2004; De Bruin et al. 2004). In addition, by postulating that Whi5 is Cln3's sole target, this model helps explain why cln1Δcln2Δclb5Δclb6Δ cells are inviable, but cln1Δcln2Δ clb5Δclb6Δsic1Δ cells are viable. Finally, by proposing that Bck2 via Ccr4 decreases the stability of WHI5 mRNAs, this model helps explain how cells deal with mutant versions of Whi5 (e.g., lacking all consensus Cdc28 phosphorylation sites) that should be Cln3 insensitive (Costanzo et al. 2004; De Bruin et al. 2004). Nonetheless, several important questions remain unanswered. First, what Whi5-independent mechanism is responsible for repressing CLN1 and CLN2 transcription in G2-phase? Second, by what mechanisms are downstream cyclins transcriptionally activated in cln3Δbck2Δwhi5Δ and cln3Δccr4Δwhi5Δ cells?
Ongoing studies continuously reinforce the concept that the genetic pathways that control cell cycle progression are amazingly complex (reviewed in Rupes 2002; Jorgensen and Tyers 2004; Cooper 2006; Bloom and Cross 2007). Nonetheless, notable progress has been made in the past few years. In particular, the discovery that Whi5 is the yeast equivalent of the pRB tumor suppressor gene further validates the similarities between yeast and mammalian cell cycle controls (Costanzo et al. 2004; De Bruin et al. 2004). In addition, the findings that pRB and G1-phase cyclins modulate cell size in higher eukaryotes have firmly established that cell size control is not a yeast-specific problem (Ohtsubo and Roberts 1993; Quelle et al. 1993; Sage et al. 2000; Potter and Xu 2001; Fang et al. 2006; Mukherji et al. 2006). While much of our current understanding regarding the function of Ccr4-Not complexes comes from work in yeast, human data suggest a role for Ccr4 in carcinogenesis. For example, the Ccr4-interacting BTG tumor suppressors downregulate cyclin D levels and inhibit proliferation via a pRb-dependent mechanism (Guardavaccaro et al. 2000; Prevot et al. 2001; Tirone 2001). Significantly, it has also recently been shown that depletion of Ccr4 in NIH3T3 cells stabilizes the mRNA for the p27 tumor suppressor gene (Morita et al. 2007). This is remarkably similar to our observation that Ccr4 modulates the timing of G1-phase cyclin expression via Whi5. Because Ccr4-Not complexes are highly conserved, elucidation of Ccr4 functions in yeast should continue to help clarify the potential cell cycle roles of Ccr4-Not complexes in mammalian cells.
Acknowledgments
We thank our anonymous reviewers for their excellent experimental suggestions and thoughtful comments. We are grateful to F. Cross, M. Tyers, M. Collart, C. Denis, R. Parker, and B. Futcher for strains and plasmids. This research was supported by grants from the Ted Nash Long Life Foundation and by National Institutes of Health grant GM077874 to B.L.S.
References
- Ausubel, F. M., 1987. Current Protocols in Molecular Biology. Greene Publishing Associates, Brooklyn, NY/Media, PA.
- Bloom, J., and F. R. Cross, 2007. Multiple levels of cyclin specificity in cell-cycle control. Nat. Rev. Mol. Cell Biol. 8 149–160. [DOI] [PubMed] [Google Scholar]
- Chang, M., D. French-Cornay, H. Y. Fan, H. Klein, C. L. Denis et al., 1999. A complex containing RNA polymerase II, Paf1p, Cdc73p, Hpr1p, and Ccr4p plays a role in protein kinase C signaling. Mol. Cell. Biol. 19 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Y. C. Chiang and C. L. Denis, 2002. CCR4, a 3′-5′ poly(A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J. 21 1414–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart, M. A., and K. Struhl, 1994. NOT1(CDC39), NOT2(CDC36), NOT3, and NOT4 encode a global-negative regulator of transcription that differentially affects TATA-element utilization. Genes Dev. 8 525–537. [DOI] [PubMed] [Google Scholar]
- Collart, M. A., and H. T. Timmers, 2004. The eukaryotic Ccr4-not complex: A regulatory platform integrating mRNA metabolism with cellular signaling pathways? Prog. Nucleic Acid Res. Mol. Biol. 77 289–322. [DOI] [PubMed] [Google Scholar]
- Cooper, K., 2006. Rb, whi it's not just for metazoans anymore. Oncogene 25 5228–5232. [DOI] [PubMed] [Google Scholar]
- Costanzo, M., J. L. Nishikawa, X. Tang, J. S. Millman, O. Schub et al., 2004. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117 899–913. [DOI] [PubMed] [Google Scholar]
