Abstract
We investigated the effect of apolipoprotein E (Apoe) on albuminuria in the males of two independent F2 intercrosses between C57BL/6J and A/J mice, using wild-type inbred strains in the first cross and B6-Apoe−/− animals in the second cross. In the first cross, we identified three quantitative trait loci (QTL): chromosome (Chr) 2 [LOD 3.5, peak at 70 cM, confidence interval (C.I.) 28–88 cM]; Chr 9 (LOD 2.0, peak 5 cM, C.I. 5–25 cM); and Chr 19 (LOD 1.9, peak 49 cM, C.I. 23–54 cM). The Chr 2 and Chr 19 QTL were concordant with previously found QTL for renal damage in rat and human. The Chr 9 QTL was concordant with a locus found in rat. The second cross, testing only Apoe−/− progeny, did not identify any of these loci, but detected two other loci on Chr 4 (LOD 3.2, peak 54 cM, C.I. 29–73 cM) and Chr 6 (LOD 2.6, peak 33 cM, C.I. 11–61 cM), one of which was concordant with a QTL found in rat. The dependence of QTL detection on the presence of Apoe and the concordance of these QTL with rat and human kidney disease QTL suggest that Apoe plays a role in renal damage.
CHRONIC kidney disease is a growing medical problem caused by various environmental and genetic factors. Identifying genes underlying common forms of kidney disease in humans has proven difficult, expensive, and time consuming. However, quantitative trait loci (QTL) for several complex traits are concordant among mice, rats, and humans, suggesting that genetic findings from animal models are relevant to human disease (Stoll et al. 2000; Sugiyama et al. 2001; Wang and Paigen 2002a,b). This has been supported by the discovery of human disease genes from candidates identified in mouse (Korstanje et al. 2004a; Hillebrandt et al. 2005). With respect to chronic kidney disease, QTL analysis using mice is likely to contribute new findings in the near future (Korstanje and DiPetrillo 2004).
The gene encoding apolipoprotein E (APOE) has been implicated in chronic kidney disease. Several human association studies have shown an association of APOE with renal damage parameters in different groups of patients (Werle et al. 1998; Araki et al. 2000; Joss et al. 2005). Additionally, we found an association between serum APOE levels and albuminuria in the general population (R. Korstanje, unpublished data). Recently, a direct, lipid independent role for APOE in the kidney and involvement in renal disease has been suggested. Chen et al. (2001) showed that APOE regulates mesangial cell proliferation in a dose-dependent fashion. APOE has an antiproliferative effect specific for mesangial cells (not endothelial cells) through the induction of matrix heparin sulfate proteoglycan (HSPG) (Chen et al. 2001). Thus, varying APOE levels or genetic variation in the APOE receptors involved in this mechanism could be expected to have an important effect on renal function and disease.
Studies of kidney disease involving Apoe−/− mice have led to contrasting results. In the study by Wen et al. (2002), Apoe−/− mice developed spontaneous glomerular lesions with macrophage accumulation, commonly with foam cell appearance, deposition of extracellular matrix, glomerular hyperplasia, and foci of mesangiolysis associated with capillary microaneurysms. On the other hand, in studies using uninephrectomy (UNX) and subtotal nephrectomy (SNX) on wild-type and Apoe−/− mice, Buzello et al. (2004) did not observe a difference in renal or glomerular injury after reduction of renal mass.
The aim of the current study is to investigate whether presence or absence of APOE would have an effect on the genetic susceptibility to albuminuria in mice. To this purpose we performed two independent F2 intercrosses between C57BL/6J mice, which do not develop albuminuria, and A/J mice, which do develop albuminuria (Qi et al. 2005). In the first cross only wild-type inbred strains B6 and A/J were used; in the second cross B6.Apoe−/− animals were crossed to A/J mice and only the Apoe−/− F2 animals were analyzed. We expected that the combination of Apoe deficiency and alleles from the susceptible A/J strain would allow us to map loci involved in the difference in susceptibility to kidney disease between B6 and A/J mice, as well as the effect of APOE on these alleles.
