Abstract
Ambient noise interferes with the propagation of acoustic signals through the environment from sender to receiver. Over the past few centuries, urbanization and the development of busy transport networks have led to dramatic increases in the levels of ambient noise with which animal acoustic communications must compete. Here we show that urban European robins Erithacus rubecula, highly territorial birds reliant on vocal communication, reduce acoustic interference by singing during the night in areas that are noisy during the day. The effect of ambient light pollution, to which nocturnal singing in urban birds is frequently attributed, is much weaker than that of daytime noise.
Keywords: urbanization, ambient noise, vocal communication, nocturnal song
1. Introduction
Across much of the world, urban areas are growing proportionately faster than any other land cover type (Meyer & Turner 1992). Such urbanization leads to dramatic changes in the structure and functioning of a diverse array of ecosystem components (Marzluff et al. 2001; Pickett et al. 2001). One important, but rather poorly studied, impact is the emission of anthropogenic noise, which has been increasing in average and peak intensity, even in many previously intensively developed regions (Berglund & Lindvall 1995). Such noise interferes with the propagation of animal acoustic signals through the environment, with potentially wide ranging consequences for those aspects of the ecologies of species that depend, proximately or ultimately, on acoustic communication (Warren et al. 2006). A range of facultative behavioural responses to anthropogenic noise have been documented, including alterations to the amplitude, frequency, timing and duration of signals to minimize acoustic competition (Slabbekoorn & Peet 2003; Brumm 2004; Foote et al. 2004; Brumm & Slabbekoorn 2005; Brumm 2006; Wood & Yezerinac 2006).
There are strong and predictable diurnal patterns in the levels of urban noise, most strikingly a reduction during the night, when human activity abates. In birds, nocturnal singing by normally diurnal species may be one way to minimize interference from ambient urban noise. However, such a response would be costly, given that singing at night leads to a large increase in metabolic rate over that associated with sleeping (Ward et al. 2003). Night singing of normally diurnal birds, particularly in urban environments, is a well-established observation (Rawson 1923; Hollom 1966; King 1966; Mitchell 1967), suggesting that some feature of urbanization drives the activity. The phenomenon has usually been attributed anecdotally to nocturnal light pollution (e.g. Hollom 1966; Stephan 1999; for a review see Molenaar et al. 2006). Miller (2006) showed that American robins Turdus migratorius began their dawn chorus earlier in areas with high levels of artificial night lighting, although to our knowledge, no study has measured the effect of urban noise on nocturnal singing.
Here, we investigate whether birds are more likely to sing at night in areas that are noisy during the day, and compare the strength of this effect with that of nocturnal light pollution, using European robins Erithacus rubecula as a case study.
2. Material and methods
We selected 121 point locations across Sheffield, England (53°22′ N, 1°20′ W) that maximized the spread of daytime noise levels based on measurements within each of the 500×500 m grid cells across the city (total urban area: 160 km2). Between 12 April and 16 June (57 and 64 locations in 2005 and 2006, respectively), each point location was visited twice, once during the day and once at night. The sequence in which each section of the city was visited was randomized to minimize non-independence among the data both spatially and in terms of the time of day or night. Onset and conclusion of night were defined as 40 min after and before the time of civil twilight (dusk and dawn), respectively.
Each survey period was of 20 min duration and surveys were conducted throughout the day and night. The distance from the point location to every singing robin was estimated with reference to features of the surrounding urban environment and after intensive practice in estimating distances in an urban setting. The number of birds singing and the number of song phrases given by each individual were noted. Ambient noise was measured at 2 min intervals throughout the survey period using a Dawe D-1405E digital sound meter (A-weighted, slow response), overall sound level being the mean of these 10 values. In no cases did robin song itself significantly impact upon measured ambient sound levels. Light levels were measured at 0, 5, 10, 15 and 20 min from the start of the survey period using an Iso-Tech Lux-1337 digital light meter. Overall light level was expressed as the mean of these five values, and square-root transformed (SQRT) for analysis.
