Abstract
We investigated the genetic architecture of variation in male sex comb bristle number, a rapidly evolving secondary sexual character of Drosophila. Twenty-four generations of divergent artificial selection for sex comb bristle number in a heterogeneous population of Drosophila melanogaster resulted in a significant response that was more pronounced in the direction of low bristle numbers. We observed a strong positive correlated response to selection in the corresponding female transverse bristle row. The correlated response in male abdominal and sternopleural bristle numbers, on the other hand, did not follow the same pattern as sex comb bristle number differences between selection lines. Relaxation-of-selection experiments along with mate choice and fecundity assays using the selection lines developed demonstrated the action of stabilizing selection on sex comb bristle number. Our results show (1) substantial genetic variation underlying sex comb bristle number variation; (2) a weak relationship between the sex comb and developmentally related, non-sex bristle systems; and (3) that sexual selection may be a driving force in sex comb evolution, indicating the potential of sex combs to diversify rapidly during population differentiation and speciation. We discuss the implications of these results for theories of genetic variation in display and nondisplay male sex traits.
MALE secondary sexual traits are one of the most rapidly diverging morphological characters of higher animals (Eberhard 1985). Their evolution is thought to be driven by sexual selection (Darwin 1871) acting through female choice (Fisher 1930; Andersson 1994), male–male competition (Parker 1970; Emlen et al. 2005), and/or sexual antagonism (Parker 1979; Chapman et al. 2003). Directional sexual selection acting on variation in male sex traits within populations, at different rates or in opposite directions across populations, can result in rapid phenotypic divergence. This can contribute to the establishment of behavioral reproductive isolation (West-Eberhard 1983; Civetta and Singh 1998; Boughman 2001). Such prezygotic barriers to mating may be more important in the early stages of species formation as compared to postzygotic isolating mechanisms such as hybrid sterility (Turelli et al. 2001; Kirkpatrick and Ravigne 2002; Coyne and Orr 2004).
The male sex comb, an array of specialized bristles on the forelegs, is one such highly variable secondary sexual trait of the melanogaster and obscura species groups of Drosophila (Kopp and True 2002; Schawaroch 2002). Behavioral studies performed in these groups have suggested that the sex combs are involved in grasping the female's abdomen or in spreading her wings during mating and that their role may even vary between species depending on morphology (Speith 1952; Cook 1977; Coyne 1985). Markow et al. (1996) found that Drosophila simulans males captured from a natural population while mating had significantly fewer sex comb teeth than males found not mating. In Drosophila bipectinata, on the other hand, mating males in similar natural conditions had a significantly increased number of sex comb teeth (Polak et al. 2004). Evidence that the number of sex comb teeth affects mating success in opposite directions in different species suggests that the high intra- and interspecific variation seen in sex comb bristle number (Coyne 1985; Kopp et al. 2003; Tatsuta and Takano-Shimizu 2006) may be driven by sexual selection.
Previous studies have investigated sex comb bristle number variation within and between species of the melanogaster complex using quantitative trait loci (QTL) mapping and gene expression analyses (Coyne 1985; True et al. 1997; Macdonald and Goldstein 1999; Nuzhdin and Reiwitch 2000; Kopp et al. 2003; Tatsuta and Takano-Shimizu 2006; Graze et al. 2007). Despite a large amount of work in this field, there remains a gap in our basic understanding of sex comb bristle number inheritance and evolution. In this study we have used artificial selection in combination with relaxation-of-selection tests, investigation of genetic correlations with other bristle traits, and measurement of various fitness components in the lines developed. Together, these experiments provide insights into the evolutionary potential of sex comb bristle number to respond to selection and the mechanisms responsible for maintenance of variation in this trait.
MATERIALS AND METHODS
All experiments were carried out at room temperature (22°–25°) with flies reared on standard cornmeal–molasses–agar medium.
Derivation of base population:
Thirty-two different lines of D. melanogaster were obtained and reared under uniform laboratory conditions for three to four generations (supplemental Table 1). Adult males were anesthetized on ice, and sex comb bristle number on both forelegs was counted in 30 males from each population under a light microscope. We used the mean of the left and right foreleg measurement as the sex comb bristle number score in all analyses. The absolute value of the numerical difference between the left and right foreleg measurement was used to calculate fluctuating asymmetry (FA) (Palmer and Strobeck 2003). To test for a relationship between degree of fluctuating asymmetry and size of the sex comb, Spearman rank correlation coefficients between FA and mean bristle number were calculated.