MATERIALS AND METHODS
Mice and phenotype characterization:
A/J, C57BL/6J (B6), and B6-129P2-Apoetm1Unc/J (B6-Apoe−/−) mice, which were backcrossed at least 12 times to B6 and are now at N12F13, were obtained from The Jackson Laboratory (Bar Harbor, ME). A/J females were mated to B6 (cross 1) or B6-Apoe−/− (cross 2) males to produce the F1 progeny; F1 mice were intercrossed to produce 381 male F2 (cross 1) and 145 male F2-Apoe−/− (cross 2) progeny. Mice were housed in a climate-controlled facility with a 14-hour:10-hour light–dark cycle with free access to food and water throughout the experiment. After weaning, mice were maintained on a chow diet (Old Guilford 234A, Guilford, CT). Spot urine samples were taken between 8 and 10 weeks, and albumin and creatinine concentrations were measured on a Beckman Synchron CX5 chemistry analyzer. Actual mouse albumin concentrations were calculated by linear regression from a standard curve generated with mouse albumin standards (Kamiya Biomedical, Seattle) (Grindle et al. 2006). All experiments were approved by The Jackson Laboratory's Animal Care and Use Committee.
Genotyping:
DNA was isolated as described previously (Korstanje et al. 2004b). Each F2 animal was genotyped using 98 SNPs (cross 1) or 140 SNPs (cross 2) polymorphic between A/J and B6 covering the whole genome (supplemental Tables 1 and 2). Genotyping was performed by KBiosciences (Hoddesdon, UK) using the Amplifluor chemistry (Serologicals, Norcross, GA).
QTL mapping and statistics:
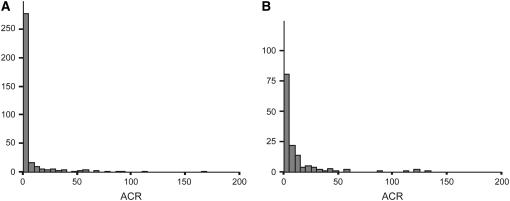
QTL analysis and genome scans were carried out using the scanone and bayesint functions of the R/qtl package (Broman et al. 2003). Urinary albumin presents a highly skewed, two-part distribution in which many individuals have a score of zero (Figure 1). Broman (2003) studied the problem of mapping such phenotypes and concluded that the nonparametric procedure (Kruglyak and Lander 1995) provides the most reliable analysis. Thus, we used the nonparametric method to analyze urinary albumin as a binary trait (Alb = 0 vs. Alb > 0). Significance of QTL LOD scores was assessed using 1000 permutations of the phenotypic data (Churchill and Doerge 1994). Significant QTL are reported at the genomewide adjusted 0.05 level (LOD 2.97 in cross 1 and LOD 3.35 in cross 2) and suggestive QTL at genomewide 0.63 level (LOD 1.93 in cross 1 and LOD 2.01 in cross 2) (Lander and Kruglyak 1995). Bayesian credible intervals for particular chromosomes are computed and reported as confidence intervals. The 10LOD is taken, rescaled to have area 1, and followed by calculating the connected interval with density above threshold having coverage matching the target probability at 0.96. The nonparametric analysis presents some limitations. It is difficult to estimate effect sizes of the QTL, multiple QTL modeling is not available, and adjustment for covariates such as sex cannot be made. Nonetheless, it is justified in the case of such an extreme phenotype distribution. Due to the extreme skew in the distribution of albumin levels, the pairscans, to test for epistasis, were highly unstable. A number of transformations were tried but did not lead to reliable results. The mode of inheritance was determined by performing an ANOVA on the mean values for the three genotypes.
Figure 1.—
Distribution of the albumin/creatinine ratio (ACR) in cross 1 (A) and cross 2 (B).
RESULTS
Renal parameters in parental and F2 animals:
Table 1 shows the values for the male parental and F2 animals. In each group, the males were more susceptible to albuminuria than the females, which had almost no detectable albuminuria (data not shown) and therefore were not analyzed in the F2. In inbred B6 mice, the presence or absence of Apoe did not affect albuminuria.
TABLE 1.