3. Results
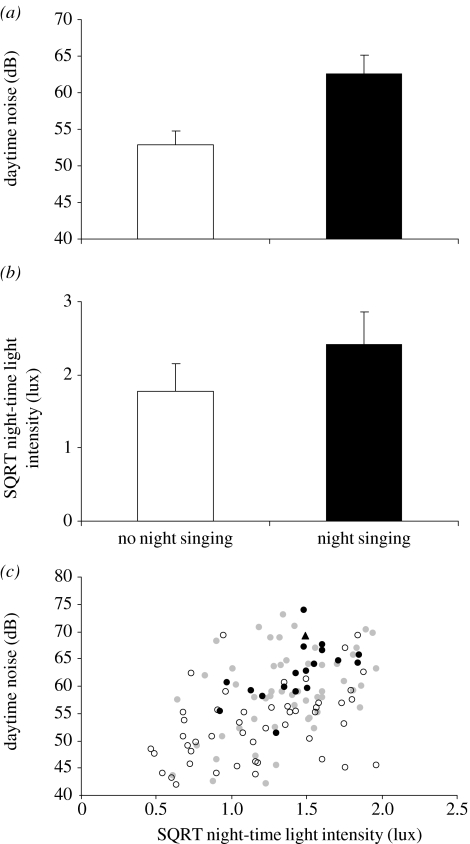
Singing robins were heard diurnally at 67 sites (76 individuals, seven sites with two individuals, one site with three), and nocturnally at 18 of these sites (20 individuals, two sites with two individuals). At one site, a bird was heard singing at night but not during the day. Across all sites, ambient noise levels were significantly lower at night (mean=49 dB, 95% CI=48–51 dB) than during the day (57 dB, 56–58 dB; t=12.44, d.f.=120, p<0.001). Diurnal noise levels were significantly higher at locations where robins sang at night than in places where they were heard singing during the day (so were known to be present), but not at night (t=5.7, d.f.=66, p<0.001; figure 1a). Singing individuals gave an average of 18.41 song phrases per sampling period during the day and 24.57 during the night, although the difference was not significant (t=0.674, d.f.=95, p=0.502). Thus, for birds that sang, nocturnal singing activity matched that of diurnal singing activity.
Figure 1.
(a) Comparison of daytime noise levels between locations where robins sang nocturnally (mean=62.68 dB, 95% CI=60.15–65.21 dB) and those where singing occurred during the day but not at night (52.87, 50.93–54.81 dB). (b) Night-time light levels at locations where nocturnal singing robins were detected (2.42, 1.98–2.86 lux) and those where singing activity was detected during the day but not at night (1.78, 1.4–2.15 lux). (c) Daytime noise and night-time light levels at 121 locations across urban Sheffield. Filled circles indicate locations with birds singing day and night, filled triangle indicates night-singing only, open circles indicate day-singing only and grey circles indicate places where no singing activity was detected by day or night.
At only one site was a bird heard singing at night but not during the day (this was a location with constant traffic noise and an average daytime noise level of 69 dB). This suggests that there was in general a high probability of detecting a singing bird, if present, even in the high noise levels prevalent during the day. Noise was very rarely constant, and over the course of the 20 min survey period, there was usually a number of lulls allowing detection of singing activity. For example, a singing robin was detected at a distance of 70 m at a location with an average noise level of 61 dB. The occurrence of night-singing birds did not change across the season, as measured by the number of days since the start of the survey period (logistic regression: b=0.013, Wald statistic=0.523, p=0.47), nor through the night, as measured by time to dusk or dawn, whichever is closer (G-test using four equal time bands: Gadj=3.7, p=0.296). Noise levels did not change with time of day (ANOVA using four equal time bands: F3,117=0.682, p=0.565) or night (F3,117=0.324, p=0.808), reflecting the heterogeneity of noise across the urban landscape.
We used two different approaches to investigate the potential biasing effect of high noise levels reducing the probability of detecting birds that were singing during the day but not heard at night. First, we removed all locations with a daytime noise level at or above 69 dB and repeated the analysis. Diurnal noise levels remained much higher at locations where robins sang nocturnally (mean=62 dB, 95% CI=59–64 dB) compared with places where birds were heard singing during the day but not at night (52, 50–54 dB; t=5.7, p<0.001). Second, there was no correlation between the distance at which singing birds were detected and ambient noise level (r=−0.189, n=67, p=0.126). We conclude from these two lines of evidence that there was a high probability of detecting singing birds in areas of high urban noise, and that variation in detectability was not driving our results.