- Cross, F. R., 1995. Starting the cell cycle: What's the point? Curr. Opin. Cell Biol. 7 790–797. [DOI] [PubMed] [Google Scholar]
- Day, A., C. Schneider and B. L. Schneider, 2004. Yeast cell synchronization. Methods Mol. Biol. 241 55–76. [DOI] [PubMed] [Google Scholar]
- de Bruin, R. A., W. H. McDonald, T. I. Kalashnikova, J. Yates, III and C. Wittenberg, 2004. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell 117 887–898. [DOI] [PubMed] [Google Scholar]
- Denis, C. L., and J. Chen, 2003. The CCR4-NOT complex plays diverse roles in mRNA metabolism. Prog. Nucleic Acid Res. Mol. Biol. 73 221–250. [DOI] [PubMed] [Google Scholar]
- Di Como, C. J., H. Chang and K. T. Arndt, 1995. Activation of CLN1 and CLN2 G1 cyclin gene expression by BCK2. Mol. Cell. Biol. 15 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirick, L., T. Bohm and K. Nasmyth, 1995. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. EMBO J. 14 4803–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Talia, S., J. M. Skotheim, J. M. Bean, E. D. Siggia and F. R. Cross, 2007. The effects of molecular noise and size control on variability in the budding yeast cell cycle. Nature 448 947–951. [DOI] [PubMed] [Google Scholar]
- Epstein, C. B., and F. R. Cross, 1994. Genes that can bypass the CLN requirement for Saccharomyces cerevisiae cell cycle START. Mol. Cell. Biol. 14 2041–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, S. C., C. de los Reyes and J. G. Umen, 2006. Cell size checkpoint control by the retinoblastoma tumor suppressor pathway. PLoS Genet. 2 e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futcher, B., 1996. Cyclins and the wiring of the yeast cell cycle. Yeast 12 1635–1646. [DOI] [PubMed] [Google Scholar]
- Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch et al., 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415 141–147. [DOI] [PubMed] [Google Scholar]
- Guardavaccaro, D., G. Corrente, F. Covone, L. Micheli, I. D'Agnano et al., 2000. Arrest of G(1)-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol. Cell. Biol. 20 1797–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, P., and M. Tyers, 2004. How cells coordinate growth and division. Curr. Biol. 14 R1014–R1027. [DOI] [PubMed] [Google Scholar]
- Jorgensen, P., J. L. Nishikawa, B. J. Breitkreutz and M. Tyers, 2002. Systematic identification of pathways that couple cell growth and division in yeast. Science 297 395–400. [DOI] [PubMed] [Google Scholar]
- Koch, C., P. Wollmann, M. Dahl and F. Lottspeich, 1999. A role for Ctr9p and Paf1p in the regulation G1 cyclin expression in yeast. Nucleic Acids Res. 27 2126–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenssen, E., N. Azzouz, A. Michel, E. Landrieux and M. A. Collart, 2007. The Ccr4-not complex regulates Skn7 through the Srb10 kinase. Eukaryot. Cell 6 2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. Y., J. H. Toyn, Y. C. Chiang, M. P. Draper, L. H. Johnston et al., 1997. DBF2, a cell cycle-regulated protein kinase, is physically and functionally associated with the CCR4 transcriptional regulatory complex. EMBO J. 16 5289–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. Y., V. Badarinarayana, D. C. Audino, J. Rappsilber, M. Mann et al., 1998. The NOT proteins are part of the CCR4 transcriptional complex and affect gene expression both positively and negatively. EMBO J. 17 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, B. A., N. Bouquin, G. F. Merrill and L. H. Johnston, 1995. A yeast transcription factor bypassing the requirement for SBF and DSC1/MBF in budding yeast has homology to bacterial signal transduction proteins. EMBO J. 14 5679–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, M., T. Suzuki, T. Nakamura, K. Yokoyama, T. Miyasaka et al., 2007. Depletion of mammalian CCR4b deadenylase triggers elevation of the p27Kip1 mRNA level and impairs cell growth. Mol. Cell. Biol. 27 4980–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji, M., R. Bell, L. Supekova, Y. Wang, A. P. Orth et al., 2006. Genome-wide functional analysis of human cell-cycle regulators. Proc. Natl. Acad. Sci. USA 103 14819–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth, K., and L. Dirick, 1991. The role of SWI4 and SWI6 in the activity of G1 cyclins in yeast. Cell 66 995–1013. [DOI] [PubMed] [Google Scholar]
