Abstract
Carotenoids are responsible for much of the yellow, orange and red pigmentation in the animal kingdom, and the importance of such coloration as an honest signal of individual quality has received widespread attention. In particular, owing to the multiple roles of carotenoids as pigments, antioxidants and immunostimulants, carotenoid-based coloration has been suggested to advertise an individual's antioxidant or immune defence capacity. However, it has recently been argued that carotenoid-based signals may in fact be advertising the availability of different antioxidants, many of which (including various vitamins, antioxidant enzymes and minerals) are colourless and so would be uninformative as components of a visual signal, yet often have greater biological activity than carotenoids. We tested this hypothesis by feeding male sticklebacks (Gasterosteus aculeatus) a diet containing a fixed level of carotenoids and either low or high, but biologically realistic levels of the colourless antioxidant vitamins C and E. High-antioxidant diet males produced significantly more intensely coloured (but not larger) carotenoid-based regions of nuptial coloration and were preferred over size-matched males of the opposite diet treatment in mate-choice trials. Furthermore, there were positive correlations between an individual's somatic antioxidant activity and signal intensity. Our data suggest that carotenoid-based ornaments may honestly signal an individual's availability of non-carotenoid antioxidants, allowing females to make adaptive mate-choice decisions.
Keywords: α-tocopherol, ascorbic acid, oxidative stress, sexual signalling
1. Introduction
Carotenoids form the basis for much of the yellow, orange and red sexual ornamentation in the animal kingdom (Goodwin 1984; Olsen & Owens 1998). However, this is not their only function. They also act as antioxidants by inactivating reactive oxygen species (ROS), thereby protecting tissues from oxidative damage (Krinsky & Yeum 2003) and can stimulate various facets of the immune system (Blount et al. 2003; Faivre et al. 2003; Grether et al. 2004; McGraw & Ardia 2004). Because carotenoids must be acquired from the diet (Hill 1991) and may be destroyed when used as antioxidants or immunostimulants, it has been suggested that they may be in limited supply for use in sexual coloration (reviews in Lozano 1994; Olsen & Owens 1998). Therefore, the quantity of carotenoids allocated to sexual ornaments (and hence the strength of sexual coloration) may signal both an individual's foraging efficiency (Hill 1991) and the extent to which it can afford to divert resources away from its antioxidant and immune defences (Lozano 1994; von Schantz et al. 1999).
However, there are a number of other substances with high levels of antioxidant activity commonly found in animals, including certain vitamins, antioxidant enzymes and minerals (Prior & Cao 1999; Fang et al. 2002; McGraw 2005). In contrast to carotenoids, these antioxidants are colourless, and therefore cannot contribute to visual displays. Nonetheless, they are probably much more important as biological protectants against ROS than carotenoids, and so their presence would indicate that an individual has good antioxidant defences (e.g. Halliwell 1996). This has led to the suggestion that rather than signalling the availability of carotenoids alone, carotenoid-based displays may reveal a more general availability of antioxidant defences (von Schantz et al. 1999; Blount et al. 2000; Hartley & Kennedy 2004). An implicit assumption of this hypothesis is that increasing the availability of non-pigmentary antioxidants should enhance the expression of carotenoid-based sexual traits.
We tested this prediction experimentally by feeding three-spined male sticklebacks (Gasterosteus aculeatus) with food containing a fixed concentration of carotenoids and either high or low, but biologically realistic, levels of other (colourless) antioxidants. During the breeding season, male sticklebacks develop a region of intense red nuptial coloration, based on carotenoids that can be limiting in the diet (Wedekind et al. 1998), and in mate-choice trials females generally prefer redder males (e.g. Milinski & Bakker 1990). Males with higher body levels of oxidative damage tend to have less colourful red patches (Pike et al. 2007), suggesting a trade-off between allocation of carotenoids to sexual signalling and somatic maintenance. We would therefore expect supplementation with additional antioxidants to enhance a male's antioxidant defences, allowing him to signal this through the development of a more carotenoid-rich (and hence more intensely coloured) signal.