Renal parameters of male parental and F2 animals from both crosses
| N | Albumin/creatinine (mg/g ± SD) | |
|---|---|---|
| B6 | 4 | 12.5 ± 7.2 |
| B6-Apoe−/− | 18 | 11.6 ± 4.4 |
| A/J | 4 | 118.2 ± 66.4 |
| F2 (wild type) | 381 | 8.9 ± 35.1 |
| F2-Apoe−/− | 145 | 13.2 ± 2.4 |
Nonparametric analyses of albuminuria:
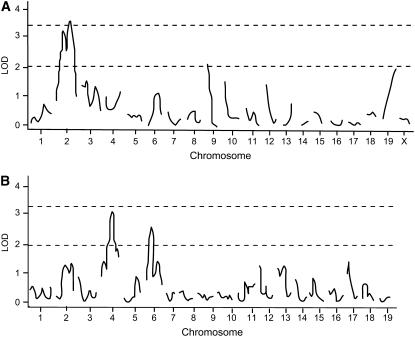
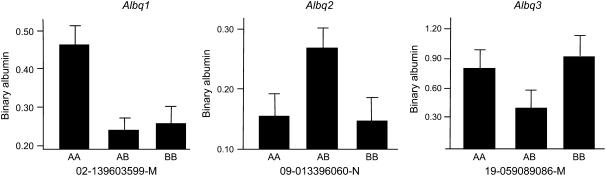
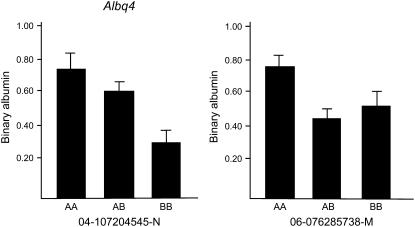
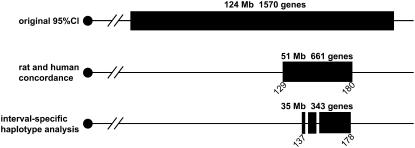
Because most F2 animals did not have detectable urinary albumin concentrations, the trait was not normally distributed (Figure 1). Therefore, we used the presence or absence of detectable urinary albumin as a binary phenotype and performed nonparametric analyses to identify loci involved in the phenotype. An overview of the QTL found in both crosses is given in Table 2, which provides the QTL peak, 95% confidence interval, locus name, LOD score, allele conferring the high value, and nearest SNP marker. The QTL were named if they were significant (LOD 2.97 in cross 1 and LOD 3.35 in cross 2) or if they were suggestive (LOD 1.93 in cross 1 and LOD 2.01 in cross 2) and found previously as homologous QTL in rat crosses. In the analysis of cross 1 we detected three loci (Figure 2A). A significant locus was found on chromosome (Chr) 2 (LOD = 3.5), while two suggestive loci were found on Chr 9 (LOD = 2.0) and Chr 19 (LOD = 1.9). The allele-effect plots of the three loci (Figure 3) show a recessive high allele from A/J at the Chr 2 locus, while for both the Chr 9 and 19 loci the heterozygotes represent the respectively susceptible and resistant phenotype. We found weak statistical evidence for a second QTL on Chr 2 (1-QTL LOD = 3.46 and 2-QTL LOD = 4.95, difference = 1.49, nominal P-value for χ2 = 0.03). However, the tests for linked QTL were underpowered and, although we cannot provide strong evidence to support a second QTL, we cannot rule it out. In the analysis of cross 2 none of these three loci was detected. Instead, two suggestive loci on Chr 4 (LOD = 3.2) and Chr 6 (LOD = 2.6) were found (Figure 2B). For both loci the A/J allele represents the susceptible phenotype but the allele is dominant at the Chr 4 locus and recessive at the Chr 6 locus (Figure 4). We reduced the interval of Albq1 by applying the bioinformatics strategy described by DiPetrillo et al. (2005). First we reduced the 95% C.I. by comparative mapping with the rat and human genome using albuminuria as the kidney disease phenotype in all three species (Figure 5). The locus is concordant with the rat Renal failure 3 (Rf3) locus on rat Chr 3 described by Shiozawa et al. (2000) and a locus on 20p13 associated with albuminuria in humans (Imperatore et al. 1998). This approach reduced the 95% C.I. of the mouse locus from 124 Mb [containing 1570 genes according to the Ensembl database (release 42)], to 51 Mb (661 genes). Second, we used the Jackson Laboratory's SNP databases (http://www.jax.org/phenome) to compare B6 and A/J haplotypes. When we exclude the regions that are identical by descent between the two strains, we can reduce the interval to three small regions with different haplotypes, which are 2, 9, and 24 Mb, respectively. Together, these regions contain 343 genes according to the Ensembl database (release 42, December 2006). As a QTL for albuminuria was previously reported in a backcross between BALB/c and KK/Ta (Shike et al. 2005), we investigated the possiblity of further narrowing the region using SNP data on BALB/cByJ and KK/HiJ. Although this cross confirmed our mapping results, it did not improve resolution.