- Ohtsubo, M., and J. M. Roberts, 1993. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science 259 1908–1912. [DOI] [PubMed] [Google Scholar]
- Pan, X., P. Ye, D. S. Yuan, X. Wang, J. S. Bader et al., 2006. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell 124 1069–1081. [DOI] [PubMed] [Google Scholar]
- Porter, S. E., T. M. Washburn, M. Chang and J. A. Jaehning, 2002. The yeast pafl-rNA polymerase II complex is required for full expression of a subset of cell cycle-regulated genes. Eukaryot. Cell 1 830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, C. J., and T. Xu, 2001. Mechanisms of size control. Curr. Opin. Genet. Dev. 11 279–286. [DOI] [PubMed] [Google Scholar]
- Prevot, D., A. P. Morel, T. Voeltzel, M. C. Rostan, R. Rimokh et al., 2001. Relationships of the antiproliferative proteins BTG1 and BTG2 with CAF1, the human homolog of a component of the yeast CCR4 transcriptional complex: involvement in estrogen receptor alpha signaling pathway. J. Biol. Chem. 276 9640–9648. [DOI] [PubMed] [Google Scholar]
- Quelle, D. E., R. A. Ashmun, S. A. Shurtleff, J. Y. Kato, D. Bar-Sagi et al., 1993. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 7 1559–1571. [DOI] [PubMed] [Google Scholar]
- Reed, S. I., 1980. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics 95 561–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, S. I., J. Ferguson and K. Y. Jahng, 1988. Isolation and characterization of two genes encoding yeast mating pheromone signaling elements: CDC72 and CDC73. Cold Spring Harb. Symp. Quant. Biol. 53(Pt. 2): 621–627. [DOI] [PubMed] [Google Scholar]
- Rowley, A., R. A. Singer and G. C. Johnston, 1991. CDC68, a yeast gene that affects regulation of cell proliferation and transcription, encodes a protein with a highly acidic carboxyl terminus. Mol. Cell. Biol. 11 5718–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupes, I., 2002. Checking cell size in yeast. Trends Genet. 18 479–485. [DOI] [PubMed] [Google Scholar]
- Sage, J., G. J. Mulligan, L. D. Attardi, A. Miller, S. Chen et al., 2000. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 14 3037–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, B. L., J. Zhang, J. Markwardt, G. Tokiwa, T. Volpe et al., 2004. Growth rate and cell size modulate the synthesis of, and requirement for, G1-phase cyclins at start. Mol. Cell. Biol. 24 10802–10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr, C. J., and J. M. Roberts, 2004. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18 2699–2711. [DOI] [PubMed] [Google Scholar]
- Tirone, F., 2001. The gene PC3(TIS21/BTG2), prototype member of the PC3/BTG/TOB family: Regulator in control of cell growth, differentiation, and DNA repair? J. Cell Physiol. 187 155–165. [DOI] [PubMed] [Google Scholar]
- Tucker, M., R. R. Staples, M. A. Valencia-Sanchez, D. Muhlrad and R. Parker, 2002. Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 21 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers, M., G. Tokiwa and B. Futcher, 1993. Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins. EMBO J. 12 1955–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland, T. J., J. R. Marks, J. A. Olson, Jr., E. M. Thompson, M. A. Resnick et al., 2004. Cell cycle progression in G1 and S phases is CCR4 dependent following ionizing radiation or replication stress in Saccharomyces cerevisiae. Eukaryot. Cell 3 430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen, H., and B. Futcher, 1999. Genetic analysis of the shared role of CLN3 and BCK2 at the G(1)-S transition in Saccharomyces cerevisiae. Genetics 153 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson et al., 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 901–906. [DOI] [PubMed] [Google Scholar]
- Woolstencroft, R. N., T. H. Beilharz, M. A. Cook, T. Preiss, D. Durocher et al., 2006. Ccr4 contributes to tolerance of replication stress through control of CRT1 mRNA poly(A) tail length. J. Cell Sci. 119 5178–5192. [DOI] [PubMed] [Google Scholar]
- Zhang, J., C. Schneider, L. Ottmers, R. Rodriguez, A. Day et al., 2002. Genomic scale mutant hunt identifies cell size homeostasis genes in S. cerevisiae. Curr. Biol. 12 1992–2001. [DOI] [PubMed] [Google Scholar]