2. Material and methods
Juvenile three-spined sticklebacks were captured with dip nets from the River Endrick, Scotland, transported back to the laboratory and allocated randomly to holding aquaria, where they were held for approximately six months until the start of the breeding season under a simulated natural photoperiod and temperature regime. Fish were fed to satiation daily on a custom-made diet containing carotenoids (at 10 μg g−1) but lacking any additional antioxidants. The use of artificial plants and algal growth control (2-chloro-4, 6-bis-(ethylamino)-s-triazine; Algae Destroyer, Aquarium Pharmaceuticals) ensured that the only antioxidants known to be available to the fish were through the diet. When males began to develop blue eye coloration (an indicator of sexual maturation, independent of the carotenoid-based signal), they were transferred to individual, visually isolated, experimental aquaria (33×18×19 cm). When a sufficient number of males (n=40) had reached maturation, they were divided equally and at random between two diet treatments. Each received the base diet (described above) supplemented with additional (non-carotenoid) antioxidants at biologically realistic levels: vitamins E (α-tocopherol acetate; Sigma, Poole, UK) and C (ascorbic acid; Sigma, Poole, UK) at concentrations of either 140 and 750 μg g−1, respectively (high-antioxidant diet) or 14 and 75 μg g−1, respectively (low-antioxidant diet). Males were provided with nesting material and shown a gravid female for 5 min twice a day for 10 days, after which time all males had completed nest building, developed nuptial coloration and entered the courtship phase.
Ten days after the start of the antioxidant-supplemented diet, experimental aquaria were rearranged into pairs so that each male was next to a size-matched individual of the opposite diet treatment (standard length: t19=0.99, p=0.34), and female preference tested using an established protocol (Milinski & Bakker 1990). Immediately following mate-choice trials, males were netted and standardized reflectance scans of their nuptial coloration obtained in order to derive stickleback-specific estimates of signal chroma (colour saturation or intensity) using psychophysical models of the stickleback's visual system (Rowe et al. 2004). This is a good predictor of an individual's carotenoid investment in sexual ornamentation (r2 =0.61; T. W. Pike 2006, unpublished data from biochemical analysis). The surface area of the nuptial signal (±0.1 mm2) was determined from digital photographs. Fish were then sacrificed with an overdose of anaesthetic (benzocaine), weighed (±0.001 g), homogenized in phosphate-buffered saline (containing 1.15% w/v potassium chloride) at 20% w/v and tested for total antioxidant activity using the Trolox Equivalent Antioxidant Capacity assay (Rice-Evans & Miller 1994). Further details of the dietary manipulation and analytical methods are given in the electronic supplementary material.
Proportional data were arcsine square root transformed and estimates of chroma normalized using log transformations prior to analysis. Mean values are presented±s.e. and n denotes the sample size.
3. Results
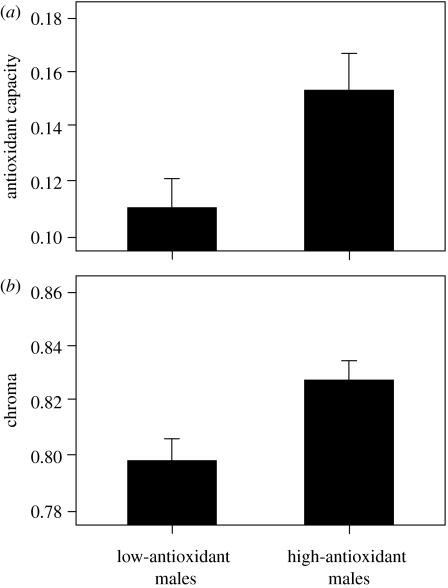
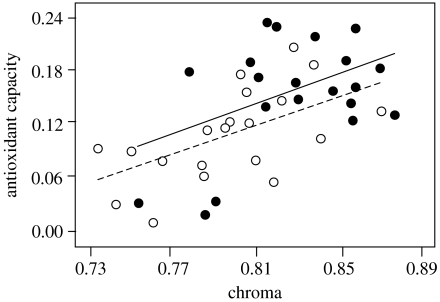
Males fed on the high-antioxidant diet had a greater antioxidant capacity than males fed low levels of antioxidants (two-sample t-test: t38=2.52, p=0.016; figure 1a). While the size of the pigmented area did not differ between treatment groups (mean±s.e. high-antioxidant males: 44.9±6.4 mm2, low-antioxidant males: 51.4±8.7 mm2; t38=0.57, p=0.57), males that received higher levels of antioxidants developed significantly more saturated regions of nuptial coloration than those fed low-antioxidant diets (t38=2.27, p=0.029; figure 1b), despite all fish receiving the same dietary concentration of carotenoids. Furthermore, signal chroma significantly predicted a male's antioxidant capacity (ANCOVA with diet treatment group as a random factor: F1,37=12.73, p=0.001; figure 2).