TABLE 2.
QTL identified for single gene genomewide scans of the two crosses
| Cross | Chra | Peak (cM) | 95% C.I. (cM) | Locus name | LODb | Susc. allele, (inheritance)c | Nearest marker |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 70 | 28–88 | Albq1 | 3.5 | A (rec) | 02-139603599-M |
| 9 | 5 | 5–25 | Albq2 | 2.0 | Het | 09-013396060-N | |
| 19 | 24 | 12–27 | Albq3 | 2.1 | A and B6 | 19-059089086-M | |
| 2 | 4 | 54 | 29–73 | Albq4 | 3.2 | A (dom) | 04-107204545-N |
| 6 | 29 | 11–61 | 2.6 | A (rec) | 06-076285738-M |
Chromosome.
Significance of QTL LOD scores was assessed using 1000 permutations of the phenotypic data (Churchill and Doerge 1994). Significant QTL are reported at the genomewide adjusted 0.05 level (LOD 2.97 in cross 1 and LOD 3.35 in cross 2) and suggestive QTL at genomewide 0.63 level (LOD 1.93 in cross 1 and LOD 2.01 in cross 2) (Lander and Kruglyak 1995).
Susceptible allele and likely mode of inheritance: rec, recessive; dom, dominant.
Figure 2.—
Genomewide scans for albuminuria using nonparametric analysis for (A) cross 1 and (B) cross 2. The dotted line at the top depicts a significant LOD score (P < 0.05) and the dotted line at the bottom a suggestive LOD score (P < 0.63).
Figure 3.—
Allele effects for the loci found in cross 1. Homozygosity for A/J alleles is represented by AA, homozygosity for C57BL/6J alleles by BB, and heterozygosity by AB. The SNP at which the allele effect is determined is shown under each graph.
Figure 4.—
Allele effects for the loci found in cross 2. Homozygosity for A/J alleles is represented by AA, homozygosity for C57BL/6J alleles by BB, and heterozygosity by AB. The SNP at which the allele effect is determined is shown under each graph.
Figure 5.—
Using comparative genomics and interval-specific haplotype analysis to narrow the chromosome 2 QTL. Comparing the C.I. of our cross with the interval on rat Chr 3 found in the (FHH × ACI) F2 intercross and the human chromosome 20 interval narrowed the region to 51 Mb. Interval-specific haplotype analysis of this region, comparing SNPs between B6 and A, resulted in three regions that have different haplotypes between the strains and contain 343 genes according to the Ensembl database (release 42).
DISCUSSION
The gene encoding apolipoprotein E (APOE) has been implicated in chronic kidney disease. Therefore, the aim of this study was to investigate whether the presence or absence of APOE would have an effect on the genes involved in kidney damage in mice. To achieve this, we crossed two strains, B6 and A/J, which are known to be near the extremes of the urinary albumin spectrum for inbred strains (Qi et al. 2005; K. DiPetrillo, unpublished results). We used wild-type B6 and A/J mice, which have identical Apoe alleles according to their genome sequence, for the first cross, whereas we used the B6-Apoe−/− knockout mice for the second cross and selected only the homozygous knockout mice in the F2 population.