To obtain a genetically variable population, the six most extreme populations according to their mean sex comb bristle number (three with the highest population mean and three with the lowest, indicated in supplemental Table 1) were crossed (supplemental Figure 1). Approximately 40 male and 40 female offspring from each population cross were pooled and allowed to interbreed for four generations to establish the base population from which we derived our replicate selection and control lines.
Artificial selection protocol:
The selection experiment consisted of two replicates (designated 1 and 2) that each included one line selected for high sex comb bristle number (high), one line selected for low bristle number (low), and one unselected control line (control). Two hundred males from the base population were scored for sex comb bristle number and the highest-scoring males, 10 for each replicate, were chosen as parents for the high lines. Similarly, the lowest-scoring males, 10 for each replicate, were used as parents for the low lines while the 10 males for each of the control lines were chosen at random. Males for each line were mated with 10 randomly chosen females (supplemental Figure 2).
Because of the low number of progeny obtained in generation 1, we scored varying numbers of males: 56 high 1, 54 high 2, 30 control 1, 30 control 2, 74 low 1, and 46 low 2 males. The 10 most extreme males from within each line were chosen as parents for the second generation. We revised the protocol and increased the number of randomly chosen females to 20. In each subsequent generation, 100 males from each line were scored and the most extreme 10 were selected. Control males and control females were chosen at random. Selection was continued in this manner for each of these lines for a total of 24 generations.
Realized heritability of male sex comb bristle number for each line was estimated by linear regression of cumulative selection response (mean sex comb bristle number for each generation added for each round of selection) on the cumulative selection differential (absolute value of parental mean minus generation mean, added to each other for each round of selection) (Falconer and Mackay 1996; Edwards et al. 2006). The first two generations of selection data were excluded because the method of selection was different. For each replicate, the coefficients of genetic (CVG) and environmental (CVE) variation were calculated as 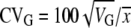 and
and 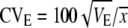 (Houle 1992). VG for each replicate was estimated as h2VP, where VP was the average phenotypic variance of the respective control line, and h2 was calculated from divergence between the high and low lines of that replicate. VE was estimated as VP − VG. The mean (
(Houle 1992). VG for each replicate was estimated as h2VP, where VP was the average phenotypic variance of the respective control line, and h2 was calculated from divergence between the high and low lines of that replicate. VE was estimated as VP − VG. The mean (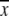 ) was the mean sex comb bristle number of the respective control line.
) was the mean sex comb bristle number of the respective control line.
Relaxed selection protocol:
Each of the four selection lines was divided into two sublines at generation 14. In the first subline, artificial selection was continued as described above. The second subline was maintained for 10 generations without further selection for sex comb bristle number. Sex comb bristle number was scored in 50 males in these relaxed sublines every alternate generation until the 10th generation after relaxation when 100 males were scored.
Correlated responses to selection:
At generation 24, we scored bristle number in the most distal transverse bristle row (TBR), the segment that corresponds to the male sex comb (Tokunaga 1962; Held et al. 2004), of both forelegs of 30 females from each line. We also scored bristle number in the fourth abdominal segment of 100 males and in the left and right sternopleural plates of 30 males from each line at generation 24. The fifth abdominal segment is more commonly used as a measure of abdominal bristle number. The sternopleural and TBR score used was the mean of the left- and right-side measurement.
Within-line fitness assays:
We tested for the effect of sexual selection on sex comb bristle number by assessing differences in mating success associated with bristle number differences within the high 2, control 2, and low 2 lines. At generation 20, virgin males and females from each line were collected within 4 hr post-eclosion using CO2 anesthesia and housed separately for 4–5 days prior to mating assays. On the basis of their sex comb bristle number, males from within each line were divided into two classes, high scoring (h) and low scoring (l), and paired in such a way as to maximize differences in sex comb bristle number. One male from each pair was marked with a notch at the base of either the right or the left wing with forceps to allow for identification. We ensured that paired treatments within a set were reciprocally marked for half the treatments. Of the 90 matings scored, 42 successful males had clipped wings and 48 had nonclipped males. These differences are not statistically significant (χ2 = 0.4, d.f. = 1, P = 0.52), confirming that notching had no significant effect on mating success. Each male pair was introduced into a vial with a female from the same line without anesthesia. We recorded male courtship behaviors, including time spent in wing vibrations (in seconds) and the number of attempted copulations. Trials were terminated if a successful copulation did not occur within 15 min. A trial was retained for statistical analysis only if both males courted the females. If one male was not active or did not get a chance to court the female because copulation occurred too soon, the trial was discarded. Thirty successful trials were recorded for each line.