Figure 1.
Mean±s.e. (a) antioxidant capacity (measured in Trolox equivalents) and (b) nuptial coloration chroma for males on the high- and low-antioxidant diet treatments.
Figure 2.
Relationship between nuptial coloration chroma and antioxidant capacity (measured in Trolox equivalents) for males on the high- (filled circles, solid line) and low-antioxidant (open circles, dashed line) diet treatments. Lines of least squares are shown.
Females significantly preferred to associate with males on the high-antioxidant diet in mate-choice trials (mean±s.e. proportion of time spent with the male on high-antioxidant diet: 0.58±0.04; one-sample t-test against a test mean of 0.5 (no preference): t19=2.21, p=0.040).
4. Discussion
As predicted, males receiving relatively high levels of dietary vitamins C and E produced more intensely coloured sexual signals and had a higher antioxidant capacity than low-antioxidant diet males (even though both received the same amount of carotenoids), and within both treatment groups there was a significant positive relationship between a male's redness and his antioxidant capacity. This suggests that carotenoid-based nuptial coloration may act as an honest signal of a male's body levels of antioxidant defences. Indeed, in mate-choice trials, females showed a significant preference for males on the high-antioxidant diet, although we cannot say unequivocally that females based their choice on the colour difference between males (for instance, high-antioxidant diet males may also have been able to maintain a higher courtship rate). Furthermore, our data cannot elucidate how females may have gained by choosing to mate with high-antioxidant diet males although various positive outcomes are possible, including enhanced male longevity (Pike et al. 2007), low-levels of oxidative sperm damage (Fraga et al. 1991) or greater disease resistance in their offspring (Barber et al. 2001).
The link between high dietary intake of vitamins C and E and the degree of ornamentation is consistent with the hypothesis that, rather than signalling the availability of carotenoids themselves, carotenoid-based displays may indicate a more general availability of antioxidant defences, where carotenoids may play a relatively minor role (von Schantz et al. 1999; Blount et al. 2000; Hartley & Kennedy 2004; Bertrand et al. 2006). Two mechanisms have been proposed to explain how carotenoid-based signals could act as honest indicators of general antioxidant levels. Males with adequate antioxidant defences could afford to divert carotenoids away from this function and instead allocate them to sexual signalling; thus males with the brightest signals should have higher non-carotenoid antioxidant levels (von Schantz et al. 1999; Blount et al. 2000; Hartley & Kennedy 2004). Alternatively, the presence of other antioxidants may mitigate against the oxidation of carotenoids, a process that alters their structure and renders them colourless (Packer 1992). Consequently, having high concentrations of intact carotenoids, and displaying them in a signal, may indicate the possession of efficient means for their protection (Hartley & Kennedy 2004). Unfortunately, our data cannot differentiate between these two alternatives, and so this provides an interesting avenue for future work.
