Abstract
In most long-lived animal species, juveniles survive less well than adults. A potential mechanism is inferior foraging skills but longitudinal studies that follow the development of juvenile foraging are needed to test this. We used miniaturized activity loggers to record daily foraging times of juvenile and adult European shags Phalacrocorax aristotelis from fledging to the following spring. Juveniles became independent from their parents 40 days post-fledging. They compensated for poor foraging proficiency by foraging for approximately 3 h d−1 longer than adults until constrained by day length in early November. Thereafter, juvenile foraging time tracked shortening day length up to the winter solstice, when foraging time of the two age classes converged and continued to track day length until early February. Few individuals died until midwinter and mortality peaked in January–February, with juvenile mortality (including some of the study birds) five times that of adults. In their last two weeks of life, juveniles showed a marked decline in foraging time consistent with individuals becoming moribund. Our results provide compelling evidence that juveniles compensate for poor foraging proficiency by increasing foraging time, a strategy that is limited by day length resulting in high winter mortality.
Keywords: immature survival, foraging efficiency, environmental constraints, moribund
1. Introduction
In most long-lived animal species, juveniles survive less well than adults and identifying the drivers of juvenile mortality is critical to understanding size and age-structure of populations (Charlesworth 1980; Stearns 1992). A major cause of juvenile mortality is thought to be inadequate foraging skills (Goss-Custard & Durell 1987; Clutton-Brock et al. 2001). Juveniles may compensate for lack of proficiency by increasing foraging time or selecting alternative prey and habitats (Sutherland et al. 1986). However, there may be costs associated with these strategies such as increased likelihood of predation or parasitic infection (Stearns 1992). Furthermore, environmental conditions will place limitations on compensation. For example, at high latitudes, opportunities for diurnal species to increase foraging time in winter may be limited by day length.
To understand the potentially complex interactions between foraging proficiency and environmental conditions on survival, longitudinal studies of individuals spanning critical periods of their lives are required. Advances in animal-borne instrumentation enable key currencies such as foraging time to be recorded over long periods, in some cases throughout an animal's life. In species that rely on their daily food intake to balance their energy requirements, foraging time is negatively related to foraging success. The European shag Phalacrocorax aristotelis, a pursuit-diving, diurnally feeding seabird, exemplifies this strategy. We used miniaturized data loggers to record daily foraging times of juveniles and adults over ten months from the end of the breeding season to the following spring. This period spanned two critical periods for juveniles, the late summer transition to independence and midwinter, when foraging time may be limited by available daylight (Daunt et al. 2006) and mortality, particularly of juveniles, is high (Harris et al. 1994). Distributions of juveniles and adults outside the breeding season overlap (Wanless & Harris 1997), and we predicted that juveniles would compensate for any lack of proficiency by increasing foraging time. However, we also predicted that high mortality in winter would be linked to a limit placed on this strategy by shortened day length.
2. Material and methods
The work was carried out on the Isle of May, Scotland (56°11′ N, 2°33′ W). Eighty nests containing broods of one to three, two to three-week-old chicks were visited between 25 and 27 May 2003. Each chick was ringed with a metal ring and a blood sample taken (under UK Home Office licence; n=160) for molecular sexing (Griffiths et al. 1996). All male chicks (n=79/160) were fitted with data loggers (global location sensing/activity; British Antarctic Survey) attached to a leg ring between 11 and 13 June. Male chicks were selected because they are more philopatric, recruit at a younger age and are larger than females (Aebischer 1986, 1995; Daunt et al. 2001), thereby increasing recapture probability and reducing risk of device-related effects. Accordingly, the percentage returning as adults (8.9%) and confirmed to have bred (28.6%) in 2006 were slightly higher than equivalent values for unsexed colour-ringed juveniles not carrying loggers (7.4%, n=1006; 21.6%, n=74). To compare juvenile and adult foraging time, we deployed identical loggers on 10 breeding males with broods of similar sizes and hatching dates to study juveniles (deployment 17–29 June).