To assess the potential role of natural selection as a counteracting force in the selected lines, we assessed the number of progeny sired by males with different sex comb bristle numbers within each line at generation 22. As described above, males from high 2, low 2, and control 2 were collected as virgins, scored for bristle number, and divided into two classes. Females were collected as virgins and aged for 4–5 days. A single male was mated to three females in a vial for 4 days, after which the parents were discarded and progeny counts were made (on day 17). If any parent was found dead at day 5, the trial was discarded. In this manner, we assayed the number of progeny sired by 30 h and 30 l males from within each line in replicate 2.
RESULTS
Genetic variation in sex comb bristle number:
The mean sex comb bristle numbers for each of the 32 different populations examined are presented in supplemental Table 1. We detected significant differences in mean bristle number among these populations (F31,928 = 22.859, P < 0.001), indicating high intraspecific variation for sex comb bristle number among these lines of D. melanogaster.
Response to artificial selection for sex comb bristle number:
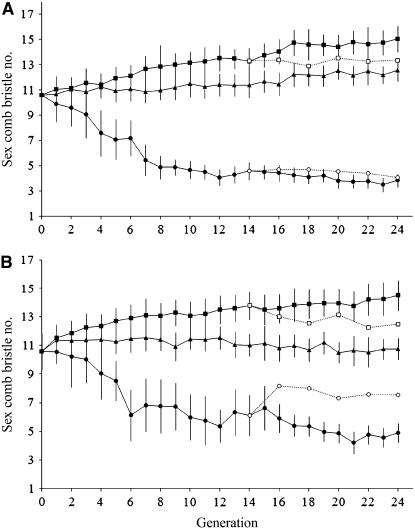
Sex comb bristle number in D. melanogaster responded to divergent artificial selection, exhibiting significant differences in mean bristle number between lines after 24 generations of selection (replicate 1: F2,297 = 4986.3, P < 0.001) (replicate 2: F2,297 = 3145.15, P < 0.001; Figures 1 and 2). The phenotypic response was greater in the direction of decreased sex comb bristle number as seen in the estimates of heritability: h2 = 0.11 ± 0.01 (P < 0.01) and h2 = 0.07 ± 0.02 (P < 0.001) for high 1 and high 2 lines, respectively, while low 1 and low 2 line heritability estimates were h2 = 0.21 ± 0.02 (P < 0.001) and h2 = 0.16 ± 0.02 (P < 0.001), respectively. Estimates of heritability derived from divergence between high and low lines were also significant (replicate 1: h2 = 0.15 ± 0.01, P < 0.001) (replicate 2: h2 = 0.09 ± 0.01, P < 0.001). We note an increase in bristle number in control 1 males (Figure 1) and this is likely due to random genetic drift since the effective sample size is small and both males and females were chosen at random for the control lines.
Figure 1.—
Response to artificial selection for male sex comb bristle number in D. melanogaster. Mean sex comb bristle numbers in high (square), low (circle), and control (triangle) lines over 24 generations in (A) replicate 1 and (B) replicate 2. Solid lines with solid symbols indicate artificial selection lines and dashed lines with open symbols indicate relaxed sublines. Error bars represent standard deviation.
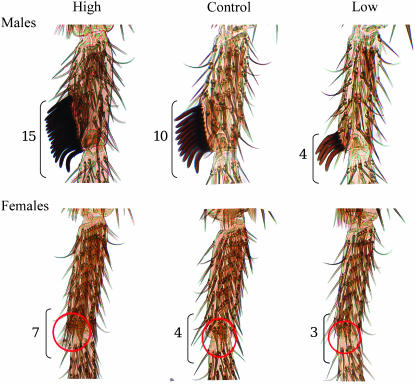
Figure 2.—
Forelegs of males (showing sex comb) and females (showing TBRs) from high, control, and low lines of D. melanogaster after 24 generations of artificial selection. Bristle number of the foreleg is indicated at the bottom left.
Stabilizing selection for sex comb bristle number:
We compared mean sex comb bristle number of the relaxed sublines after 10 generations of relaxation with those of the paired source population before relaxation, at generation 14. Mean sex comb bristle number did not change significantly in both high 1 relaxed subline (t = 1.41, P = 0.96) and low 1 relaxed subline (t = 1.96, P = 0.312) after 10 generations of relaxation (Figure 1). Mean sex comb bristle number in the high 2 relaxed subline regressed toward control levels (t = 9.67, P < 0.001) while mean bristle number in low 2 relaxed subline showed an increase in the direction of the controls (t = 7.61, P < 0.001) (Figure 1). This demonstrates the action of net stabilizing selection acting to maintain intermediate bristle numbers in these lines.
Correlated responses to selection:
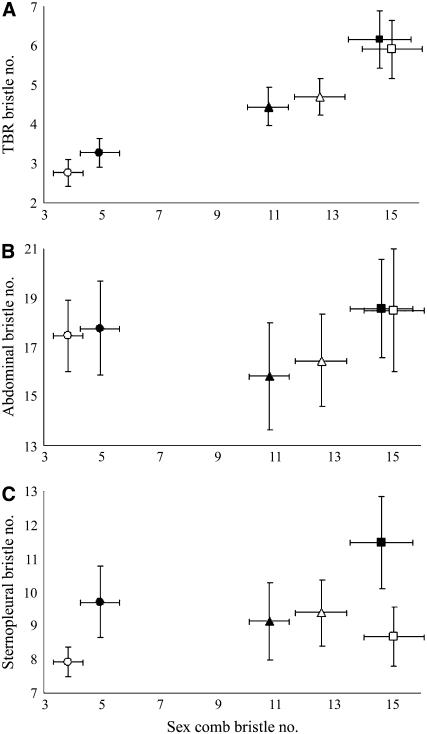
Significant differences in female distal TBR bristle numbers were seen in both replicate 1 (F2,87 = 251.3, P < 0.001) and replicate 2 (F2,87 = 220.14, P < 0.001; Figures 2 and 3A). These differences followed the same pattern as sex comb bristle number differences between selection lines; both high lines exhibited significantly higher TBR bristle numbers than their respective control lines, which had significantly more bristles than their respective low lines [Tukey's honestly significantly different (HSD) test, P < 0.01 for all comparisons]. In contrast, the differences in abdominal bristle number (replicate 1: F2,297 = 30.33, P < 0.001 and replicate 2: F2,297 = 48.17, P < 0.001) (Figure 3B) and sternopleural bristle number (replicate 1: F2,87 = 14.7, P < 0.001 and replicate 2: F2,87 = 28.8, P < 0.001) (Figure 3C) were not entirely consistent with the pattern of changes in sex comb bristle number. Mean abdominal bristle numbers of males from low 1 and low 2 lines were significantly higher than those of males from control 1 and control 2 lines, respectively (Tukey's HSD test, P < 0.001). Sternopleural bristle numbers did not change significantly from the respective control lines in high 1 (Tukey's HSD test, P = 0.12) and low 2 (Tukey's HSD test, P = 0.52) lines in response to selection for sex comb bristle number. All other comparisons of abdominal and sternopleural bristle numbers within replicates were statistically significant at P < 0.01 (Tukey's HSD test). These results show a strong positively correlated response to selection for sex comb bristle number in the homologous female TBRs but a weaker, inconsistent response in the abdominal and sternopleural bristles.
Figure 3.—
Correlated responses to divergent selection for sex comb bristle number. Mean sex comb bristle numbers are plotted against (A) mean female distal TBR bristle numbers, (B) mean male abdominal bristle numbers, and (C) mean male sternopleural bristle numbers of high (square), low (circle), and control (triangle) lines at generation 24 in replicate 1 (open) and replicate 2 (solid). Error bars represent standard deviation.
Sexual selection against extremely low sex comb bristle numbers:
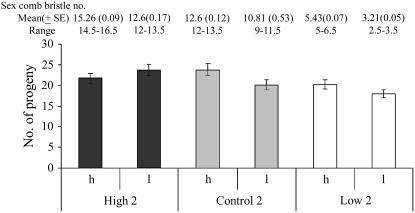
Females from high 2, low 2, and control 2 lines were given a choice between males that differed in sex comb bristle number from within their line to assess differences in mating success associated with differences in sex comb bristle number. Means of sex comb bristle numbers, time spent in wing vibration, and numbers of attempted copulations of successful vs. unsuccessful males within each line were compared using a Wilcoxon paired sample test (Table 1). The only comparison that showed a significant difference was mean sex comb bristle numbers of low 2 males: successful males from the low 2 line had more sex comb teeth than unsuccessful males (χ2 = 19.2, d.f. = 1, P < 0.01) (Table 1, Figure 4). More l males were successful as compared to the h males in the high 2 (χ2 = 1.2, d.f. = 1, P = 0.27) and control 2 lines (χ2 = 0.13, d.f. = 1, P = 0.71) (Figure 4), but these differences were not significant.
TABLE 1.
