Abstract
Humans are uniquely endowed with the ability to engage in accurate, high-momentum throwing. Underlying this ability is a unique morphological adaptation that enables the characteristic rotation of the arm and pelvis. What is unknown is whether the psychological mechanisms that accompany the act of throwing are also uniquely human. Here we explore this problem by asking whether free-ranging rhesus monkeys (Macaca mulatta), which lack both the morphological and neural structures to throw, nonetheless recognize the functional properties of throwing. Rhesus not only understand that human throwing represents a threat, but that some aspects of a throwing event are more relevant than others; specifically, rhesus are sensitive to the kinematics, direction and speed of the rotating arm, the direction of the thrower's eye gaze and the object thrown. These results suggest that the capacity to throw did not coevolve with psychological mechanisms that accompany throwing; rather, this capacity may have built upon pre-existing perceptual processes. These results are consistent with a growing body of work showing that non-human animals often exhibit perceptual competencies that do not show up in their motor responses, suggesting evolutionary dissociations between the systems of perception that provide understanding of the world and those that mediate action on the world.
Keywords: throwing, action perception, action production, phylogeny
1. Introduction
Humans, but not other animals, are endowed with the capacity for accurate, high-momentum throwing, a morphological specialization with highly significant adaptive consequences in fighting and prey capture. Furthermore, evolutionary biologists and neuroscientists have suggested that the evolution of throwing abilities has resulted in neural changes linked to fine motor control. These changes, in turn, have enabled the evolution of a suite of uniquely human abilities such as language, music, technological virtuosity and non-kin cooperation (Calvin 1983; Bingham 1999). An assumption underlying this theoretical framework is that the capacity to throw coevolved with the psychological mechanisms that allow observers to interpret or understand throwing, including details of the motion and object thrown. An alternative hypothesis is that the capacity to throw evolved independently of these psychological mechanisms. On this view, non-human animals that lack the musculature and neural machinery to throw would nonetheless be capable of reasoning about someone who can throw.
Decoupling systems of motor output from systems of perception or comprehension have proved invaluable in evolutionary, developmental and neurobiological studies, focused on language, music, tool use and mathematics. For example, non-human animals can compute algebraic rules and non-adjacent relationships, aspects that enter into human language processing, but never appear in non-human animals' natural communicative repertories (Hauser et al. 2002; Newport et al. 2004). Thus, these capacities did not evolve specifically for human language, but rather, reflect general processes of the primate auditory and conceptual systems. Similarly, though animals never create explicit numerical symbols or their operators, they compute numerosities using non-linguistic computational mechanisms (Brannon & Terrace 1998). These results highlight the point that the absence of a particular motor pattern in a given species need not indicate a deficiency in this species' ability to understand the pattern when generated by someone or something else. This general point leads to the specific question motivating the present study: does our unique throwing ability depend on both uniquely human motor and perceptual capacities or did these two aspects of throwing evolve independently? If they evolved independently, then non-human animals lacking the capacity to throw may nonetheless understand and reason correctly about observed throwing actions. We test this hypothesis by exploring how a non-throwing primate, the rhesus monkey (Macaca mulatta), responds to the act of throwing by a human, as well as to the changes to various properties of the throw.
2. Material and methods
We tested adult rhesus monkeys living on the island of Cayo Santiago, Puerto Rico. These individuals have some experience with humans throwing overhand, as this action is deployed in cases where an animal threatens an experimenter, and the experimenter throws but never hits the individual with a small rock. None of the authors in the present study have ever seen a researcher on the island throw any other way than overhand, and for one of the authors (M.D.H.), observations go back to 1987. Although this does not mean that these rhesus have never witnessed another type of throwing action (e.g. underhand), their predominant experience is with overhand throwing.
In each condition, we tested 20 monkeys, each recognized by distinctive ear notches and tattoos. For each trial, an experimenter approached to 10 m of a lone monkey and set up a video camera. Once the subject was attending, the experimenter performed the throwing action. In the initial condition, this involved showing a rock held in one hand and initiating a complete throwing action, but without releasing the rock. The dependent measure for all conditions was the subject's displacement from the starting position to 0.5 m or more; this is a measure of avoidance associated with the detection of a potential threat (Walk et al. 1957; Schiff et al. 1962). Two coders scored all of the trials from video recordings; trials were excluded from the final analysis when the coders disagreed.
(a) Description of throws
Overhand throw. The experimenter performed an overhand throwing motion towards the subject (figure 1).
Preparation phase only. The experimenter moved his arm up and away from the subject.
Preparation and rotation phases. The experimenter moved his arm up and away from the subject and then moved his arm forward towards the subject.
Extension phase only. The experimenter extended his forearm towards the subject.
Underhand throw. The experimenter performed an underhand throwing motion towards the subject.
Overhand (off target). The experimenter began the throw looking at the subject with his body facing 90° away. He then performed the overhand throwing motion 90° away from the subject.
Push. The experimenter moved his arm directly backwards and then forward towards the subject.
