Abstract
When the diminutive skeleton of Homo floresiensis was found on the Indonesian island of Flores, it was interpreted as an island dwarf, conforming to the ‘island rule’ that large animals evolve smaller size on islands, but small animals tend to get larger. However, previous studies of the island rule have not included primates, so the extent to which insular primate populations undergo size change was unknown. We use a comparative database of 39 independently derived island endemic primate species and subspecies to demonstrate that primates do conform to the island rule: small-bodied primates tend to get larger on islands, and large-bodied primates get smaller. Furthermore, larger species undergo a proportionally greater reduction in size on islands.
Keywords: insular dwarf, comparative method, Homo floresiensis
1. Introduction
The ‘island rule’ is the name given to the observation that small-bodied species tend to evolve towards gigantism on islands, but larger-bodied species tend towards dwarfism on islands (Foster 1964; Van Valen 1973). This graded trend from gigantism in smaller species to dwarfism in larger species is predominantly a feature of mammals (Foster 1964; Van Valen 1973; Heaney 1978; Lomolino 1985, 2005), though it has also been reported in birds (Clegg & Owens 2002) and snakes (Boback 2003). However, the generality of the island rule has been questioned, by highlighting single species or whole mammalian orders that do not follow the rule (Van Valen 1973; Heaney 1978; Lomolino 1985; Meiri et al. 2004). This is not surprising since the proposed determinants of the island rule—resource requirements, predation avoidance, inter- and intraspecific competition—may vary between taxonomic groups (MacArthur & Wilson 1963; Van Valen 1973; Heaney 1978; Lomolino 1985, 2005; Smith 1992; Clegg & Owens 2002; Palkovacs 2003; Raia & Meiri 2006).
In particular, while there are spectacular cases of island dwarfism in other mammalian taxa, such as the Stegadon elephants found on Flores (one species of which was less than one-tenth the size of modern Asian elephants; van den Bergh et al. 2001), there are few obvious cases of island dwarf primates. This is important because the surprisingly small stature of the newly discovered hominin from the island of Flores in Indonesia, Homo floresiensis, has been explained as a consequence of island dwarfism (Brown et al. 2004). However, this claim has been refuted on the grounds that the degree of size reduction is greater than would be expected from insular dwarfing (e.g. Jacob et al. 2006; Martin et al. 2006a,b). Yet, there has been no analysis of the degree of size reduction expected in island primates against which these hypotheses can be evaluated. Our aim in this study is to provide a comparative study of body size in island primates against which claims of island dwarfing can be evaluated.
2. Material and methods
We searched the literature and online databases and consulted experts to identify insular primate populations that were reported to be distinct in some way from their mainland relatives, indicating a sufficient degree of genetic isolation from the mainland population to permit the potential evolution of body size. Since the island rule is considered less likely to be observed on very large islands (Lomolino 2005), we considered only taxa endemic to islands with an area of less than 100 000 km2: this excluded primates endemic to Madagascar, Borneo, Java and Sumatra (all of which are treated as ‘mainland’ in this study). Primate taxonomy is constantly changing, so rather than relying on any single taxonomic treatment, we accepted any recognizably distinct island taxon, regardless of its formal taxonomic status. To provide a comparison for the evolution of differences in body size, we selected the closest mainland relative of each island endemic population using a combination of published phylogenies, taxonomies, distribution data and consultation with experts (see electronic supplementary material for details).
We collected two datasets (see electronic supplementary material for details). The first dataset consists of phylogenetically independent pairs of island and mainland taxa for which body mass measurements were available (table 1 in electronic supplementary material). In addition, we chose pairs of island and mainland primates for which head–body length or skull measurements were available, using taxonomy and distribution data to select independent pairs where phylogenies were unavailable (table 2 in electronic supplementary material). Island area was taken from the literature or measured from the base map in ArcGIS v. 9 (tables 1 and 2 in electronic supplementary material). We were unable to include time since the isolation of the island from the mainland population, as this information was not available for most of the species included in this study. However, most of the islands in this study are ‘land-bridge’ islands likely to have been isolated from the mainland only since the last glacial maximum.
