Abstract
A general model for the early recognition and colocalization of homologous DNA sequences is proposed. We show, on thermodynamic grounds, how the distance between two homologous DNA sequences is spontaneously regulated by the concentration and affinity of diffusible mediators binding them, which act as a switch between two phases corresponding to independence or colocalization of pairing regions.
CHROMOSOME recognition and pairing are general features of nuclear organization. In particular, these phenomena have a prominent role (and are comparatively better studied) in meiosis, the specialized cell division necessary for the production of haploid gametes from diploid nuclei. During the prophase of the first meiotic division, homologous chromosomes identify each other and pair via a still mysterious long-distance reciprocal recognition process (Zickler and Kleckner 1998; Gerton and Hawley 2005; Zickler 2006).
Many hypotheses exist on the mechanisms underlying the early stages of coalignment of homologs along their length (see references in Zickler and Kleckner 1998; Gerton and Hawley 2005; Zickler 2006). A longstanding idea is that pairing may occur via unstable interactions, such as a direct physical contact between DNA duplexes (the “kissing model”; see, e.g., Kleckner and Weiner 1993). Pairing initially based on nonpermanent interactions has the important advantage of preventing ectopic association between nonhomologous chromosomes and avoiding topologically unacceptable entanglements, leaving space for adjustments (Kleckner and Weiner 1993). Several mechanisms could contribute to the outcome of the process, e.g., constrained motion of chromosomes in territories, bouquet formation at telomeres, and tethering to the nuclear envelope. While chromosome full alignment includes several stages, the early physical contact and colocalization could be driven by specific chromosomal regions bridged by molecular mediators. In this complex scenario, though, the crucial question on the mechanical origin of early recognition and pairing remains unexplained.
Here we explore the thermodynamic properties of a recognition/pairing mechanism based on weak, biochemically unstable interactions between specific DNA sequences and molecular mediators binding them. We show that randomly diffusing molecules can produce a long-distance interaction mechanism whereby homologous sequences spontaneously recognize and become tethered to each other. This colocalization mechanism is tunable by two “thermodynamic switches,” namely the concentration of molecular mediators and their affinity for their binding sites. When threshold values in the concentration, or affinity, of mediators are exceeded, homologous sequences are joined together; otherwise they move independently.
MODEL
Our model includes (see Figure 1) two homolog segments involved in mutual recognition and pairing, described as self-avoiding bead chains, a well-established model of polymer physics (Doi and Edwards 1984), and a concentration, c, of Brownian molecular factors having a chemical affinity, EX, for them. We investigate the thermodynamic properties of the system by Monte Carlo (MC) computer simulations (Binder 1997). For computational purposes, chromosomal segments and molecules are placed in a volume consisting of a cubic lattice with spacing d0 (our space unit, of the order of the molecular factors length) and linear sizes Lx = 2L, Ly = L, and Lz = L (see Figure 1). In each simulation, the “beads” of the chromosomal segments start from a straight, vertical line configuration, at a distance L from each other, and molecular mediators from a random initial distribution. Diffusing molecules randomly move from one to a nearest-neighbor vertex on the lattice. On each vertex no more than one particle can be present at a given time. The chromosomal segments diffuse as well on such a lattice, performing a Brownian motion under the constraint that two proximal beads on the string must be within a distance 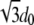 from each other (i.e., on next or nearest-next neighboring sites on the lattice). For the sake of simplicity, we disregard here the rest of the chromosomes and DNA segment ends are constrained to move tethered to the bottom and top planes of the system volume (Figure 1). When neighboring a chromosomal chain, molecules interact with it via a binding energy EX. Below, we discuss mainly the case where EX is of the order of a “weak” hydrogen bond-like energy, say 3 kJ/mol, which at room temperature corresponds to EX = 1.2kT (Watson et al. 2003). In our simulations, at each time unit (corresponding to a MC lattice sweep) the probability of a particle moving to a neighboring empty site is proportional to the Arrhenius factor r0 exp(−ΔE/kT), where ΔE is the energy barrier in the move, k the Boltzmann constant, and T the temperature (Stanley 1971; Watson et al. 2003). The factor r0 is the reaction kinetic rate, depending on the nature of the molecular factors and of the surrounding viscous fluid, and sets the timescale. We employ r0 = 30 sec−1, a typical value in biochemical kinetics. Averages are over up to 2048 runs from different initial configurations.
