Abstract
Adaptive theory predicts that mothers would be advantaged by adjusting the sex ratio of their offspring in relation to their offspring's future reproductive success. Studies investigating sex ratio variation in mammals have produced notoriously inconsistent results, although recent studies suggest more consistency if sex ratio variation is related to maternal condition at conception, potentially mediated by changes in circulating glucose level. Consequently, we hypothesized that change in condition might better predict sex ratio variation than condition per se. Here, we investigate sex ratio variation in feral horses (Equus caballus), where sex ratio variation was previously shown to be related to maternal condition at conception. We used condition measures before and after conception to measure the change in condition around conception in individual mothers. The relationship with sex ratio was substantially more extreme than previously reported: 3% of females losing condition gave birth to a son, whereas 80% of those females that were gaining condition gave birth to a son. Change in condition is more predictive of sex ratio than actual condition, supporting previous studies, and shows the most extreme variation in mammals ever reported.
Keywords: evolutionary theory, sex allocation, sex ratios, maternal investment
1. Introduction
Variation in the production of sons and daughters is a key variable in life-history and evolutionary theory, and sex ratios at birth and hatching do vary (e.g. reviews by Clutton-Brock & Iason 1986; Cameron 2004; Sheldon & West 2004). Adaptive theories predict systematic variation in the sex ratio when the fitness returns of producing sons and daughters vary between individual parents (Trivers & Willard 1973; Clark 1978). The Trivers–Willard hypothesis (TWH; Trivers & Willard 1973) has been one of the most influential of these hypotheses. The TWH suggests that, where one sex has more variable reproductive success (males in polygynous species): (i) mothers in good condition with more resources to invest would be advantaged by producing sons, as highly competitive sons would out-compete highly competitive daughters, who are constrained to a lower reproductive rate and (ii) mothers with less resources to invest would be advantaged by producing a daughter, as a daughter would out-reproduce an unsuccessful son.
The hypothesis is logically appealing and has been extensively tested in a variety of taxa (e.g. reviews: mammals, Cameron 2004; Sheldon & West 2004; birds, Ewen et al. 2004). In mammals, few studies have produced conclusive results either confirming or refuting the hypothesis, and the inconsistent results have proved difficult to interpret (Clutton-Brock & Iason 1986; Frank 1990; Festa-Bianchet 1996; Hewison & Gaillard 1999; Brown 2001). Recent reviews have suggested that this is due to variation in the timing of the measure, with measures taken at conception providing the strongest support (Cameron 2004; Sheldon & West 2004), and inconsistencies in the measures themselves (Cameron 2004).
Recently, a mechanism for sex ratio adjustment was hypothesized (Cameron 2004) and tested (Cameron et al. submitted). Briefly, male and female conceptuses are sexually dimorphic in their response to glucose (Gutiérrez-Adán et al. 2001) and in their ability to survive in mediums with different glucose concentrations (Larson et al. 2001). Added glucose enhances male conceptus growth and development, but inhibits female conceptus development.
Experimental manipulation of glucose levels resulted in significant variation in sex ratios, but the change in glucose levels was more predictive than glucose levels per se (Cameron et al. submitted). Therefore, Cameron et al. (submitted) suggested that changes in condition during early conceptus development rather than actual condition may be more important for determining sex ratio variation. A previous study also showed that change in condition from birth of previous offspring to conception was more predictive of sex ratio variation than actual condition (Roche et al. 2006). These studies provide evidence that change in condition may be an overlooked variable in studies of sex ratio variation.
Here, we use a population of free-ranging feral horses (Equus caballus) already known to show significant variation in birth sex ratio in relation to condition at conception (Cameron et al. 1999) to investigate the influence of change in condition around conception on the birth sex ratio.
2. Material and methods
Feral horses inhabiting the Kaimanawa Mountains and the surrounding areas in the central North Island of New Zealand were studied between August 1994 and 1999. The maternal investment (e.g. Cameron & Linklater 2000; Cameron et al. 2000, 2003) and social structure (e.g. Linklater et al. 1999, 2000; Linklater & Cameron 2000) of the subpopulation in the Moawhango River basin were studied intensively and over 400 horses were individually identifiable by either freeze brands on their rumps or natural markings.
Sex ratio adjustment was previously investigated in relation to a range of factors, including body condition at conception, and described in detail in Cameron et al. (1999). In the initial study, we calculated body condition at conception by backdating from date of foaling (accurate to ±5 days) by the average gestation length ±1 s.d. (336±10 days; Kiltie 1982). Here, we investigated the change in condition from pre-conception (conception −20±10 days) to post-conception (conception +20±10 days) for foals born in 1995, 1996 and 1997. Consequently, the scores in this study did not overlap with the values previously reported.
Body condition scores (BCS) were estimated by visual body fat distribution on an 11-point scale (0–5 with 0.5 gradations; Carroll & Huntingdon 1988; Rudman & Keiper 1991). Body condition scoring is a reliable index of body fat percentage (r=0.81; Henneke et al. 1983). Visual BCS correlate more strongly with body fat and live weight in horses than other ungulates (e.g. Berry et al. 2006), because readily used fat is carried subcutaneously in equids rather than around internal organs (Huntingdon & Cleland 1992). BCS were recorded with the aid of 10–15× binoculars or a 15–60× telescope every time a horse was seen, provided visibility was good. Interobserver reliability was high (r=0.91). Only mares that had their BCS recorded at least twice during each 20-day period were used in the analysis, and modal BCS was used, resulting in a sample size of 118 births. BCS ranged from 1 to 3.5, with a mean of 2.5. Foals were sexed by sighting external genitalia, which are visible in both sexes from birth.
3. Results
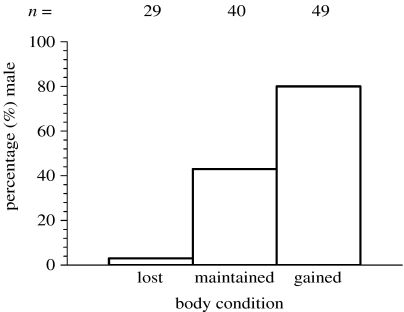
Mothers that conceived sons tended to gain condition (0.38±0.04), whereas mothers of daughters tended to lose condition (−0.16±0.05; t=8.21, d.f.=116, p<0.001). When mothers were categorized by whether they were losing condition (n=29), maintaining their condition (n=40) or gaining condition (n=49) around conception, the results were striking; only 3% of females losing condition conceived a son, whereas 80% of those gaining condition conceived a son (Fisher's exact test, p<0.001; figure 1). These results differed from 50 : 50 for mothers losing condition (binomial test, p<0.0001) and gaining condition (binomial test, p<0.0001), but not for mothers maintaining their condition (binomial test, p=0.16). This variation in sex ratio is substantially greater than previously observed from investigating condition scores at conception (poor condition 18.5% male and good condition 57% male; Cameron et al. 1999).
Figure 1.
Direction of condition change from pre-conception to post-conception in relation to offspring sex ratio in horses.
4. Discussion
Horses represent a good test of the TWH (Cameron et al. 1999), since litter size is fixed at one (Platt 1978), and post-natal variation in maternal investment in relation to maternal condition occurs (Cameron & Linklater 2000). Sexual size dimorphism at birth is also minimal (Duncan 1992), hence neither sex is markedly more costly in utero. There is no difference in foaling rates in relation to condition at conception (Cameron et al. 1999). Therefore, it is unlikely that our results could be explained by differential loss of the more costly sex during gestation as proposed by Myers (1978), particularly since sex ratio deviates significantly in both directions, towards sons when females are gaining condition and towards daughters when females are losing condition. Previous studies have shown variation in sex ratios in horses, in relation to food availability (Monard et al. 1997) and condition around conception (Cameron et al. 1999).
The results provide the strongest mammalian support for the TWH that we know of. The birth sex ratio varies over a 77% range depending on whether mothers were gaining or losing condition around conception. Change in condition is more predictive of offspring sex than condition per se, consistent with speculation that changes in circulating glucose levels mediate sex ratios (Cameron 2004; Gutiérrez-Adán et al. 2006; Cameron et al. submitted). Only one study has previously reported on the influence of change in condition on sex ratios, which was over the period from birth of the previous offspring to conception of the current offspring, which showed equally convincing results in a domestic species (Roche et al. 2006). From an evolutionary perspective, changing condition around conception would be more predictive of condition during the peak period of investment, which in mammals is during lactation (Oftedal 1985). Consequently, there is correspondence between facultative birth sex ratio adjustment and a mother's future ability to invest. Many of the inconsistencies in studies of sex ratios might be explained if a change in condition were responsible for observed sex ratio patterns, rather than condition per se. This has implications for both evolutionary theory and species management (e.g. Robertson et al. 2006). Future studies should investigate change in condition as a predictor of sex ratio variation.
Acknowledgments
The data were collected while we were students in the Ecology Group of the Institute of Natural Resources at Massey University, funded by the Department of Conservation, New Zealand (contract 1850). Many thanks to Kevin Stafford and Ed Minot for their support, and to two anonymous reviewers for their helpful comments. All procedures were approved by the Massey University Animal Ethics Committee.
References
- Berry D.P, MacDonald K.A, Penno J.W, Roche J.R. Association between body condition score and live weight in pasture-based Holstein–Friesian dairy cows. J. Dairy Res. 2006;73:487–491. doi: 10.1017/S0022029906002020. doi:10.1017/S0022029906002020 [DOI] [PubMed] [Google Scholar]
- Brown G.R. Sex-biased investment in nonhuman primates: can Trivers & Willard's theory be tested? Anim. Behav. 2001;61:683–694. doi:10.1006/anbe.2000.1659 [Google Scholar]
- Cameron E.Z. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. B. 2004;271:1723–1728. doi: 10.1098/rspb.2004.2773. doi:10.1098/rspb.2004.2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron E.Z, Linklater W.L. Individual mares bias investment in sons and daughters in relation to condition. Anim. Behav. 2000;60:359–367. doi: 10.1006/anbe.2000.1480. doi:10.1006/anbe.2000.1480 [DOI] [PubMed] [Google Scholar]
- Cameron E.Z, Linklater W.L, Stafford K.J, Veltman C.J. Birth sex ratios relate to mare condition at conception in Kaimanawa horses. Behav. Ecol. 1999;10:472–475. doi:10.1093/beheco/10.5.472 [Google Scholar]
- Cameron E.Z, Linklater W.L, Stafford K.J, Minot E.O. Aging and improving reproductive success in horses: declining residual reproductive value or just older and wiser? Behav. Ecol. Sociobiol. 2000;47:243–249. doi:10.1007/s002650050661 [Google Scholar]
- Cameron E.Z, Linklater W.L, Stafford K.J, Minot E.O. Social group and maternal behaviour in feral horses, Equus caballus. Behav. Ecol. Sociobiol. 2003;53:92–101. [Google Scholar]
- Cameron, E. Z., Lemons, P., Bateman, P. W. & Bennett, N. C. Submitted. Experimental alteration of litter sex ratios in a mammal. [DOI] [PMC free article] [PubMed]
- Carroll C.L, Huntingdon P.J. Body condition scoring and weight estimation in horses. Equine Vet. J. 1988;20:41–45. doi: 10.1111/j.2042-3306.1988.tb01451.x. [DOI] [PubMed] [Google Scholar]
- Clark A.B. Sex ratio and local resource competition in a prosimian primate. Science. 1978;201:163–165. doi: 10.1126/science.201.4351.163. doi:10.1126/science.201.4351.163 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Iason G.R. Sex ratio variation in mammals. Q. Rev. Biol. 1986;61:339–374. doi: 10.1086/415033. doi:10.1086/415033 [DOI] [PubMed] [Google Scholar]
- Duncan P. Springer; New York, NY: 1992. Horses and grasses: the nutritional ecology of Equids and their impact on the Camargue. [Google Scholar]
- Ewen J.G, Cassey P, Moller A.P. Facultative primary sex ratio variation: a lack of evidence in birds? Proc. R. Soc. B. 2004;271:1277–1282. doi: 10.1098/rspb.2004.2735. doi:10.1098/rspb.2004.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa-Bianchet M. Offspring sex ratio studies of mammals: does publication depend upon the quality of the research or the direction of the results? Ecoscience. 1996;3:42–44. [Google Scholar]
- Frank S.A. Sex allocation theory for birds and mammals. Annu. Rev. Ecol. Syst. 1990;21:13–55. doi:10.1146/annurev.es.21.110190.000305 [Google Scholar]
- Gutiérrez-Adán A, Granados J, Pintado B, de la Fuente J. Influence of glucose on the sex ratio of bovine IM/IVF embryos cultured in vitro. Reprod. Fert. Dev. 2001;13:361–365. doi: 10.1071/rd00039. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Adán A, Perez-Crespo M, Fernandez-Gonzalez R, Ramirez M.A, Moreira P, Pintado B, Lonergan P, Rizos D. Developmental consequences of sexual dimorphism during pre-implantation embryonic development. Reprod. Domest. Anim. 2006;41:54–62. doi: 10.1111/j.1439-0531.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- Henneke D.R, Potter G.D, Kreider J.L, Yeates B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983;15:371–372. doi: 10.1111/j.2042-3306.1983.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Hewison A.J.M, Gaillard J.M. Successful sons or advantaged daughters? The Trivers–Willard model and sex biased maternal investment in ungulates. Trends Ecol. Evol. 1999;14:229–234. doi: 10.1016/s0169-5347(99)01592-x. doi:10.1016/S0169-5347(99)01592-X [DOI] [PubMed] [Google Scholar]
- Huntingdon P, Cleland F. Agmedia; Melbourne, Australia: 1992. Horse sense: the Australian guide to horse husbandry. [Google Scholar]
- Kiltie R.A. Intraspecific variation in the mammalian gestation period. J. Mammal. 1982;63:646–652. doi:10.2307/1380270 [Google Scholar]
- Larson M.A, Kimura K, Kubisch H.M, Roberts R.M. Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-tau. Proc. Natl Acad. Sci. USA. 2001;98:9677–9682. doi: 10.1073/pnas.171305398. doi:10.1073/pnas.171305398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linklater W.L, Cameron E.Z. Tests for cooperative behaviour between stallions. Anim. Behav. 2000;60:731–743. doi: 10.1006/anbe.2000.1525. doi:10.1006/anbe.2000.1525 [DOI] [PubMed] [Google Scholar]
- Linklater W.L, Cameron E.Z, Minot E.O, Stafford K.J. Stallion harassment and the mating system of horses. Anim. Behav. 1999;58:295–306. doi: 10.1006/anbe.1999.1155. doi:10.1006/anbe.1999.1155 [DOI] [PubMed] [Google Scholar]
- Linklater W.L, Cameron E.Z, Stafford K.J, Veltman C.J. Social and spatial structure and range use by Kaimanawa wild horses (Equus caballus: Equidae) New Zeal. J. Ecol. 2000;24:139–152. [Google Scholar]
- Monard A.-M, Duncan P, Fritz H, Feh C. Variations in the birth sex ratio and neonatal mortality in a natural herd of horses. Behav. Ecol. Sociobiol. 1997;41:243–249. doi:10.1007/s002650050385 [Google Scholar]
- Myers J.H. Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? Am. Nat. 1978;112:381–388. doi:10.1086/283280 [Google Scholar]
- Oftedal O.T. Pregnancy and lactation. In: Hudson R.J, White R.G, editors. Bioenergetics of wild herbivores. CRC Press; Boca Raton, FL: 1985. pp. 215–238. [Google Scholar]
- Platt H. The Animal Health Trust; Newmarket, UK: 1978. A survey of perinatal mortality and disorders in the Thoroughbred. [Google Scholar]
- Robertson B.C, Elliot G.P, Eason D.K, Clout M.N, Gemmell N.J. Sex allocation theory aids species conservation. Biol. Lett. 2006;2:229–231. doi: 10.1098/rsbl.2005.0430. doi:10.1098/rsbl.2005.0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J.R, Lee J.M, Berry D.P. Pre-conception energy balance and secondary sex ratio—partial support for the Trivers–Willard hypothesis in dairy cows. J. Dairy Sci. 2006;89:2119–2125. doi: 10.3168/jds.S0022-0302(06)72282-2. [DOI] [PubMed] [Google Scholar]
- Rudman R, Keiper R.R. The body condition of feral ponies on Assateague Island. Equine Vet J. 1991;23:453–456. doi: 10.1111/j.2042-3306.1991.tb03760.x. [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, West S.A. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 2004;163:40–54. doi: 10.1086/381003. doi:10.1086/381003 [DOI] [PubMed] [Google Scholar]
- Trivers R.L, Willard D. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. doi:10.1126/science.179.4068.90 [DOI] [PubMed] [Google Scholar]