Analysis of cross 1 between A/J and B6 mice identified one significant locus and two suggestive loci; in cross 2 between A/J and B6-Apoe−/− mice, these loci did not reach the suggestive level and two different loci were identified. The significant Chr 2 locus maps to the same region as the previously reported albuminuria QTL that was found in a KK/Ta × (BALB/c × KK/Ta) F1 backcross (Shike et al. 2005) with a similar LOD score (3.5). In a review, we previously discussed concordance of renal damage loci between mouse, rat, and human (Korstanje and DiPetrillo 2004). Most of the loci found in the current crosses are concordant with rat loci for renal parameters (Table 3). The Chr 2 region is homologous with the distal part of rat Chr 3 containing QTL for kidney mass and renal function (Shiozawa et al. 2000). The homologous human region (20p13) is also associated with albuminuria (Imperatore et al. 1998). Using this concordance to narrow the interval region by comparative mapping significantly reduces the number of candidate genes (Figure 5). The Chr 9 locus is homologous to a part of rat Chr 8 to which a QTL for urinary albumin excretion was mapped in three independent crosses (Poyan Mehr et al. 2003; Schulz et al. 2003). The QTL mapped to Chr 19 is concordant with the rat Renal failure 1 (Rf1) locus on Chr 1, which was mapped in four independent rat crosses (Brown et al. 1998; Shiozawa et al. 2000; Garrett et al. 2003; Schulz et al. 2003) and is also concordant with a human locus (19q13) affecting renal disease (Freedman et al. 2002) and creatinine clearance (Hunt et al. 2002). Finally, the Chr 4 region found in cross 2 is concordant with QTL for renal function (Moreno et al. 2003), kidney mass (Ueno et al. 2003), and renin concentration (Bilusic et al. 2004) mapped to the homologous region on rat Chr 5. Because we performed the QTL analysis on two independent crosses, there might be some confounders that could lead to differences in the analysis. For this study, confounders such as different mouse rooms (in the same building) and different seasonal effects could influence the results. However, we do not believe that the variability caused by these factors substantially affects the results. Evidence for this comes from comparing published QTL crosses for blood pressure in B × A populations (Sugiyama et al. 2001; Woo and Kurtz 2003). Although the independent crosses were conducted at different institutions under different dietary and environmental conditions—one cross used only males and the other used both sexes, the population sizes differed by more than fourfold, and one cross was a backcross while the other an intercross—the experiments produced similar results. The QTL with the highest LOD scores identified by Woo and Kurtz (2003) were also detected by Sugiyama et al. (2001), using one-quarter the population size. The differences between the crosses in our study were fewer than between these published crosses. Thus, we believe that the presence or absence of Apoe is the primary reason for the different QTL identified in cross 1 vs. cross 2, suggesting that the absence of APOE modifies the QTL involved in albuminuria. The strain that we used has been backcrossed 12 times, leaving a theoretical 0.05% of the 129 genome in the mice. Although chances are small, it would be possible for a 129 allele to contribute to the susceptibility (or resistance) to albuminuria. We do not have data of a cross between A/J and 129 to test this. However, we do have data from a cross between B6 and 129 for albuminuria, in which a QTL was found on Chr18 (S. Sheehan, unpublished results). This suggests that the only difference between B6 and 129 with respect to albuminuria is on Chr 18; we did not observe this QTL in our crosses (Figure 2).
TABLE 3.
Concordance between the mouse urinary albumin QTL and QTL found for related phenotypes in rat and human
| QTL | Chr | Peak (cM) | Concordance in rat | Concordance in human |
|---|---|---|---|---|
| Albq1 | 2 | 70 | Chr 3 for UAE, UPE | Chr 20p13 for ACR |
| Albq2 | 9 | 5 | Chr 8 for UAE, UPE | |
| Albq3 | 19 | 24 | Chr 1 for UAE, UPE | Chr 10q23 for ESRD, CC |
| Albq4 | 4 | 54 | Chr 5 for CC, KM |
UAE, urinary albumin excretion; UPE, urinary protein excretion; ACR, urinary albumin-to-creatinine ratio; ESRD, end-stage renal disease; CC, creatinine clearance; KM, kidney mass.