from each other (i.e., on next or nearest-next neighboring sites on the lattice). For the sake of simplicity, we disregard here the rest of the chromosomes and DNA segment ends are constrained to move tethered to the bottom and top planes of the system volume (Figure 1). When neighboring a chromosomal chain, molecules interact with it via a binding energy EX. Below, we discuss mainly the case where EX is of the order of a “weak” hydrogen bond-like energy, say 3 kJ/mol, which at room temperature corresponds to EX = 1.2kT (Watson et al. 2003). In our simulations, at each time unit (corresponding to a MC lattice sweep) the probability of a particle moving to a neighboring empty site is proportional to the Arrhenius factor r0 exp(−ΔE/kT), where ΔE is the energy barrier in the move, k the Boltzmann constant, and T the temperature (Stanley 1971; Watson et al. 2003). The factor r0 is the reaction kinetic rate, depending on the nature of the molecular factors and of the surrounding viscous fluid, and sets the timescale. We employ r0 = 30 sec−1, a typical value in biochemical kinetics. Averages are over up to 2048 runs from different initial configurations.
Figure 1.—
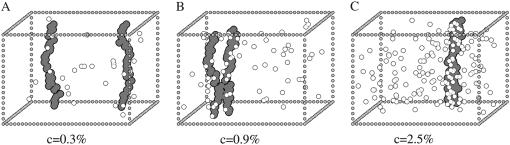
Pictures of typical configurations, from computer simulations, of the model system at thermodynamic equilibrium, in the two described phases discussed in Figure 2 (A, independent motion; C, colocalization) and their intermediate crossover region (B), for the shown values of the concentration of molecular mediators, c (here EX = 1.2kT).
RESULTS
First we show how the interaction of chromosomes with molecular mediators drives colocalization. To this aim, we calculated the thermodynamic equilibrium value of the average square distance (relative to the system linear size L) between the two chromosomal segments,
 |
(1) |
where N is the number of beads in each string (here N = L) and 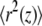 is the average (over MC simulations) of the square distance of the beads at “height” z. The average value of d2 is maximal when the two “chromosomes” float independently and decreases if parts of the polymers become colocalized, approaching zero when a perfect alignment is attained.
is the average (over MC simulations) of the square distance of the beads at “height” z. The average value of d2 is maximal when the two “chromosomes” float independently and decreases if parts of the polymers become colocalized, approaching zero when a perfect alignment is attained.
The equilibrium distance, d2, depends on the concentration, c, of mediators. At low concentration (see Figure 2; e.g., c < c1) d2 has a value of the order of the system size (∼40% of L2), corresponding to the expected average distance of two independent strings undergoing Brownian motion in a box of size L; a typical configuration for c = 0.3% is shown in Figure 1A). Indeed, the physical basis for the independence of chromosomes exposed to a low concentration of mediating molecules is intuitive: pairing can occur when bridges are formed by molecules attached to couples of binding sites. A single bridging event, however, can be statistically quite unlikely since weak bonds are biochemically unstable and to form a bridge a diffusing molecule must first find (and bind) a site on one chromosome and then together they have to successfully encounter the second one.