We used two basic approaches to test whether the body size of island primates differed consistently from that of their mainland relatives. Firstly, we used the non-parametric sign test and Wilcoxon signed-rank test to ask whether the direction and degree of size difference between island and mainland primates were non-randomly distributed. Secondly, we tested whether the degree of island dwarfing was related to mainland body size by plotting mainland against island body size and then examining the slope of the relationship using reduced major axis (RMA) regression (Sokal & Rohlf 1995). We tested for the effects of island area on the degree of island dwarfing (ratio of island to mainland body size, Si) using ordinary least squares regression. We also tested the relationship between the degree of sexual size dimorphism in mainland taxa and Si. (see electronic supplementary material for details and additional statistical analyses).
3. Results
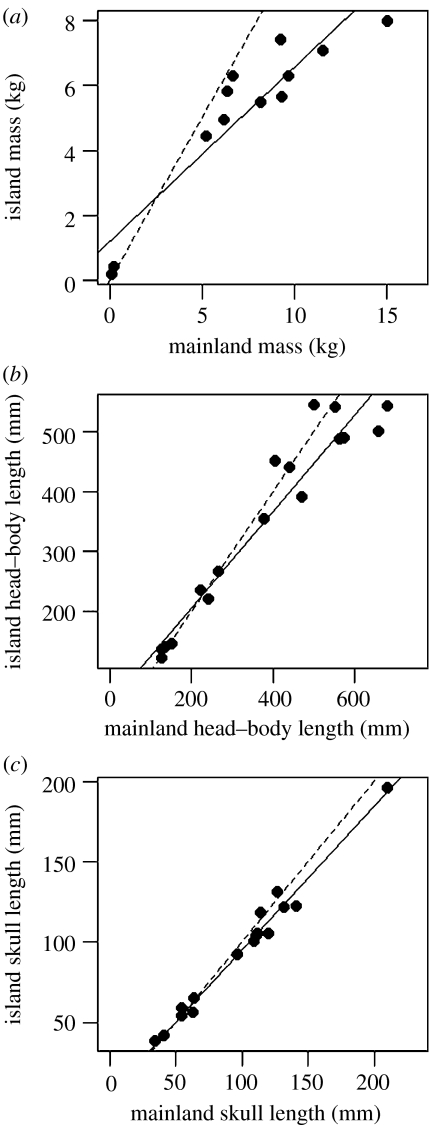
These data provide clear evidence that primates follow the island rule. For the mass dataset, all small island primate species (less than 5 kg; see electronic supplementary material) are larger than their closest mainland relatives, and all other island species are smaller than their closest mainland relatives. This relationship is significant under sign tests and Wilcoxon tests for average body mass (psign=0.014; pWilcoxon=0.019), male body mass (psign=0.006; pWilcoxon=0.006) and female body mass (psign=0.002; pWilcoxon=0.004). The same patterns are observed for head–body length (psign=0.048; pWilcoxon=0.016) and skull size (psign=0.048; pWilcoxon=0.011; table 3 in electronic supplementary material). These relationships are supported by slopes of significantly less than 1 for the relationship between mainland and island body masses, head–body lengths and skull lengths (figure 1), indicating that larger-bodied taxa undergo a greater proportional size reduction on islands. There was no evidence of a relationship between island area and degree of body size change. Taxa with more pronounced sexual dimorphism in head–body length showed a greater degree of head–body length reduction on islands (p=0.016); however, this pattern was not observed for the body mass data (p=0.441; table 4 in electronic supplementary material).
Figure 1.
Relationship between island and mainland primate body sizes. Slopes of RMA regression lines fitted through the comparisons (solid lines) are significantly less than 1 (dashed lines) for: (a) mass (n=12, slope=0.58 (95% confidence intervals on slope 0.42–0.73), pslope=1=0.0007); (b) head–body length (n=17, slope=0.75 (0.62–0.88), pslope=1=0.023) and (c) skull length (n=16, slope=0.9 (0.83–0.97), pslope=1=0.014).
4. Discussion
Our analysis confirms that primates do undergo predictable shifts in body size when confined to islands. These observed changes in body size occur on islands not very distant from larger landmasses and over relatively short time-scales. Most of our comparisons are between subspecies, which in some cases may be less than 10 000 years old (Foster 1964; Smith 1992; Groves 2001), and virtually all of the islands included here were separated from the mainland after the last glacial maximum, probably less than 12 000 years ago.