Sex comb bristle number, time spent in wing vibration, and attempted copulations of successful and unsuccessful males
| Bristle no. of sex combs
|
Time in wing vibration (sec)
|
No. of attempted copulations
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Line | Successful | Unsuccessful | P | Successful | Unsuccessful | P | Successful | Unsuccessful | P |
| High 2 | 14.15 (1.6) | 14.78 (1.38) | t = 176, P = 0.24 | 25 (14.74) | 20.76 (14.4) | t = 186.5, P = 0.36 | 2.8 (2.3) | 2.8 (1.56) | t = 214, P = 0.87 |
| Control 2 | 12.13 (1.3) | 12.26 (1.62) | t = 231.5, P = 0.98 | 14.8 (12.8) | 9.46 (8.6) | t = 157.5, P = 0.13 | 1.6 (1.52) | 1.46 (1.57) | t = 202, P = 0.75 |
| Low 2 | 5.21 (0.71) | 3.53 (0.73) | t = 50, P = 0** | 14.8 (9.15) | 19.06 (12.61) | t = 141, P = 0.06 | 2.63 (2.15) | 2.93 (1.92) | t = 173, P = 0.35 |
Data are from mating trials between high-scoring (h) and low-scoring (l) males from within high 2, control 2, and low 2 lines with mean (±SD) given. Comparisons between successful and unsuccessful males were performed using a Wilcoxon paired sample test. **P < 0.01.
Figure 4.—
Numbers of high-scoring (h) and low-scoring (l) males that were successful in mating trials conducted within each selection line. Mean (±SE) and range of sex comb bristle numbers of all (30) males from each class are indicated above bars.
The number of progeny sired by males with different sex comb bristle numbers within high 2, low 2, and control 2 lines was assessed to uncover fecundity differences between these males (Figure 5). We detected a significant effect of line in assaying differences in fecundity (F2,175 = 4.82, P = 0.009) and found that low 2 males had significantly lower fertility than high 2 (Tukey's HSD test; P < 0.01) males. Within a line, however, multiple t-tests showed no significant difference in the number of progeny sired by high-scoring and low-scoring males within high 2 (t = 1.69, P = 0.09), low 2 (t = 1.98, P = 0.05), or control 2 (t = 1.89, P = 0.07)
Figure 5.—
Mean number of progeny (±SE) sired by high-scoring (h) and low-scoring (l) males from within each selection line. Mean (±SE) and range of sex comb bristle numbers of all (30) males from each class are indicated above bars.
DISCUSSION
Genetic architecture of sex comb bristle number variation in D. melanogaster:
The large differences in sex comb bristle number between the geographically widespread populations of D. melanogaster used in our study, coupled with the rapid, robust phenotypic response to artificial selection, show that there is substantial additive genetic variance underlying this trait. The magnitude of the realized heritability, however, is relatively low (∼0.1) when compared to other morphological traits such as abdominal bristle number (h2 ∼ 0.5; Clayton et al. 1957) and body size (h2 ∼ 0.4; Robertson 1957) in Drosophila. The genetic and environmental coefficients of variation, respectively, were 3.36 and 7.99 for replicate 1 and 2.96 and 7.87 for replicate 2. These values show that the low heritability of male sex comb bristle number is due to a higher proportion of environmental variance, rather than to a lack of genetic variation, which is a typical feature of many secondary sexual traits (Alatalo et al. 1988; Pomiankowski and Moller 1995).
The response to selection was highly asymmetrical with both low lines showing a greater per-generation decrease in sex comb bristle number as compared to the increase in bristle number in the high lines. Such a response could have resulted from the action of selection (natural and/or sexual) operating along with the artificial selection applied. The regression of bristle numbers toward intermediate levels in both high 2 and low 2 on relaxation of selection shows that sex comb bristle number is under net stabilizing selection in these lines. The lack of a significant response to relaxation in replicate 1 may be due to lower levels of genetic variation (Figure 1).
We measured correlated responses to selection for sex comb bristle number in other developmentally related mechanosensory bristle systems to assess the extent of genetic linkage between them. The last TBR of the female is homologous to the male sex comb (Tokunaga 1962; Held et al. 2004), and here we observed a strong indirect response to selection. Changes in male abdominal and sternopleural bristle numbers, on the other hand, were not entirely consistent with the pattern of differences seen in sex comb bristle number after 24 generations of selection. It appears that selection may have altered the frequency of a few loci affecting bristle number in general, but that there remain major loci affecting the sex comb system specifically that are not shared with other mechanosensory bristle systems. This weak relationship suggests reduced developmental constraint on the sex combs to evolve in concert with other, non-sex, bristle systems. The sex combs appear to be a sexual modification evolving relatively independently of related bristle systems, which could enable them to evolve rapidly, and potentially with exaggeration.
Males from the low 2 line appear to be unfit in comparison to males from both high 2 and control 2 lines. Low 2 males had lower fecundity than males from control 2 and high 2, and within the low 2 line, males with extremely low bristle numbers had reduced mating success. This could be due to the accumulation of deleterious alleles during the selection process. Sexual selection against small combs could also be responsible, maintaining higher frequencies of alleles for greater bristle numbers in the base population, which would explain the greater response in the downward direction when artificial selection was applied. Another interesting observation is that, although not significant, successful males from within the control 2 and high 2 lines had fewer sex comb teeth than unsuccessful males (Table 1, Figure 4). This is similar to the trend seen in natural populations of D. simulans, where mating males had significantly fewer sex comb teeth (Markow et al. 1996). Sexual selection appears to be an important driving force in sex comb evolution and it would be worthwhile to further investigate this in the melanogaster subgroup to help understand its potential role in species divergence.
Theories of genetic variation for male sexual traits:
Sexual selection acting on male sex traits is expected to lead to rapid fixation of favorable alleles and depletion of heritable genetic variation in such traits (Borgia 1979; Taylor and Williams 1982). However, in contrast to this expectation, it has been shown that additive genetic variation not only is maintained, but is actually higher in male sex traits as compared to non-sex traits (Pomiankowski and Moller 1995). Different hypotheses have been proposed to explain this persistence of genetic variance in male traits but few studies have attempted to empirically test these hypotheses. We find high levels of genetic variation underlying sex comb bristle number, allowing us to assess if, and how, current theories of genetic variation for sexual traits apply to this trait.
Pomiankowski and Moller (1995) have proposed that fitness increases exponentially as a sexually selected trait becomes exaggerated, which favors an increase in phenotypic variance through the evolution of modifiers that can increase the number of genes and their effect on the trait. According to this hypothesis, the high additive genetic variance in male sex traits is a consequence of continual directional selection while traits subject to stabilizing selection should have reduced levels of genetic variation due to modifiers that restrict the number of loci and their effects. However, this explanation fails to consider that the exaggeration of a sexual trait does not continue indefinitely and that, after initial spread, most sexually selected traits are expected to be under stabilizing selection (Kirkpatrick and Ryan 1991; Andersson 1994). Indeed, sex comb bristle number shows only a limited increase in the high lines in spite of the strong artificial selection applied and appears to be under stabilizing selection in the base population.
Alternatively, the “genic capture” hypothesis (Rowe and Houle 1996) proposes that the expression of male sex traits is costly and condition dependent and thus involves a large number of genes in the genome, which provides an inexhaustible source of variation in such traits. For this explanation to apply, the secondary sexual trait must be condition dependent, but our results fail to find evidence to support this in the sex comb: A negative phenotypic correlation between size and degree of FA is expected for costly, condition-dependent secondary sexual traits, since males of high condition should be able to simultaneously maximize size and minimize FA of sexual traits (Manning and Hartley 1991; Moller and Pomiankowski 1993; Tomkins and Simmons 2003). We did not detect a significant negative correlation in any of the 32 lines that we tested (supplemental Table 1). In a previous study, Polak et al. (2004) found a positive relationship between sex comb bristle number and FA in lab-reared populations of D. bipectinata.
Male secondary sexual traits can be classified into two types: (1) costly display traits subject to female choice and (2) traits not used for display but to coerce or drive females to mate with or without females having any control (Singh and Kulathinal 2005). The absence of a negative relationship between FA and size suggests that the trait may not be a typical costly, condition-dependent display trait (Moller and Cuervo 2003). Instead, it has been proposed that the combs help to grasp the female (Speith 1952), suggesting that males use them to control females during copulation. Singh and Kulathinal (2005) recently proposed the “male sex drive” hypothesis, which offers a more general explanation for maintenance of variation in different types of male traits. Complementary to female choice, it is the concept that males are the sex that develops new strategies (morphological, physiological, and behavioral) to mate and pass on offspring. This male drive to secure mates and reproduce leads to the recapture of any mutations affecting any male trait involved in sex- and reproduction-related functions. This selection-driven continuous input of new mutations would compensate for loss of genetic variation.