Figure 2.—
(Top) The equilibrium chromosome average square distance, d2, is shown as a function of the concentration of binding molecules, c (here the molecule/chromosome affinity is EX = 1.2kT): for  , d2 approaches values as big as the system size and chromosomes are randomly and independently diffusing (horizontal dotted lines give the values found for pure random walks); for c > ctr, d2 rapidly decays to zero, showing that they have colocalized. Around ctr there is a crossover regime, approximately between c1 and c2, where chromosomes tend to align since d2 is smaller than in the region where they move independently, but its fluctuations, Δd2, are of the order of d2; here chromosomes are only transiently colocalizing. (Bottom) A similar behavior is found when d2 is plotted as a function of the chemical affinity, EX, shown here for c = 0.1%.
, d2 approaches values as big as the system size and chromosomes are randomly and independently diffusing (horizontal dotted lines give the values found for pure random walks); for c > ctr, d2 rapidly decays to zero, showing that they have colocalized. Around ctr there is a crossover regime, approximately between c1 and c2, where chromosomes tend to align since d2 is smaller than in the region where they move independently, but its fluctuations, Δd2, are of the order of d2; here chromosomes are only transiently colocalizing. (Bottom) A similar behavior is found when d2 is plotted as a function of the chemical affinity, EX, shown here for c = 0.1%.
Figure 2 shows, however, that when c is higher than a threshold value, ctr (for EX = 1.2kT, 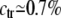 ), d2 collapses to zero: this is the sign that the two chromosomes have colocalized; a typical picture of the system state, for c = 2.5%, is shown in Figure 1C. Actually, when c is high enough, chances increase to form multiple bridges and, as they reinforce each other, configurations where molecules hold together the two polymers become stabilized. The threshold concentration value, ctr, corresponds to the point where such a positive mechanism becomes winning and can be approximately defined by the inflection point of the curve d2(c). Similar to phase transitions in finite-size systems (Stanley 1971; Binder 1997) (see below), around ctr there is a crossover region that can be located, for instance, between the concentrations c1 and c2 (see Figure 2) defined by the criterion that d2 is close within 5% to the random or zero plateau value (for EX = 1.2kT,
), d2 collapses to zero: this is the sign that the two chromosomes have colocalized; a typical picture of the system state, for c = 2.5%, is shown in Figure 1C. Actually, when c is high enough, chances increase to form multiple bridges and, as they reinforce each other, configurations where molecules hold together the two polymers become stabilized. The threshold concentration value, ctr, corresponds to the point where such a positive mechanism becomes winning and can be approximately defined by the inflection point of the curve d2(c). Similar to phase transitions in finite-size systems (Stanley 1971; Binder 1997) (see below), around ctr there is a crossover region that can be located, for instance, between the concentrations c1 and c2 (see Figure 2) defined by the criterion that d2 is close within 5% to the random or zero plateau value (for EX = 1.2kT, 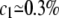 and
and 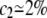 ).
).
In Figure 2, along with the distance between chromosomes, d2, we plot the squared fluctuations of the distance (i.e., its statistical variance), Δd2(c), as a function of the concentration of mediators. For c < c1, both d2(c) and Δd2(c) have the nonzero value found for noninteracting Brownian strings in the independent diffusion regime (Δd2 ∼ 30%); instead, Δd2(c) = 0 for c > c2 in the tight colocalization regime. Interestingly, in the crossover region, d2(c) is smaller than in the purely random regime, although it has marked fluctuations [Δd2(c) can be even >d2(c)]. This situation is illustrated by a picture of a typical configuration, for c = 0.9%, shown in Figure 1B. In such an intermediate regime chromosome couples are continuously formed and disrupted.
Summarizing, our results show that colocalization is spontaneously induced by the “collective” binding of molecular mediators and occurs only when c is above a critical value, ctr, i.e., in the “colocalization phase.” Conversely, when c < ctr, d2(c) has the same value found for two noninteracting Brownian strings. This is the “random phase,” where chromosomes are independent. The concentration of mediators acts as a switch between the two phases, while around the critical threshold chromosomes undergo transient interactions.