Although we chose to manipulate levels of vitamins E and C, there are several other antioxidants that might have had similar effects (Prior & Cao 1999; Fang et al. 2002; Bertrand et al. 2006). However, it is likely to be particularly important for animals to balance the amounts of vitamin C, vitamin E and carotenoids in their bodies, since they may provide synergic protection against lipid peroxidation (Barclay et al. 1983). Furthermore, our results do not preclude the antioxidant action of carotenoids themselves or suggest that they are necessarily unimportant outside of their signalling role. Indeed, dietary carotenoids in sticklebacks have been shown to be important in combating oxidative stress and regulating lifespan in the absence of any other major antioxidants (Pike et al. 2007), while carotenoid metabolites are functionally important in vertebrate vision and are essential for a wide range of gene activation and regulatory events, including the regulation of immune response genes (e.g. Garbe et al. 1992).
Acknowledgments
This research adhered to the Association for the study of Animal Behaviour Guidelines for the use of animals in research, and was performed under licence from the UK Home Office. We thank Stirling University's Institute of Aquaculture for providing the antioxidant-free food, J. Laurie and G. Adam for their help in animal husbandry, P. McLachlan for his help in the chemical analyses and D. McLennan and an anonymous referee for their useful comments on the manuscript. The work was funded by a grant from the Natural Environment Research Council (to N.B.M, J.D.B and J.L.). J.D.B. was supported by a Royal Society University Research Fellowship.
Supplementary Material
This file contains further details on the preparation of the experimental diets, analysis of ornament coloration, mate-choice assay and determination of antioxidant capacity
References
- Barber I, Arnott S.A, Braithwaite V.A, Andrew J, Huntingford F.A. Indirect fitness consequences of mate choice in sticklebacks: offspring of brighter males grow slowly but resist parasitic infections. Proc. R. Soc. B. 2001;268:71–76. doi: 10.1098/rspb.2000.1331. doi:10.1098/rspb.2000.1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay L.R.C, Locke S.J, MacNeil J.M. The antioxidation of unsaturated lipids in micelles: synergism of inhibitors vitamin C and E. Can. J. Chem. 1983;61:1288–1290. doi:10.1139/v83-225 [Google Scholar]
- Bertrand S, Faivre B, Sorci G. Do carotenoid-based sexual traits signal the availability of non-pigmentary antioxidants? J. Exp. Biol. 2006;209:4414–4419. doi: 10.1242/jeb.02540. doi:10.1242/jeb.02540 [DOI] [PubMed] [Google Scholar]
- Blount J.D, Møller A.P, Houston D.C. Why egg yolk is yellow. Trends Ecol. Evol. 2000;15:47–49. doi: 10.1016/s0169-5347(99)01774-7. doi:10.1016/S0169-5347(99)01774-7 [DOI] [PubMed] [Google Scholar]
- Blount J.D, Metcalfe N.B, Birkhead T.R, Surai P.F. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300:125–127. doi: 10.1126/science.1082142. doi:10.1126/science.1082142 [DOI] [PubMed] [Google Scholar]
- Faivre B, Grégoire A, Préault M, Cézilly F, Sorci G. Immune activation rapidly mirrored in a carotenoid-based secondary sexual trait. Science. 2003;300:103. doi: 10.1126/science.1081802. doi:10.1126/science.1081802 [DOI] [PubMed] [Google Scholar]
- Fang Y.Z, Yang S, Wu G. Free radicals, antioxidants and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. doi:10.1016/S0899-9007(02)00916-4 [DOI] [PubMed] [Google Scholar]
- Fraga C.G, Motchnik P.A, Shigenaga M.K, Helbock H.J, Jacobs R.A, Ames B.N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl Acad. Sci. USA. 1991;88:11 003–11 006. doi: 10.1073/pnas.88.24.11003. doi:10.1073/pnas.88.24.11003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe A, Buck J, Hammerling U. Retinoids are important cofactors in T-cell activation. J. Exp. Med. 1992;176:109–117. doi: 10.1084/jem.176.1.109. doi:10.1084/jem.176.1.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T.W. Chapman and Hall; New York, NY: 1984. The biochemistry of the carotenoids, vol. 11, animals. Tunicates and fish. [Google Scholar]