APOE, a component of very low-density lipoprotein (VLDL) particles as they are secreted from the liver, is acquired by chylomicrons soon after their synthesis and secretion by the small intestine (Mahley and Rall 2000). APOE accomplishes its lipid transport and delivery function mainly through binding to several receptors like the LDL receptor (LDLR), VLDL receptor (VLDLR), and megalin (LRP2). This binding of lipoproteins to the receptors is enhanced by lipoprotein lipase (LPL) (Stevenson et al. 2001). Both the receptors and LPL have been implicated previously in renal damage (Leheste et al. 1999; Sato et al. 2002). In addition to its possible role in the kidney through lipid metabolism, recent data show that APOE also has direct affects on mesangial cell proliferation and extracellular matrix expansion. Mesangial expansion is a key feature in the pathogenesis of renal diseases (Couser and Johnson 1994). Moreover, Apoe knockout mice show increased mesangial cell proliferation and matrix formation compared with wild-type mice or Apob-overexpressing mice, which have elevated plasma cholesterol and triglycerides. This suggests that lack of APOE, rather than hyperlipidemia, contributes to mesangial expansion (Chen et al. 2001). In line with these findings, in vitro experiments show that APOE inhibits mesangial cell proliferation and apoptosis induced by oxidized LDL. In addition, APOE induces the mesangial matrix heparin sulfate proteoglycan (Chen et al. 2001). Loss of anionic heparin sulfate proteoglycan in the basement membrane and mesangial matrix is associated with disruption of the filtration barrier (Tamsma et al. 1994).
Finding one significant QTL in the presence of Apoe and only two (different) suggestive QTL in the absence of Apoe suggests that the underlying gene of the Chr 2 QTL is dependent on Apoe to cause increased albumin/creatine ratio (ACR). When APOE is absent, this dependency gets lost and the Chr 2 locus no longer contributes to the difference in susceptibility. Instead, ACR is determined by other (Apoe-independent) loci.
In conclusion, we detected five loci using nonparametric analyses for albuminuria in a cross between A/J and either B6 or B6-Apoe−/− mice. However, the loci detected depend on the presence or absence of APOE. Only one of the loci (Chr 2) was significant, but all loci, except for the locus on Chr 6, are concordant with QTL for renal damage in rat, and two of the loci (Chr 2 and Chr 19) are concordant with associations found in human. The dependence on APOE suggests the involvement of this pathway in renal disease.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK-69381 and National Institute of General Medical Sciences grant GM-070683.
References
- Araki, S., D. K. Moczulski, L. Hanna, L. J. Scott, J. H. Warram et al., 2000. APOE polymorphisms and the development of diabetic nephropathy in type 1 diabetes: results of case-control and family-based studies. Diabetes 49 2190–2195. [DOI] [PubMed] [Google Scholar]
- Bilusic, M., A. Bataillard, M. R. Tschannen, L. Gao, N. E. Barreto et al., 2004. Mapping the genetic determinants of hypertension, metabolic diseases, and related phenotypes in the lyon hypertensive rat. Hypertension 44 695–701. [DOI] [PubMed] [Google Scholar]
- Broman, K. W., 2003. Mapping quantitative trait loci in the case of a spike in the phenotype distribution. Genetics 163 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman, K. W., H. Wu, S. Sen and G. A. Churchill, 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19 889–890. [DOI] [PubMed] [Google Scholar]
- Brown, D. M., R. P. Van Dokkum, M. R. Korte, M. G. McLauglin, M. Shiozawa et al., 1998. Genetic control of susceptibility for renal damage in hypertensive fawn-hooded rats. Ren. Fail. 20 407–411. [DOI] [PubMed] [Google Scholar]