A similar effect is found when, for a given (high enough) concentration, c, the chemical affinity, EX, of binding sites is changed (see Figure 2, bottom): when EX is smaller than a threshold value, Etr, the two polymers float independently one from the other. Around Etr a crossover region is found, and as soon as EX > Etr, an effective attraction between polymers is established and they are spontaneously colocalized. Another potential layer of regulation of the system is the number of binding sites for molecular mediators. In fact, a reduction in the number of binding sites produces the same effect as a reduction in the affinity of mediators; that is, chromosomes become unable to find and bind each other.
The pairing mechanisms illustrated above have a thermodynamics origin. It is a “phase transition” (Stanley 1971) occurring when entropy loss due to polymer colocalization is compensated by particle energy gain as they bind both polymers, the lower the EX, the higher the concentration, c, required. Actually, the transition is found in a broad region of the (EX, c) plane, as shown in Figure 3 where the system phase diagram is plotted in a range of typical biochemical values of weak binding energies EX. For very low values of EX the colocalization can be, instead, impossible. The overall properties of such a phase diagram (independent vs. colocalized chromosomes) are robust to changes in the model details, although the precise location of the different phases can be affected (Stanley 1971). Summarizing, when soluble mediators bind a specific recognition sequence on homologous chromosomes, recognition and colocalization of homologs can occur, as a result of a robust and general thermodynamic phenomenon, namely a phase transition occurring in the system. The higher the affinity of mediators for chromosomal binding sites, the lower is the threshold concentration of mediators that promotes colocalization (see Figure 3).
Figure 3.—
This phase diagram shows the state of the two chromosomes at thermodynamic equilibrium in a range of values of chemical affinity and concentration of their molecular mediators, i.e., in the (EX, c) plane. For small EX and c, chromosomes move independently while, above a transition region, they spontaneously colocalize. The transition line, ctr(EX), is marked by the thick black line. Colocalization, thus, can be spontaneously attained by upregulation of mediator concentration, c, or of molecule chemical affinity, EX, to chromosomal sequences.
DISCUSSION
We described a general colocalization mechanism, grounded in thermodynamics, whereby specific regions of a pair of chromosomes can spontaneously recognize each other and align. Physical juxtaposition is mediated by sequence-specific molecular factors that bind DNA via weak, nonpermanent, biochemical interactions. When the concentration/affinity of molecular mediators is above a critical threshold, an effective attraction between their binding regions is generated, leading to a close alignment; otherwise chromosomes float away from each other by Brownian motion. In the threshold crossover region, pairing sites undergo transient interactions: the average distance is shorter than in the purely random regime, but marked fluctuations are observed.
In our simulations, the two homologous pairing regions are described as polymers diffusing with their ends tethered to the upper and lower planes of the system box. This recalls telomeres tethering to the nuclear envelope observed at meiosis. While it is not a prerequisite for the switch mechanism, on the other hand, it can enhance the switch effects (Zickler and Kleckner 1998; Gerton and Hawley 2005; Zickler 2006). Releasing such a constraint does not change the general results, but pairing regions would collapse in a more disordered geometry. The overall properties of the phase diagram (independent vs. colocalized chromosomes) are robust to changes in the model details (M. Nicodemi and A. Prisco, unpublished results). A model including many pairs of chromosomes has longer equilibration times, as expected in a crowded environment, yet its phase diagram is unchanged. The scenario is also unaltered in the case of mediators that interact with each other and aggregate.
An implication of this model is that a cell can regulate the initiation of homologous chromosome interaction by upregulating the concentration of mediators or their affinity for DNA sites (e.g., through changes in the chromatin or by a chemical modification of the mediator). This switch has general and robust roots in a thermodynamics phase transition (Stanley 1971), irrespective of the ultimate molecular and biochemical basis. In real cells, specific short chromosomal regions (“pairing centers”) could mediate the early steps of homolog recognition and act as a seed and a reference point to a subsequent stable long-scale chromosomal pairing, which could involve additional mechanisms. A speculation is that the threshold effect can be exploited to ensure a precise control of pairing formation/release, while the presence of a crossover region in concentration to reduce undesired entanglements. The initial binding molecules could, in turn, help the sequences in recruiting complexes used later for other purposes (e.g., in pairing stabilization, synapsis, and recombination).