- Grether G.F, Kasahara S, Kolluru G.R, Cooper E.L. Sex-specific effects of carotenoid intake on the immunological response to allografts in guppies (Poecilia reticulata) Proc. R. Soc. B. 2004;271:45–49. doi: 10.1098/rspb.2003.2526. doi:10.1098/rspb.2003.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Vitamin C: antioxidant or pro-oxidant in vivo? Free Rad. Res. 1996;25:439–454. doi: 10.3109/10715769609149066. [DOI] [PubMed] [Google Scholar]
- Hartley R.C, Kennedy M.W. Are carotenoids a red herring in sexual display? Trends Ecol. Evol. 2004;19:353–354. doi: 10.1016/j.tree.2004.04.002. doi:10.1016/j.tree.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Hill G.E. Plumage colouration is a sexually selected indicator of male quality. Nature. 1991;350:337–339. doi:10.1038/350337a0 [Google Scholar]
- Krinsky N.I, Yeum K.J. Carotenoid–radical interactions. Biochem. Biophys. Res. Commun. 2003;305:754–760. doi: 10.1016/s0006-291x(03)00816-7. doi:10.1016/S0006-291X(03)00816-7 [DOI] [PubMed] [Google Scholar]
- Lozano G.A. Carotenoids, parasites, and sexual selection. Oikos. 1994;70:309–311. doi:10.2307/3545643 [Google Scholar]
- McGraw K.J. The antioxidant function of many animal pigments: are there consistent health benefits of sexually selected colorants? Anim. Behav. 2005;69:757–764. doi:10.1016/j.anbehav.2004.06.022 [Google Scholar]
- McGraw K.J, Ardia D.R. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 2004;162:704–712. doi: 10.1086/378904. doi:10.1086/378904 [DOI] [PubMed] [Google Scholar]
- Milinski M, Bakker T.C.M. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature. 1990;344:330–333. doi:10.1038/344330a0 [Google Scholar]
- Olsen V.A, Owens I.P.F. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 1998;13:510–514. doi: 10.1016/s0169-5347(98)01484-0. doi:10.1016/S0169-5347(98)01484-0 [DOI] [PubMed] [Google Scholar]
- Packer L. Academic Press; London, UK: 1992. Carotenoids. Part A, chemistry, separation, quantitation, and antioxidation. [Google Scholar]
- Pike T.W, Blount J.D, Bjerkeng B, Lindström J, Metcalfe N.B. Carotenoids, oxidative stress and female mating preference for longer-lived males. Proc. R. Soc. B. 2007;274:1591–1596. doi: 10.1098/rspb.2007.0317. doi:10.1098/rspb.2007.0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior R.L, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radical Biol. Med. 1999;27:1173–1181. doi: 10.1016/s0891-5849(99)00203-8. doi:10.1016/S0891-5849(99)00203-8 [DOI] [PubMed] [Google Scholar]
- Rice-Evans C, Miller N.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994;234:279–293. doi: 10.1016/0076-6879(94)34095-1. [DOI] [PubMed] [Google Scholar]
- Rowe M.P, Banbe C.L, Loew E.R, Phillips J.B. Optimal mechanisms for finding and selecting mates: how threespine stickleback (Gasterosteus aculeatus) should encode male throat colors. J. Comp. Physiol. A. 2004;190:241–256. doi: 10.1007/s00359-004-0493-8. doi:10.1007/s00359-004-0493-8 [DOI] [PubMed] [Google Scholar]
- von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. B. 1999;266:1–12. doi: 10.1098/rspb.1999.0597. doi:10.1098/rspb.1999.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedekind C, Meyer P, Frischknecht M, Niggli U.A, Pfander H. Different carotenoids and potential information content of red coloration of male three-spined stickleback. J. Chem. Ecol. 1998;24:787–801. doi:10.1023/A:1022365315836 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains further details on the preparation of the experimental diets, analysis of ornament coloration, mate-choice assay and determination of antioxidant capacity