- Buzello, M., C. S. Haas, F. Hauptmann, M. L. Gross, J. Faulhaber et al., 2004. No aggravation of renal injury in apolipoprotein E knockout mice (ApoE(−/−)) after subtotal nephrectomy. Nephrol. Dial. Transplant. 19 566–573. [DOI] [PubMed] [Google Scholar]
- Chen, G., L. Paka, Y. Kako, P. Singhal, W. Duan et al., 2001. A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J. Biol. Chem. 276 49142–49147. [DOI] [PubMed] [Google Scholar]
- Churchill, G. A., and R. W. Doerge, 1994. Empirical threshold values for quantitative trait mapping. Genetics 138 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couser, W. G., and R. J. Johnson, 1994. Mechanisms of progressive renal disease in glomerulonephritis. Am. J. Kidney Dis. 23 193–198. [DOI] [PubMed] [Google Scholar]
- DiPetrillo, K., X. Wang, I. M. Stylianou and B. Paigen, 2005. Bioinformatics toolbox for narrowing rodent quantitative trait loci. Trends Genet. 21 683–692. [DOI] [PubMed] [Google Scholar]
- Freedman, B. I., S. S. Rich, H. Yu, B. H. Roh and D. W. Bowden, 2002. Linkage heterogeneity of end-stage renal disease on human chromosome 10. Kidney Int. 62 770–774. [DOI] [PubMed] [Google Scholar]
- Garrett, M. R., H. Dene and J. P. Rapp, 2003. Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J. Am. Soc. Nephrol. 14 1175–1187. [DOI] [PubMed] [Google Scholar]
- Grindle, S., C. Garganta, S. Sheehan, J. Gile, A. Lapierre et al., 2006. Validation of high-throughput methods for measuring blood urea nitrogen and urinary albumin concentrations in mice. Comp. Med. 56 482–486. [PubMed] [Google Scholar]
- Hillebrandt, S., H. E. Wasmuth, R. Weiskirchen, C. Hellerbrand, H. Keppeler et al., 2005. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat. Genet. 37 835–843. [DOI] [PubMed] [Google Scholar]
- Hunt, S. C., S. J. Hasstedt, H. Coon, N. J. Camp, R. M. Cawthon et al., 2002. Linkage of creatinine clearance to chromosome 10 in Utah pedigrees replicates a locus for end-stage renal disease in humans and renal failure in the fawn-hooded rat. Kidney Int. 62 1143–1148. [DOI] [PubMed] [Google Scholar]
- Imperatore, G., R. L. Hanson, D. J. Pettitt, S. Kobes, P. H. Bennett et al., 1998. Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes 47 821–830. [DOI] [PubMed] [Google Scholar]
- Joss, N., A. Jardine, D. Gaffney and J. M. Boulton-Jones, 2005. Influence of apolipoprotein E genotype on progression of diabetic nephropathy. Nephron. Exp. Nephrol. 101 e127–e133. [DOI] [PubMed] [Google Scholar]
- Korstanje, R., and K. DiPetrillo, 2004. Unraveling the genetics of chronic kidney disease using animal models. Am. J. Physiol. Renal. Physiol. 287 F347–F352. [DOI] [PubMed] [Google Scholar]
- Korstanje, R., P. Eriksson, A. Samnegard, P. G. Olsson, K. Forsman-Semb et al., 2004. a Locating Ath8, a locus for murine atherosclerosis susceptibility and testing several of its candidate genes in mice and humans. Atherosclerosis 177 443–450. [DOI] [PubMed] [Google Scholar]
- Korstanje, R., R. Li, T. Howard, P. Kelmenson, J. Marshall et al., 2004. b Influence of sex and diet on quantitative trait loci for HDL cholesterol levels in an SM/J by NZB/BlNJ intercross population. J. Lipid Res. 45 881–888. [DOI] [PubMed] [Google Scholar]
- Kruglyak, L., and E. S. Lander, 1995. A nonparametric approach for mapping quantitative trait loci. Genetics 139 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander, E., and L. Kruglyak, 1995. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat. Genet. 11 241–247. [DOI] [PubMed] [Google Scholar]
- Leheste, J. R., B. Rolinski, H. Vorum, J. Hilpert, A. Nykjaer et al., 1999. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 155 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley, R. W., and S. C. Rall, Jr., 2000. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1 507–537. [DOI] [PubMed] [Google Scholar]