From our findings of significant heritable genetic variation, a weak relationship with other non-sex bristle systems, and evidence of intraspecific sexual selection in D. melanogaster, we can conclude that the sex comb has the potential to diversify rapidly. The results presented here raise the possibility that sex comb bristles may not be a typical display trait and force us to think about the maintenance of genetic variation in display vs. nondisplay traits. Our study lays the foundation for further work, providing experimental material to further analyze the genetic architecture, functional significance, and evolutionary dynamics of the sex comb in Drosophila.
Acknowledgments
We thank Marisa Melas and Maria Abou Chakra for foreleg images and Artyom Kopp and Joel Atallah for helpful suggestions and protocols. We are thankful to Wilfried Haerty, Ben Evans, Jonathon Stone, Carlo Artieri, Richard Morton, the associate editor, and three anonymous reviewers for their valuable comments on the manuscript. This work was supported by a Natural Sciences and Engineering Research Council of Canada grant to R.S.S.
References
- Alatalo, R. V., J. Hoglund and A. Lunnberg, 1988. Patterns of variation in tail ornament size in birds. Biol. J. Linn. Soc. Lond. 34 363–374. [Google Scholar]
- Andersson, M., 1994. Sexual Selection. Princeton University Press, Princeton, NJ.
- Borgia, G., 1979. Sexual selection and the evolution of mating systems, pp. 19–80 in Sexual Selection and Reproductive Competition, edited by M. S. Blum and N. A. Blum. Academic Press, New York.
- Boughman, J. W., 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411 944–948. [DOI] [PubMed] [Google Scholar]
- Chapman, T., G. Arnqvist, J. Bangham and L. Rowe, 2003. Sexual conflict. Trends Ecol. Evol. 18 41–47. [Google Scholar]
- Civetta, A., and R. S. Singh, 1998. Sex related genes, directional sexual selection and speciation. Mol. Biol. Evol. 15 901–909. [DOI] [PubMed] [Google Scholar]
- Clayton, G. A., J. A. Morris and A. Robertson, 1957. An experimental check on quantitative genetical theory. I. Short term responses to selection. J. Genet. 55 131–151. [Google Scholar]
- Cook, R. M., 1977. Behavioral role of the sex combs in Drosophila melanogaster and Drosophila simulans. Behav. Genet. 7 349–357. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1985. Genetic studies of three sibling species of Drosophila with relationship to theories of speciation. Genet. Res. 46 169–192. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 2004. Speciation. Sinauer Associates, Sunderland, MA.
- Darwin, C., 1871. The Descent of Man, and Selection in Relation to Sex. John Murray, London.
- Eberhard, W. G., 1985. Sexual Selection and Animal Genitalia. Harvard University Press, Cambridge, MA.
- Edwards, A. C., S. M. Rollmann, T. J. Morgan and T. F. C. Mackay, 2006. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2 e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen, D. J., J. Marangelo, B. Ball and C. W. Cunnigham, 2005. Diversity in the weapons of sexual selection: horn evolution in the beetle genus Onthophagus (Coleoptera: Scarabaeidae). Evolution 59 1060–1084. [PubMed] [Google Scholar]
- Falconer, D. S., and T. F. C. Mackay, 1996. Introduction to Quantitative Genetics. Longman, New York.
- Fisher, R. A., 1930. The Genetical Theory of Natural Selection. Clarendon, Oxford.
- Graze, R. M., O. Barmina, D. Tufts, E. Naderi, K. L. Harmon et al., 2007. New candidate genes for sex comb divergence between Drosophila mauritiana and Drosophila simulans. Genetics 176 2561–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held, L. I., M. J. Grimson and Z. Du, 2004. Proving an old prediction: the sex comb rotates at 16 to 24 hours after pupariation. Dros. Inf. Serv. 87 76–78. [Google Scholar]
- Houle, D., 1992. Comparing the evolvability and variability of quantitative traits. Genetics 130 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick, M., and V. Ravigne, 2002. Speciation by natural and sexual selection: models and experiments. Am. Nat. 159 22–35. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, M., and M. J. Ryan, 1991. The evolution of mating preferences and the paradox of the lek. Nature 350 33–38. [Google Scholar]
- Kopp, A., and J. R. True, 2002. Evolution of male sexual characters in the Oriental Drosophila melanogaster species group. Evol. Dev. 4 278–291. [DOI] [PubMed] [Google Scholar]
- Kopp, A., R. M. Graze, S. Xu, S. B. Carroll and S. V. Nuzhdin, 2003. Quantitative trait loci responsible for variation in sexually dimorphic traits in Drosophila melanogaster. Genetics 163 771–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, S. J., and D. B. Goldstein, 1999. A quantitative genetic analysis of male sexual traits distinguishing the sibling species D. simulans and D. sechellia. Genetics 153 1683–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, J. T., and M. A. Hartley, 1991. Symmetry and ornamentation are correlated in the peacock's train. Anim. Behav. 42 1020–1021. [Google Scholar]
- Markow, T. A., D. Bustoz and S. Pitnick, 1996. Sexual selection and a secondary sexual character in two Drosophila species. Anim. Behav. 52 759–766. [Google Scholar]
- Moller, A. P., and J. J. Cuervo, 2003. Asymmetry, size, and sexual selection: factors affecting heterogeneity in relationships between asymmetry and sexual selection, pp. 262–275 in Developmental Instability: Causes and Consequences, edited by M. Polak. Oxford University Press, New York.