There is some evidence that taxa with a greater degree of sexual dimorphism undergo a proportionally greater reduction in size on islands, possibly reflecting a role of intraspecific competition as a determinant of the island rule. Sexual dimorphism in primates has been considered an indicator of degree of intraspecific competition (Lindenfors 2002; Isaac 2005), thus may be expected to change in response to changes in the level of competition pressure on islands, as predicted under the island rule (Van Valen 1973; Lomolino 1985).
What implications do these findings have for interpreting the Flores hominin? We can make three relevant observations. Firstly, H. floresiensis remains have been reported from Flores (area=14 300 km2) from a period of between 20 000 and 80 000 years (Brown et al. 2004; Morwood et al. 2005). Our results suggest that this is a sufficient length of time for a significant reduction in primate body size. However, the long isolation of Flores from the mainland (probably since the Mid-Pliocene; see Argue et al. 2006) suggests that the hominins may have arrived after the separation of the island from the mainland, which makes estimating population isolation times difficult. Secondly, the degree of size reduction observed in H. floresiensis, when compared with Homo sapiens and Homo erectus, falls within the range observed for other island primate species. For the mass dataset, the three largest island species (over 7 kg) are 52, 61 and 80% of the size of their mainland counterparts. The predicted mass of H. floresiensis is around 55% of the mass of modern Indonesian H. sapiens, around 52% of the estimated mass of Indonesian H. erectus and similar in body size to some australopithecines (see electronic supplementary material). Thirdly, although the type specimen of H. floresiensis (LB1) has an extremely small skull for a member of Homo, its skull length relative to head–body length is within the range expected for an island dwarf primate.
Our results suggest that the hypothesis that H. floresiensis represents an insular dwarf race of hominids cannot be rejected on the grounds of degree of size reduction alone. However, these results cannot be used to reject the alternative hypothesis that LB1 is a microcephalic individual, nor confirm or reject the claim that the Flores hominins represent a new species of Homo (Argue et al. 2006; Martin et al. 2006a; Richards 2006). In particular, it is important to note that the most intense debates about H. floresiensis have focused not on absolute stature or skull length but on the relatively small brain volume of LB1, the only specimen with a relatively complete skull. The encephalization quotient calculated from the estimated brain and body mass of LB1 is very low compared with other Homo species, and the size of the skull of LB1 relative to its predicted stature neither appears to follow a human ontogenetic scale (a human child of the stature of LB1 has a much larger brain) nor resembles the relative proportions of human pygmies (whose brains are comparatively large for their small bodies).
Examples of insular dwarf elephants and bovids have been used to argue both that hominid brains should shrink comparatively less (Martin et al. 2006a) or more (Brown et al. 2004) than their stature. We are unable to provide a direct test of these hypotheses due to lack of comparative data on brain volume for most of the primates included here. However, these results do suggest that other primate species undergo dramatic reduction in body mass, body length and skull length over comparatively short time periods when confined to islands, even relatively large islands that are not far from the mainland.
Acknowledgments
We are grateful to Colin Groves, John Welch and Shai Meiri for their helpful comments on data, analysis and interpretation.
Supplementary Material
Supplementary methods
References
- Argue D, Donlon D, Groves C, Wright R. Homo floresiensis: microcephalic, pygmoid, Australopithecus, or Homo? J. Hum. Evol. 2006;51:360–374. doi: 10.1016/j.jhevol.2006.04.013. doi:10.1016/j.jhevol.2006.04.013 [DOI] [PubMed] [Google Scholar]
- Boback S.M. Body size evolution in snakes: evidence from island populations. Copeia. 2003;1:81–94. doi:10.1643/0045-8511(2003)003[0081:BSEISE]2.0.CO;2 [Google Scholar]