- Moreno, C., P. Dumas, M. L. Kaldunski, P. J. Tonellato, A. S. Greene et al., 2003. Genomic map of cardiovascular phenotypes of hypertension in female Dahl S rats. Physiol. Genomics 15 243–257. [DOI] [PubMed] [Google Scholar]
- Poyan Mehr, A., A. K. Siegel, P. Kossmehl, A. Schulz, R. Plehm et al., 2003. Early onset albuminuria in Dahl rats is a polygenetic trait that is independent from salt loading. Physiol. Genomics 14 209–216. [DOI] [PubMed] [Google Scholar]
- Qi, Z., H. Fujita, J. Jin, L. S. Davis, Y. Wang et al., 2005. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54 2628–2637. [DOI] [PubMed] [Google Scholar]
- Sato, T., K. Liang and N. D. Vaziri, 2002. Down-regulation of lipoprotein lipase and VLDL receptor in rats with focal glomerulosclerosis. Kidney Int. 61 157–162. [DOI] [PubMed] [Google Scholar]
- Schulz, A., D. Standke, L. Kovacevic, M. Mostler, P. Kossmehl et al., 2003. A major gene locus links early onset albuminuria with renal interstitial fibrosis in the MWF rat with polygenetic albuminuria. J. Am. Soc. Nephrol. 14 3081–3089. [DOI] [PubMed] [Google Scholar]
- Shike, T., T. Gohda, M. Tanimoto, M. Kobayashi, Y. Makita et al., 2005. Chromosomal mapping of a quantitative trait locus for the development of albuminuria in diabetic KK/Ta mice. Nephrol. Dial. Transplant. 20 879–885. [DOI] [PubMed] [Google Scholar]
- Shiozawa, M., A. P. Provoost, R. P. van Dokkum, R. R. Majewski and H. J. Jacob, 2000. Evidence of gene-gene interactions in the genetic susceptibility to renal impairment after unilateral nephrectomy. J. Am. Soc. Nephrol. 11 2068–2078. [DOI] [PubMed] [Google Scholar]
- Stevenson, F. T., G. C. Shearer and D. N. Atkinson, 2001. Lipoprotein-stimulated mesangial cell proliferation and gene expression are regulated by lipoprotein lipase. Kidney Int. 59 2062–2068. [DOI] [PubMed] [Google Scholar]
- Stoll, M., A. E. Kwitek-Black, A. W. Cowley, Jr., E. L. Harris, S. B. Harrap et al., 2000. New target regions for human hypertension via comparative genomics. Genome Res. 10 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama, F., G. A. Churchill, D. C. Higgins, C. Johns, K. P. Makaritsis et al., 2001. Concordance of murine quantitative trait loci for salt-induced hypertension with rat and human loci. Genomics 71 70–77. [DOI] [PubMed] [Google Scholar]
- Tamsma, J. T., J. van den Born, J. A. Bruijn, K. J. Assmann, J. J. Weening et al., 1994. Expression of glomerular extracellular matrix components in human diabetic nephropathy: decrease of heparan sulphate in the glomerular basement membrane. Diabetologia 37 313–320. [DOI] [PubMed] [Google Scholar]
- Ueno, T., J. Tremblay, J. Kunes, J. Zicha, Z. Dobesova et al., 2003. Gender-specific genetic determinants of blood pressure and organ weight: pharmacogenetic approach. Physiol. Res. 52 689–700. [PubMed] [Google Scholar]
- Wang, X., and B. Paigen, 2002. a Comparative genetics of atherosclerosis and restenosis: exploration with mouse models. Arterioscler. Thromb. Vasc. Biol. 22 884–886. [DOI] [PubMed] [Google Scholar]
- Wang, X., and B. Paigen, 2002. b Quantitative trait loci and candidate genes regulating HDL cholesterol: a murine chromosome map. Arterioscler. Thromb. Vasc. Biol. 22 1390–1401. [DOI] [PubMed] [Google Scholar]
- Wen, M., S. Segerer, M. Dantas, P. A. Brown, K. L. Hudkins et al., 2002. Renal injury in apolipoprotein E-deficient mice. Lab. Invest. 82 999–1006. [DOI] [PubMed] [Google Scholar]
- Werle, E., W. Fiehn and C. Hasslacher, 1998. Apolipoprotein E polymorphism and renal function in German type 1 and type 2 diabetic patients. Diabetes Care 21 994–998. [DOI] [PubMed] [Google Scholar]
- Woo, D. D., and I. Kurtz, 2003. Mapping blood pressure loci in (A/J x B6)F2 mice. Physiol. Genomics 15 236–242. [DOI] [PubMed] [Google Scholar]