- Moller, A. P., and A. Pomiankowski, 1993. Fluctuating asymmetry and sexual selection. Genetica 89 267–279. [Google Scholar]
- Nuzhdin, S. V., and S. G. Reiwitch, 2000. Are the same genes responsible for intra- and interspecific variability for sex comb tooth number in Drosophila? Heredity 84 97–102. [DOI] [PubMed] [Google Scholar]
- Palmer, R. A., and C. Strobeck, 2003. Fluctuating asymmetry analyses revisited, pp. 279–319 in Developmental Instability: Causes and Consequences, edited by M. Polak. Oxford University Press, New York.
- Parker, G. A., 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45 525–567. [Google Scholar]
- Parker, G. A., 1979. Sexual selection and sexual conflict, pp. 123–166 in Sexual Selection and Reproductive Competition in Insects, edited by M. S. Blum and N. A. Blum. Academic Press, London.
- Polak, M., W. T. Starmer and L. L. Wolf, 2004. Sexual selection for size and symmetry in a diversifying secondary sexual character in Drosophila bipectinata. 58 597–607. [PubMed]
- Pomiankowski, A., and A. P. Moller, 1995. A resolution of the lek paradox. Proc. R. Soc. Lond. B Biol. Sci. 260 21–29. [Google Scholar]
- Robertson, F. W., 1957. Studies in quantitative inheritance. XI. Genetic and environmental correlation between body size and egg production in Drosophila melanogaster. J. Genet. 55 428–443. [DOI] [PubMed] [Google Scholar]
- Rowe, L., and D. Houle, 1996. The lek paradox and the capture of genetic variance by condition-dependent traits. Proc. R. Soc. Lond. B Biol. Sci. 263 1415–1421. [Google Scholar]
- Schawaroch, V., 2002. Phylogeny of a paradigm lineage: the Drosophila melanogaster species group. Biol. J. Linn. Soc. Lond. 76 21–37. [Google Scholar]
- Singh, R. S., and R. J. Kulathinal, 2005. Male sex drive and the masculinization of the genome. BioEssays 27 518–525. [DOI] [PubMed] [Google Scholar]
- Speith, H. T., 1952. Mating behavior within the genus Drosophila (Diptera). Bull. Am. Mus. Nat. Hist. 99 395–474. [Google Scholar]
- Tatsuta, H., and T. Takano-Shimizu, 2006. Genetic architecture of variation in sex-comb tooth number in Drosophila simulans. Genet. Res. 87 93–107. [DOI] [PubMed] [Google Scholar]
- Taylor, P. D., and G. C. Williams, 1982. The lek paradox is not resolved. Theor. Popul. Biol. 22 392–409. [Google Scholar]
- Tokunaga, C., 1962. Cell lineage and differentiation on the male foreleg of Drosophila melanogaster. Dev. Biol. 4 489–516. [DOI] [PubMed] [Google Scholar]
- Tomkins, J. L., and L. W. Simmons, 2003. Fluctuating asymmetry and sexual selection: paradigm shifts, publication bias, and observer expectation, pp. 231–261 in Developmental Instability: Causes and Consequences, edited by M. Polak. Oxford University Press, New York.
- True, J. R., J. Liu, L. F. Stam, Z. B. Zeng and C. C. Laurie, 1997. Quantitative genetic analysis of divergence in male secondary sexual traits between Drosophila simulans and Drosophila mauritiana. Evolution 51 81–832. [DOI] [PubMed] [Google Scholar]
- Turelli, M., N. H. Barton and J. A. Coyne, 2001. Theory and speciation. Trends Ecol. Evol. 16 330–343. [DOI] [PubMed] [Google Scholar]
- West-Eberhard, M. J., 1983. Sexual selection, social competition, and speciation. Q. Rev. Biol. 58 155–183. [Google Scholar]