Push (with preparation phase). This throw was the same as the ‘push’ throw except the experimenter first moved his arm up and away from the subject.
Overhand (slow motion). The experimenter performed the overhand throw towards the subject at one-third of the speed of the normal overhand throw.
No object. The experimenter showed the subject that his hand was empty and then performed an overhand throwing motion towards the subject.
Food object. The experimenter showed the subject an apple in his hand and then performed an overhand throwing motion towards the subject.
No attention. The experimenter ensured that the subject was attending and then looked 90° away from the subject and performed an overhand throw towards the subject.
Figure 1.
Sample frames from the subject's point of view of the throwing actions.
3. Results
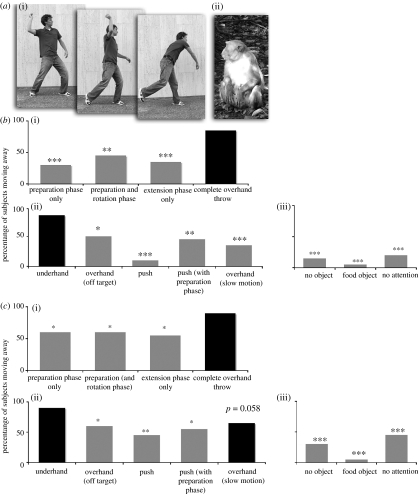
Rhesus spontaneously evaluated throwing actions according to the most functionally relevant properties of the action. Eighty-five per cent of the subjects showed the avoidance response to the complete overhand throw (figure 2b). In contrast, rhesus showed a significantly lower level of avoidance to preparation (30%), extension (35%) and preparation with rotation (45%). Thus, rhesus are highly attentive to the kinematics of throwing, responding most strongly when all the three phases emerge in succession.
Figure 2.
(a) (i) human performing the three phases of an overhand throw: preparation; rotation; and extension. (ii) a rhesus monkey. (b) Percentage of avoidance to: (i), the three phases of throwing and to the complete overhand throw; (ii), the throwing actions testing trajectory, direction, torque and speed; and (iii), the throwing action testing the presence of an object, the type of object and visual attention. (c) Percentage of subjects showing any type of agitation to the same throws. Grey bars signify statistically significant differences compared with the complete overhand throw with a rock (chi-square, two-tailed predictions); ***p<0.001; **p<0.01; *p<0.05.
Rhesus responded with the same level of avoidance to the relatively unfamiliar underhand throw (85%) as they did to the familiar overhand throw. In contrast, they showed significantly less avoidance to the ‘pushing’ throw (10%), a motion that lacks sufficient torque to serve as a threat. This reduction in avoidance held even when the pushing throw was preceded by the preparation phase of the throw (45%). Rhesus also showed significantly less avoidance to the familiar overhand throw performed at slower speed (35%) or in a different direction from the subject (50%), as well as when the experimenter performed the overhand throw with an empty hand (15%) or a soft food object (5%), all of which signify little threat. Finally, rhesus perceived an overhand throw as less threatening when the experimenter looked away from the subject (20%), suggesting that they understand that in order to throw accurately, one must direct visual attention towards the target goal.
To provide an additional response measure, we asked whether subjects showed any visible sign of agitation to the throws. We counted an agitation response as (i) any type of visible startle, (ii) threatening behaviour towards the experimenter, (iii) any sign of fear (i.e. fear grimace, turning away from the experimenter), or (iv) standing or moving away. To be included, these behaviours needed to occur within 2 s of completing the throw. Coding the data using this more sensitive measure led to the same qualitative pattern of behaviour towards the various throws as described above (figure 2c).
4. Discussion
The goal of the present study was to ask whether the capacity to throw coevolved with the psychological mechanisms that accompany throwing. Surprisingly, our results show that despite the fact that rhesus monkeys cannot throw themselves, they nevertheless respond correctly to observed throwing actions. In comparison to the overhand throw performed with a rock, rhesus showed significantly less avoidance to the throwing actions when the kinematics, trajectory, speed or direction of the rotating arm were changed such that the throw no longer represented a threat to the subject. Furthermore, rhesus showed less avoidance to the overhand throw when it was performed with an empty hand or a soft food object, as well as when the experimenter was looking away from the target location. Thus, rhesus were responding not only to learned associations between throwing motions and negative consequences, because many of these throws were nearly identical in motion, but nonetheless yielded different behavioural responses. Instead, our results suggest that rhesus evaluate throwing events by parsing them into at least three relevant components: the kinematics of the motor action; the thrower's attention; and the object held.
Because rhesus monkeys do not throw themselves and seem to lack the morphological structures that support throwing, we conclude that at least some of the psychological mechanisms that accompany human throwing abilities built upon pre-existing capacities that evolved for other domains, such as spatial reasoning, social interaction and object representation. This conclusion does not rule out the possibility that once the motor programmes and relevant anatomy for throwing evolved, the capacities to comprehend throwing underwent a further round of evolution and fine tuning. However, prior to this potential evolutionary refinement, our species was equipped with perceptual and conceptual mechanisms that allowed observers to evaluate actions that they could not produce themselves.
These results can be interpreted as challenging a currently dominant position in philosophy and neuroscience that interprets the capacity to understand the semantics of observed actions (i.e. the agent's intentions and goals) as intimately connected with the ability to produce these actions, an interpretation supported by mirror neurons in the premotor cortex that respond to both the observation and production of actions (Rizzolatti & Craighero 2004). Our results force a rejection of the strong version of this claim: if understanding of an action only comes from the ability to perform the action, then rhesus monkeys, lacking the ability to throw, should not have the ability to understand the meaning and functional properties of throwing. The data presented leave little room to question their understanding, even though many questions remain concerning how this knowledge is acquired, whether it is shared with more distantly related species, and its underlying neural substrates. One possibility is that despite the fact that non-human animals cannot throw, the motor system nonetheless plays some role in reasoning about observed throwing (Ferrari et al. 2005). An alternate possibility is that primates access separate mechanisms, with distinct neural substrates (Buccino et al. 2004), for understanding the meaning of actions within and beyond their own motor repertoire. Independent of these competing hypotheses, our results show that non-human primates are able to make spontaneous, rapid and accurate inferences about the potential outcomes of actions that fall unambiguously outside of both their natural and potential motor repertoires.
In sum, our results build on a growing body of work showing that in several domains of knowledge, animals have rich conceptual representations that guide perception and categorization in the absence of matched and coordinated capacities for motor output. These dissociations may correspond to the separate ventral and dorsal neural pathways which guide visual perception and vision for action, respectively, and appear to have evolved separately (Milner & Goodale 2006). Based on these evolutionary dissociations between the systems of perception that provide understanding of the world and those that mediate action on the world, we propose that the evolutionary origins of many uniquely human capacities can be understood partially in terms of evolved processes for integrating information between perception and action.
Acknowledgments
This research adheres to the Animal Behaviour Society Guidelines for the Use of Animals in Research, the legal requirements of the country in which the work was carried out and all institutional guidelines.
The project described was supported by Grant Number CM-5 P40 RR003640-20 from the National Centre for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. Further support comes from grants from the NIH (NRSA), as well as funding from the Guggenheim and Jeffrey Epstein to M.D.H. We thank Melissa Gerald for facilitating our research on Cayo Santiago.
References
- Bingham P.M. Human uniqueness: a general theory. Q. Rev. Biol. 1999;74:133–169. doi:10.1086/393069 [Google Scholar]
- Brannon E.M, Terrace H.S. Ordering of the numerosities 1–9 by monkeys. Science. 1998;282:746–749. doi: 10.1126/science.282.5389.746. doi:10.1126/science.282.5389.746 [DOI] [PubMed] [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro C.A, Rizzolatti G. Neural circuits involved in the recognition of actions performed by non-conspecifics: an fMRI study. J. Cognit. Neurosci. 2004;16:114–126. doi: 10.1162/089892904322755601. doi:10.1162/089892904322755601 [DOI] [PubMed] [Google Scholar]
- Calvin W.H. McGraw-Hill; New York, NY: 1983. The throwing Madonna. [Google Scholar]
- Ferrari P.F, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J. Cognit. Neurosci. 2005;17:212–226. doi: 10.1162/0898929053124910. doi:10.1162/0898929053124910 [DOI] [PubMed] [Google Scholar]
- Hauser M.D, Chomsky N, Fitch W.T. The faculty of language: what is it, who has it, and how did it evolve? Science. 2002;298:1569–1579. doi: 10.1126/science.298.5598.1569. doi:10.1126/science.298.5598.1569 [DOI] [PubMed] [Google Scholar]
- Milner A.D, Goodale M.A. 2nd edn. Oxford University Press; Oxford, UK: 2006. The visual brain in action. [Google Scholar]
- Newport E.L, Hauser M.D, Spaepen G, Aslin R.N. Learning at a distance: II. Statistical learning of non-adjacent dependencies in a nonhuman primate. Cognit. Psychol. 2004;49:85–117. doi: 10.1016/j.cogpsych.2003.12.002. doi:10.1016/j.cogpsych.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror–neuron System. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. doi:10.1146/annurev.neuro.27.070203.144230 [DOI] [PubMed] [Google Scholar]
- Schiff W, Caviness J.A, Gibson J.J. Persistent fear responses in rhesus monkeys to the optical stimulus of “looming”. Science. 1962;136:982–963. doi: 10.1126/science.136.3520.982. doi:10.1126/science.136.3520.982 [DOI] [PubMed] [Google Scholar]
- Walk R.D, Gibson E.J, Tighe T.J. Behavior of light- and dark-reared rats on a visual cliff. Science. 1957;126:80–81. doi: 10.1126/science.126.3263.80-a. doi:10.1126/science.126.3263.80-a [DOI] [PubMed] [Google Scholar]
Notice of correction
Figure 1 is now presented in the correct form. 26 February 2007