We found an elevation in night-time light intensity at places where birds sang nocturnally (t=2.3, d.f.=47.21, p=0.026; figure 1b), but night-time light was not significant in a multiple logistic regression model including both terms as predictors of the occurrence of night-time singing (p=0.947; table 1). The two variables were only weakly positively related (r2=0.202). Though nocturnal singing did occur primarily in strongly illuminated areas, it was limited to those areas that were also noisy during the day and was absent in most well-lit places that were relatively quiet during the day (figure 1c). This is not what would be predicted if light was the dominant effect. We therefore conclude that daytime noise had a much greater effect on nocturnal singing activity than night-time light levels.
Table 1.
Results of a full logistic regression model investigating the effects of daytime noise and night-time light levels on nocturnal singing.
| b (s.e.) | Wald statistic | p | |
|---|---|---|---|
| daytime noise | 0.127 (0.042) | 9.214 | 0.002 |
| night-time light | −0.016 (0.241) | 0.005 | 0.947 |
4. Discussion
Mean daytime noise levels were ca 10 dB (i.e. an order of magnitude) higher in places where birds continued to sing nocturnally (figure 1a), suggesting that birds were either (i) singing at night to reduce total time spent singing against acoustic competition or (ii) taking advantage of quieter conditions during the night to give additional signalling. Resolution of these two mechanisms will require detailed fieldwork monitoring individual singing activity over time. That daytime noise was a stronger predictor of nocturnal singing than night-time light hints at a very different mechanism driving the phenomenon. If ambient light levels drive nocturnal singing, then it can be viewed as a passive by-product of a physiological response to the external stimulus, whereas if nocturnal singing occurs in response to daytime noise, it suggests a facultative behavioural response. Since light from point sources will be blocked by physical barriers, it is possible that average light conditions across the territory differed from our sampled measurement. However, several of the night-singing birds were located in very dark conditions, and there was no consistent tendency for singing birds to be located close to light sources.
Robins sing at 2–9 kHz (Hoelzel 1986), and while urban noise is concentrated at the lower end of this range, any increase in urban noise will impact directly on the ability of robin song to propagate through the environment. Playback of songs of heterospecifics resulted in short-term adjustments to timing of song delivery in nightingales Luscinia megarhynchos (Brumm 2006), and male Eleutherodactylus tree frogs reduced short-term acoustic interference by calling within silent or lower intensity intervals between broadcast synthetic call notes (Zelick & Narins 1982, 1983). Greenfield (1988) documents a shift to nocturnal stridulating in the katydid Neoconocephalus spiza in the presence of diurnal-stridulating competitors. Our findings show for the first time that birds can dramatically alter their diel pattern of communication to take advantage of temporal fluctuations in ambient anthropogenic noise.
Urban noise levels continue to increase, through use of more powerful sources of noise, greater geographical spread and mobility of noise sources, and a greater proportion of the day being exposed (Berglund & Lindvall 1995). For example, acoustic energy levels in urban Sheffield doubled in the decade prior to 2001 (Sharp 2002). Our data suggest that such developments will further increase acoustic interference with animal communication, with important consequences for behavioural patterns in urban species.
Acknowledgments
This work was supported by EPSRC grant GR/S20529/1 to the CityForm Research Consortium. K.J.G. holds a Royal Society-Wolfson Research Merit Award. We are grateful to C. Flockhart for her assistance with data collection, and B. Hatchwell, K. Evans, M. Dallimer, T. Longcore and two anonymous reviewers for their comment and discussion.
References
- Berglund B, Lindvall T, editors. Community noise. World Health Organization; Stockholm, Sweden: 1995. [Google Scholar]
- Brumm H. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 2004;73:434–440. doi:10.1111/j.0021-8790.2004.00814.x [Google Scholar]
- Brumm H. Signalling through acoustic windows: nightingales avoid interspecific competition by short-term adjustment of song timing. J. Comp. Physiol. A. 2006;192:1279–1285. doi: 10.1007/s00359-006-0158-x. doi:10.1007/s00359-006-0158-x [DOI] [PubMed] [Google Scholar]
- Brumm H, Slabbekoorn H. Acoustic communication in noise. Adv. Stud. Behav. 2005;35:151–209. doi:10.1016/S0065-3454(05)35004-2 [Google Scholar]
- Foote A.D, Osborne R.W, Hoelzel A.R. Whale-call response to masking boat noise. Nature. 2004;428:910. doi: 10.1038/428910a. doi:10.1038/428910a [DOI] [PubMed] [Google Scholar]
- Greenfield M.D. Interspecific acoustics interactions among katydids Neoconocephalus: inhibition-induced shifts in diel periodicity. Anim. Behav. 1988;36:684–695. doi:10.1016/S0003-3472(88)80151-9 [Google Scholar]
- Hoelzel A.R. Song characteristics and response to playback of male and female robins Erithacus rubecula. Ibis. 1986;128:115–127. [Google Scholar]
- Hollom P.A.D. Nocturnal singing and feeding by robins in winter. Br. Birds. 1966;59:501–502. [Google Scholar]
- King B. Nocturnal singing and feeding by robins in winter. Br. Birds. 1966;59:501–502. [Google Scholar]
- Marzluff J.M, Bowman R, Donnelly R, editors. Avian ecology and conservation in an urbanizing world. Kluwer Academic Publishers; Boston, MA: 2001. [Google Scholar]
- Meyer W.B, Turner B.L., II Human population growth and global land-use/cover change. Annu. Rev. Ecol. Syst. 1992;23:39–61. doi:10.1146/annurev.es.23.110192.000351 [Google Scholar]
- Miller M.W. Apparent effects of light pollution on singing behavior of American robins. Condor. 2006;108:130–139. doi:10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2 [Google Scholar]
- Mitchell K.D.G. Nocturnal activity of city blackbird. Br. Birds. 1967;60:373–374. [Google Scholar]
- Molenaar J.G.de, Sanders M.E, Jonkers D.A. Road lighting and grassland birds: local influence of road lighting on a black-tailed godwit population. In: Rich C, Longcore T, editors. Ecological consequences of artificial night lighting. Island Press; Washington, DC: 2006. pp. 114–136. [Google Scholar]
- Pickett S.T.A, Cadenasso M.L, Grove J.M, Nilon C.H, Pouyat R.V, Zipperer W.C, Costanza R. Urban ecological systems: linking terrestrial ecological, physical and socioeconomic components of metropolitan areas. Annu. Rev. Ecol. Syst. 2001;32:127–157. doi:10.1146/annurev.ecolsys.32.081501.114012 [Google Scholar]
- Rawson H.E. A bird's song in relation to light. Trans. Hertfordshire Nat. Hist. Soc. Field Club. 1923;17:363–365. [Google Scholar]
- Sharp D. Silencing cities. J. Urban Health. 2002;79:162–163. doi: 10.1093/jurban/79.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn H, Peet M. Birds sing at a higher pitch in urban noise. Nature. 2003;424:267. doi: 10.1038/424267a. doi:10.1038/424267a [DOI] [PubMed] [Google Scholar]
- Stephan, B. 1999 Die Amsel Die Neue Brehm-Bücherei, Bd. 95, Westarp Wissenschaften, Hohenwarsleben.
- Ward S, Speakman J.R, Slater P.J.B. The energy cost of song in the canary, Serinus canaria. Anim. Behav. 2003;66:893–902. doi:10.1006/anbe.2003.2250 [Google Scholar]
- Warren P.A, Katti M, Ermann M, Brazel A. Urban bioacoustics: it's not just noise. Anim. Behav. 2006;71:491–502. doi:10.1016/j.anbehav.2005.07.014 [Google Scholar]
- Wood W.E, Yezerinac S.M. Song sparrow (Melospiza melodia) song varies with urban noise. Auk. 2006;123:650–659. doi:10.1642/0004-8038(2006)123[650:SSMMSV]2.0.CO;2 [Google Scholar]
- Zelick R.D, Narins P.M. Analysis of acoustically evoked call suppression behaviour in a Neotropical treefrog. Anim. Behav. 1982;30:728–733. doi:10.1016/S0003-3472(82)80144-9 [Google Scholar]
- Zelick R.D, Narins P.M. Intensity discrimination and the precision of call timing in two species of Neotropical treefrogs. J. Comp. Physiol. A. 1983;153:403–412. doi:10.1007/BF00612594 [Google Scholar]