In the present model individual mediators do not need to be strongly binding to glue homologous chromosomes together, and any molecules with above-threshold affinity can induce attraction. Specificity of colocalization among many chromosome pairs could be, indeed, obtained by sets of molecules binding, with higher affinities, specific homologous sequences. While the molecular mediators considered here are supposed to have more than one “DNA-binding domain,” proteins that can bind a single DNA site, but are able to make protein–protein interactions, could also mediate colocalization. As a pair of linked proteins is, in fact, a single molecular mediator, the thermodynamics picture is unchanged. Finally, direct DNA duplex interactions (Kleckner and Weiner 1993) could replace, or help, binding molecules. A duplex kissing site would correspond in our model to a binding site with a molecular mediator already attached, so the overall behavior should be similar.
Experimental discoveries on meiotic pairing have accomplished huge progress, but the mechanisms for homolog early coalignment are still unclear (Zickler and Kleckner 1998; Gerton and Hawley 2005; Zickler 2006). In Caenorhabditis elegans, for instance, homologs' proper pairing is primarily regulated by special telomeric regions, known as pairing centers (PCs) (McKim et al. 1988; Villeneuve 1994; MacQueen et al. 2005). Homologous PCs interact, during early prophase, with HIM/ZIM Zn-finger proteins that are necessary to mediate pairing (Phillips et al. 2005; Phillips and Dernburg 2006). Specific sites and proteins are also involved in meiotic pairing of Drosophila. In males, on the X and Y chromosomes, a 240-bp repeated sequence in the intergenic spacer of rDNA acts as a pairing center, and autosomes pair, as well, by the interaction of a number of sites (see references in Gerton and Hawley 2005; Zickler 2006). A similar behavior is observed in Drosophila females (Hawley et al. 1992; Dernburg et al. 1996). In Drosophila males, special proteins, SNM and MNM, have been also discovered that bind X–Y and autosomal pairing sites at prophase I and are required for pairing (Thomas et al. 2005). The question is open whether the present model applies to such an experimental scenario. In a picture where pairing is mediated by unstable interactions, thermodynamics dictates, anyway, a precise framework showing that minimal “ingredients,” such as soluble DNA-binding molecules and homologous arrays of binding sites, can in fact be sufficient for pairing if the balance of mediator concentration and DNA affinity is appropriate.
Our thermodynamic-switch theory is prone to experimental tests (e.g., the existence of threshold effects in mediator concentration, c). It can be exploited, as well, for a quantitative understanding of the effects on pairing, e.g., of deletions (which can be modeled here by reducing the binding-site number within L), or of chemical modifications of binding sequences (modeled by changes in EX), and to guide the search for candidates for chromosomal sites and interaction mediators. Finally, the general message of the model may be applicable to various cellular processes that involve the spatial reorganization of DNA in nuclear space (e.g., organization of chromosomal loci and territories, juxtaposition of DNA sequences in transcriptional regulation, somatic pairing, and pairing of X chromosomes at the onset of X inactivation; Zickler and Kleckner 1998; Gerton and Hawley 2005; Bacher et al. 2006; Xu et al. 2006; Zickler 2006; Lanctôt et al. 2007; Misteli 2007; Nicodemi and Prisco 2007a,b).
Acknowledgments
We thank N. Kleckner and A. Storlazzi for very helpful discussions and critical reading of the manuscript.
References
- Bacher, C. P., M. Guggiari, B. Brors, S. Augui, P. Clerc et al., 2006. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat. Cell Biol. 8 293–299. [DOI] [PubMed] [Google Scholar]
- Binder, K., 1997. Applications of Monte Carlo methods to statistical physics. Rep. Prog. Phys. 60 487. [Google Scholar]
- Dernburg, A. F., J. W. Sedat and R. S. Hawley, 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86 135–146. [DOI] [PubMed] [Google Scholar]
- Doi, M., and S. F. Edwards, 1984. The Theory of Polymer Dynamics. Clarendon Press, Oxford.
- Gerton, J. L., and R. S. Hawley, 2005. Homologous chromosome interactions in meiosis: diversity amidst conservation. Nat. Rev. Genet. 6 477–487. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., H. Irick, A. E. Zitron, D. A. Haddox, A. Lohe et al., 1992. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13 440–467. [DOI] [PubMed] [Google Scholar]
- Kleckner, N., and B. M. Weiner, 1993. Potential advantages of unstable interactions for pairing of chromosomes in meiotic, somatic, and premeiotic Cells. Cold Spring Harbor Symp. Quant. Biol. LVIII 553–565. [DOI] [PubMed] [Google Scholar]
- Lanctôt, C., T. Cheutin, M. Cremer, G. Cavalli and T. Cremer, 2007. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat. Rev. Genet. 8 104–115. [DOI] [PubMed] [Google Scholar]
- MacQueen, A. J., C. M. Phillips, N. Bhalla, A. M. Villeneuve and A. F. Dernburg, 2005. Chromosome sites play dual roles to establish homologous synapsis during meiosis in C. elegans. Cell 123 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim, K. S., A. M. Howell and A. M. Rose, 1988. The effects of translocations on recombination frequency in Caenorhabditis elegans. Genetics 120 987–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli, T, 2007. Beyond the sequence: cellular organization of genome function. Cell 128 787–800. [DOI] [PubMed] [Google Scholar]
- Nicodemi, M., and A. Prisco, 2007. a A symmetry breaking model for X chromosome inactivation. Phys. Rev. Lett. 98 108–104. [DOI] [PubMed] [Google Scholar]
- Nicodemi, M., and A. Prisco, 2007. b Self-assembly and DNA binding of the blocking factor in X chromosome inactivation. PLoS Comp. Biol. 3 2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, C. M., and A. F. Dernburg, 2006. A family of zinc-finger proteins is required for chromosome-specific pairing and synapsis during meiosis in C. elegans. Dev. Cell 11 817–829. [DOI] [PubMed] [Google Scholar]
- Phillips, C. M., C. Wong, N. Bhalla, P. M. Carlton, P. Weiser et al., 2005. HIM-8 binds to the X chromosome pairing center and mediates chromosome-specific meiotic synapsis. Cell 123 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, H.E., 1971. Introduction to Phase Transitions and Critical Phenomena. Clarendon Press, Oxford.
- Thomas, S. E., M. Soltani-Bejnood, P. Roth, R. Dorn, J. M. Logsdon, Jr. et al., 2005. Identification of two proteins required for conjunction and regular segregation of achiasmate homologs in Drosophila male meiosis. Cell 123 555–568. [DOI] [PubMed] [Google Scholar]
- Villeneuve, A. M., 1994. A cis-acting locus that promotes crossing over between X chromosomes in Caenorhabditis elegans. Genetics 136 887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, J. D., T. A. Baker, S. P. Bell, A. Gann, M. Levine et al., 2003. Molecular Biology of the Gene. Benjamin-Cummings, Menlo Park, CA.
- Xu, N., C.-L. Tsai and J. T. Lee, 2006. Transient homologous chromosome pairing marks the onset of X inactivation. Science 311 1149–1152. [DOI] [PubMed] [Google Scholar]
- Zickler, D., 2006. From early homologue recognition to synaptonemal complex formation. Chromosoma 115 158–174. [DOI] [PubMed] [Google Scholar]
- Zickler, D., and N. Kleckner, 1998. The leptotene-zygotene transition of meiosis. Annu. Rev. Genet. 32 619–697. [DOI] [PubMed] [Google Scholar]