- Brown P, Sutikna T, Morwood M.J, Soejono R.P, Jatmiko, Wayhu Saptomo E, Awe Due R. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature. 2004;431:1055–1061. doi: 10.1038/nature02999. doi:10.1038/nature02999 [DOI] [PubMed] [Google Scholar]
- Clegg S.M, Owens I.P.F. The “island rule” in birds: medium body size and its ecological explanation. Proc. R. Soc. B. 2002;269:1359–1365. doi: 10.1098/rspb.2002.2024. doi:10.1098/rspb.2002.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.B. Evolution of mammals on islands. Nature. 1964;202:234–235. doi:10.1038/202234a0 [Google Scholar]
- Groves C.P. Smithsonian Institution Press; Washington, DC: 2001. Primate taxonomy. [Google Scholar]
- Heaney L.R. Island area and body size of insular mammals: evidence from the tri-coloured squirrel (Callosciurus prevosti) of southeast Asia. Evolution. 1978;32:29–44. doi: 10.1111/j.1558-5646.1978.tb01096.x. doi:10.2307/2407408 [DOI] [PubMed] [Google Scholar]
- Isaac J.L. Potential causes and life-history consequences of sexual size dimorphism in mammals. Mamm. Rev. 2005;35:101–115. doi:10.1111/j.1365-2907.2005.00045.x [Google Scholar]
- Jacob T, Indriati E, Soejono R.P, Hsu K, Frayer D.W, Eckhardt R.B, Kuperavage A.J, Thorne A, Henneberg M. Pygmoid Australomelanesian Homo sapiens skeletal remains from Liang Bua, Flores: population affinities and pathological abnormalities. Proc. Natl Acad. Sci. USA. 2006;103:13 421. doi: 10.1073/pnas.0605563103. doi:10.1073/pnas.0605563103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenfors P. Sexually antagonistic selection on primate size. J. Evol. Biol. 2002;15:595–607. doi:10.1046/j.1420-9101.2002.00422.x [Google Scholar]
- Lomolino M.V. Body size of mammals on islands: the island rule reexamined. Am. Nat. 1985;125:310–316. doi:10.1086/284343 [Google Scholar]
- Lomolino M.V. Body size evolution in insular vertebrates: generality of the island rule. J. Biogeogr. 2005;32:1683–1699. doi:10.1111/j.1365-2699.2005.01314.x [Google Scholar]
- MacArthur R.H, Wilson E.O. An equilibrium theory of insular zoogeography. Evolution. 1963;17:373–387. doi:10.2307/2407089 [Google Scholar]
- Martin R.D, MacLarnon A.M, Phillips J.L, Dobyns W.B. Flores hominid: new species or microcephalic dwarf? Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006a;288A:1123–1145. doi: 10.1002/ar.a.20389. doi:10.1002/ar.a.20389 [DOI] [PubMed] [Google Scholar]
- Martin R.D, MacLarnon A.M, Phillips J.L, Dussubieux L, Williams P.R, Dobyns W.B. Comment on “The Brain of LB 1, Homo floresiensis”. Science. 2006b;312:999. doi: 10.1126/science.1121144. doi:10.1126/science.1121144 [DOI] [PubMed] [Google Scholar]
- Meiri S, Dayan T, Simberloff D. Body size of insular carnivores: little support for the island rule. Am. Nat. 2004;163:469–479. doi: 10.1086/382229. doi:10.1086/382229 [DOI] [PubMed] [Google Scholar]
- Morwood M.J, et al. Further evidence for small-bodied hominins from the Late Pleistocene of Flores, Indonesia. Nature. 2005;437:1012–1017. doi: 10.1038/nature04022. doi:10.1038/nature04022 [DOI] [PubMed] [Google Scholar]
- Palkovacs E.P. Explaining adaptive shifts in body size on islands: a life history approach. Oikos. 2003;103:37–44. doi:10.1034/j.1600-0706.2003.12502.x [Google Scholar]
- Raia P, Meiri S. The island rule in mammals: paleontology meets ecology. Evolution. 2006;60:1731–1742. doi:10.1554/05-664.1 [PubMed] [Google Scholar]
- Richards G.D. Genetic, physiologic and ecogeographic factors contributing to variation in Homo sapiens: Homo floresiensis reconsidered. J. Evol. Biol. 2006;19:1744–1767. doi: 10.1111/j.1420-9101.2006.01179.x. doi:10.1111/j.1420-9101.2006.01179.x [DOI] [PubMed] [Google Scholar]
- Smith F.A. Evolution of body size among woodrats from Baja California, Mexico. Funct. Ecol. 1992;6:265–273. doi:10.2307/2389516 [Google Scholar]
- Sokal R.R, Rohlf F.J. WH Freeman & Co; New York, NY: 1995. Biometry. [Google Scholar]
- van den Bergh, G. D., Vos, J. & Morwood, M. J. 2001 Elephantoidea in the Indonesian region: new Stegodon findings from Flores. The World of Elephants: Proc. 1st Int. Cong. pp. 623–627.
- Van Valen L. Pattern and the balance of nature. Evol. Theory. 1973;1:31–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods