Abstract
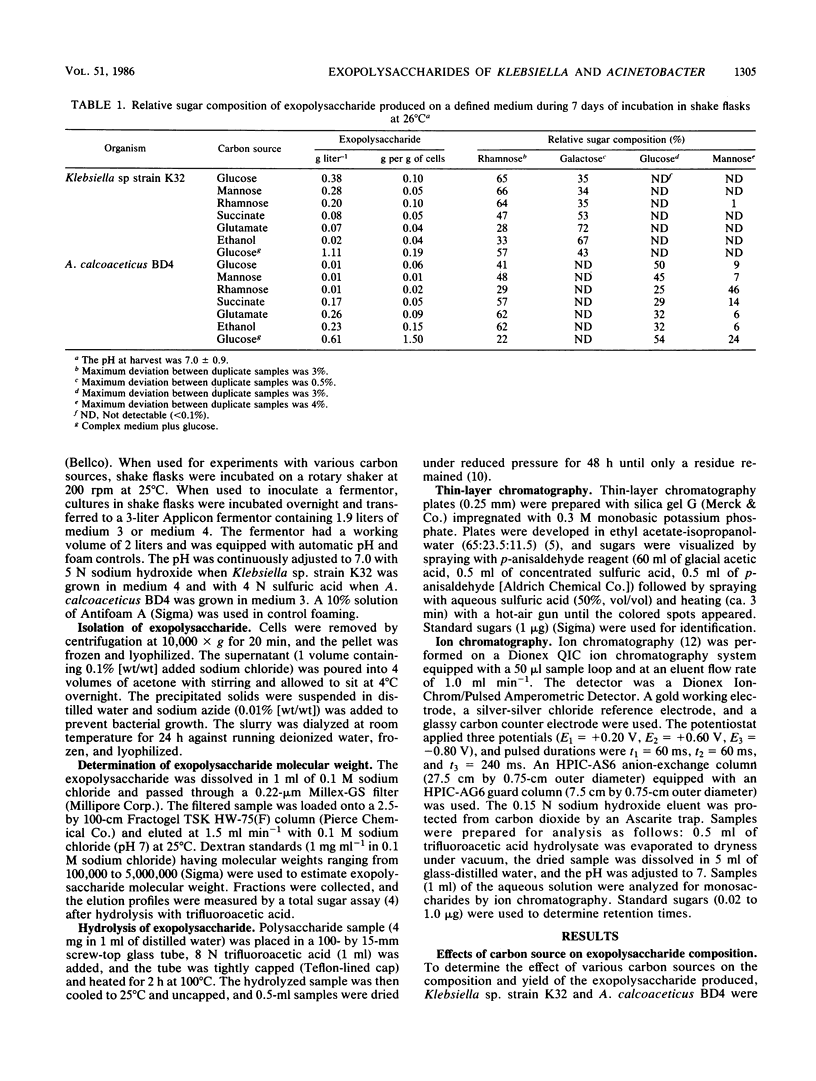
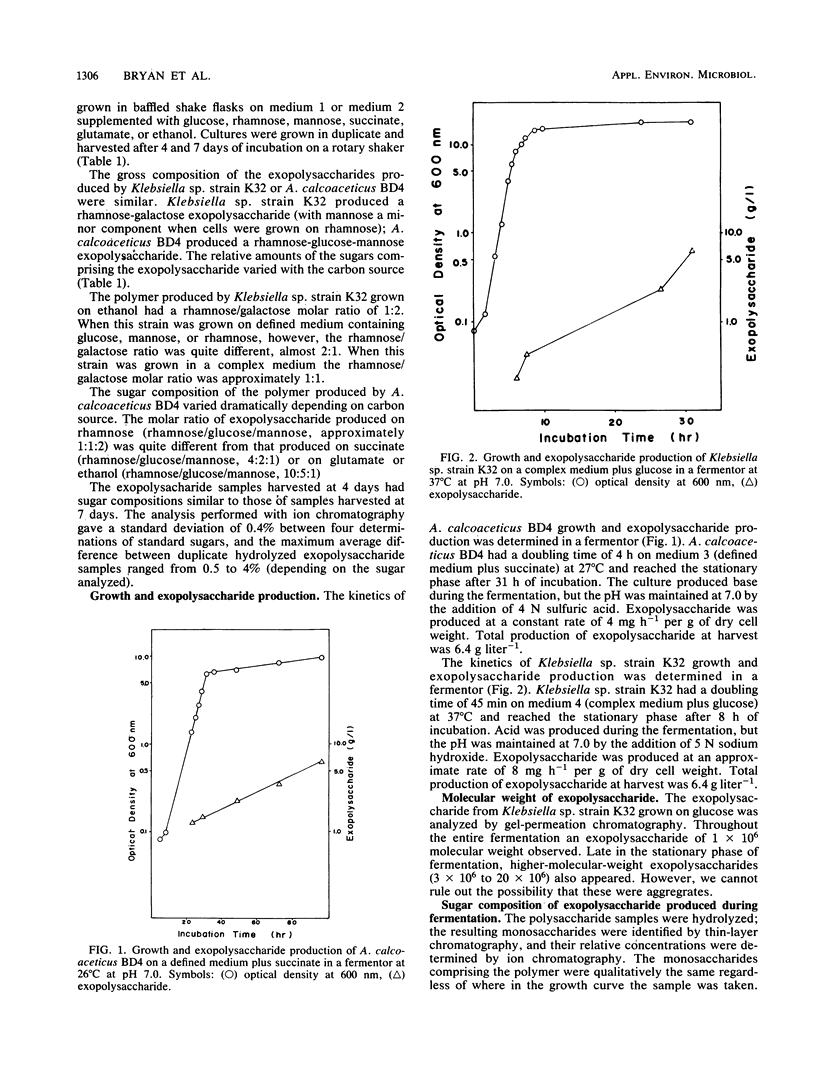
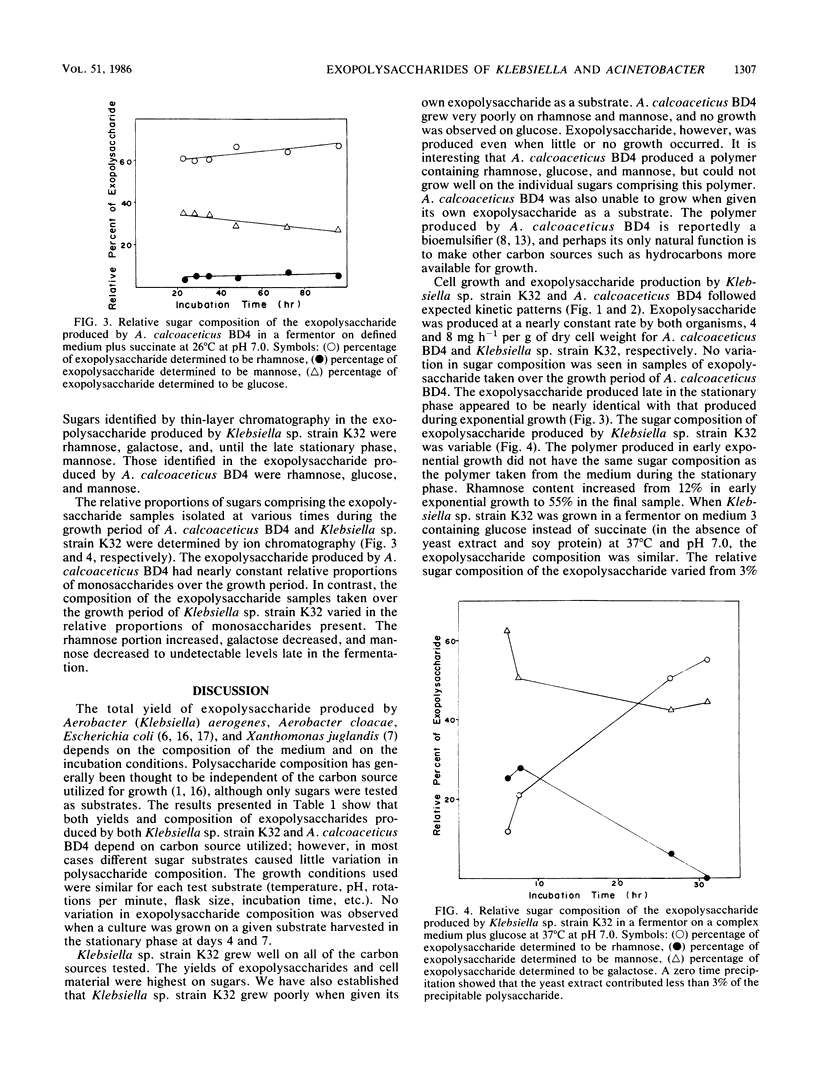
The exopolysaccharides produced by Klebsiella sp. strain K32 and Acinetobacter calcoaceticus BD4 under different growth conditions have been analyzed for sugar composition. The first use of ion chromatography for the quantitative determination of microbial exopolysaccharide composition is reported. Klebsiella sp. strain K32 produced a polymer composed of rhamnose, galactose, and mannose early in its fermentation. The composition of the polymer varied markedly depending on the growth stage of the organism. Klebsiella sp. strain K32 grown in a fermentor produced a polymer which was rich in mannose during early exponential growth in a complex medium, but in the late stationary phase it did not contain detectable levels of mannose. The rhamnose present in the polymer increased from 12 to 55% over the course of growth, whereas galactose decreased from 63 to 45%. A. calcoaceticus BD4 produced a polymer containing rhamnose, glucose, mannose throughout its growth and stationary phase. Klebsiella sp. strain K32 and A. calcoaceticus BD4 were grown on various carbon sources in shake flasks. The polymer yield and composition from both organisms were found to vary with the carbon source. The exopolysaccharide with the highest mannose composition was obtained by using rhamnose as a carbon source for both organisms. These and other data suggest that regulatory changes caused by growth on different substrates result in either the production of a different distribution of polymers or a change in exopolysaccharide structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS G. A., MARTIN S. M. EXTRACELLULAR POLYSACCHARIDES OF SERRATIA MARCESCENS. Can J Biochem. 1964 Oct;42:1403–1413. doi: 10.1139/o64-152. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., WILKINSON J. F. The influence of cultural conditions on polysaccharide production by Aerobacter aerogenes. J Gen Microbiol. 1953 Oct;9(2):174–189. doi: 10.1099/00221287-9-2-174. [DOI] [PubMed] [Google Scholar]
- Drucker D. B. Bacterial cell wall analysis. II. Qualitative analysis of cell wall hydrolysates. Lab Pract. 1969 Dec;18(12):1288–1289. [PubMed] [Google Scholar]
- Kaplan N., Rosenberg E. Exopolysaccharide Distribution of and Bioemulsifier Production by Acinetobacter calcoaceticus BD4 and BD413. Appl Environ Microbiol. 1982 Dec;44(6):1335–1341. doi: 10.1128/aem.44.6.1335-1341.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. C., Casida L. E. GELRITE as a Gelling Agent in Media for the Growth of Thermophilic Microorganisms. Appl Environ Microbiol. 1984 Feb;47(2):427–429. doi: 10.1128/aem.47.2.427-429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Schweizer T. F. A quantitative determination by capillary gas-liquid chromatography of neutral and amino sugars (as O-methyloxime acetates), and a study on hydrolytic conditions for glycoproteins and polysaccharides in order to increase sugar recoveries. Anal Biochem. 1984 Oct;142(1):58–67. doi: 10.1016/0003-2697(84)90516-5. [DOI] [PubMed] [Google Scholar]
- Omar A. S., Weckesser J., Mayer H. Different polysaccharides in the external layers (capsule and slime) of the cell envelope of Rhodopseudomonas capsulata Sp11. Arch Microbiol. 1983 Dec;136(4):291–296. doi: 10.1007/BF00425219. [DOI] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Characterization of the organism and polysaccharide. J Bacteriol. 1961 May;81:688–693. doi: 10.1128/jb.81.5.688-693.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON J. F., DUDMAN W. F., ASPINALL G. O. The extracellular polysaccharide of Aerobacter aerogenes A3 (S1) (Klebsiella type 54). Biochem J. 1955 Mar;59(3):446–451. doi: 10.1042/bj0590446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON J. F., DUGUID J. P., EDMUNDS P. N. The distribution of polysaccharide production in Aerobacter and Escherichia strains and its relation to antigenic character; with a note on the influence of potassium deficiency upon production of polysaccharide by Aerobacter aerogenes. J Gen Microbiol. 1954 Aug;11(1):59–72. doi: 10.1099/00221287-11-1-59. [DOI] [PubMed] [Google Scholar]