Seven loggers were retrieved from juveniles, five found dead during their first winter, one found dead in the second winter and one recaptured as a breeding adult in 2006. Eight were retrieved from live adults. Comparisons between juveniles and adults were made from fledging to 30 April 2004. Loggers recorded proportion of time in seawater at 10 min intervals, which we summarized as hours in the water per day. This measure is a reliable proxy for foraging time because shags have a partially wettable plumage and enter the water principally to forage (Grémillet et al. 1998; see electronic supplementary material). Foraging time, when unconstrained by extrinsic or intrinsic limitations (see electronic supplementary material) is also a proxy for foraging success because shags do not carry substantial fat reserves and need to balance energy requirements on a daily basis (Grémillet et al. 2003). For juveniles, we defined fledging as the first date birds entered the water and death as the day after the last recorded foraging.
Magnitude and timing of mortality were determined from the proportion of colour-ringed adults (n=764) and juveniles (n=1085) known to be alive at the end of the 2003 breeding season subsequently found dead between 1 August 2003 and 30 April 2004 (see electronic supplementary material).
3. Results
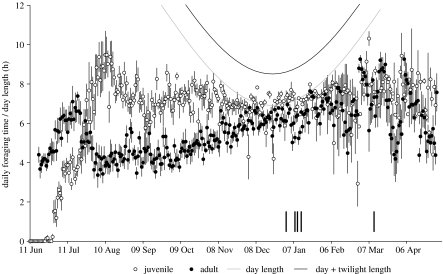
Juveniles fledged at a mean age of 54.9 days (range 53–57) and spent on average 99 min in the water. Thereafter, foraging time increased rapidly at a rate of 12 min d−1, peaking 40 days later at 9–10 h (figure 1). At fledging, male parents were spending a mean of 4 h d−1foraging. Thereafter, adult foraging time also increased but at a slower rate (6 min d−1; t13=2.84; p=0.01), for a shorter period (25 days; t13=3.22; p<0.01) and thus to a lower maximum (7 h). Foraging time then declined in both groups, although the juvenile decrease only started when adult foraging time had returned to pre-fledging levels.
Figure 1.
Mean (±s.e.) daily foraging time of juveniles (open circles) and adults (filled circles) from deployment to end April 2004, excluding the 15 days prior to death (figure 2). Day length (grey line) and day plus twilight length (black line) at the Isle of May's latitude (aa.usno.navy.mil) and juvenile death dates (thick, vertical lines) also shown.
As day length shortened during autumn, both age groups allocated progressively more time to foraging (0.4 and 1 min d−1 for juveniles and adults, respectively; t13=1.74; p=0.10). However, juvenile daily foraging times were on average almost 3 h longer (t13=4.83; p<0.001), and by early November, juveniles were using almost all available daylight for foraging (figure 1). Thereafter, juvenile foraging time tracked shortening day length, converging with adult values in early December, and both groups continued to track day length until early February (t13=0.41; p=0.69). From then until the end of April, foraging time in both age classes was variable but showed no systematic trend, so the proportion of daylight spent foraging declined (figure 1).
Few dead colour-ringed birds were reported before the winter solstice. However, substantial numbers died in the second half of winter, peaking in January–February, with mortality of juveniles five times that of adults (Table 1). These temporal and age effects were mirrored in the logger birds.
Table 1.
Mortality totals of juveniles and adults from August 2003 to April 2004. Owing to the delay between bird death and corpse finding (19.2 days for logger birds, range 0–36 days), peak mortality likely occurred in January–February (see electronic supplementary material). Among colour-ringed individuals, there was no difference in timing of mortality between age classes (Fisher Exact Test, before and after winter solstice, p=1.00), but a significant difference in magnitude (Χ2=26.7, p<0.001).
| juveniles | adults | ||||
|---|---|---|---|---|---|
| month | date found (ringed birds) | date found (logger birds) | date died (logger birds) | date found (ringed birds) | date found (logger birds) |
| August | 2 | 0 | 0 | 0 | 0 |
| September | 1 | 0 | 0 | 0 | 0 |
| October | 2 | 0 | 0 | 0 | 0 |
| November | 4 | 0 | 0 | 0 | 0 |
| December | 2 | 0 | 0 | 2 | 0 |
| January | 10 | 1 | 4 | 0 | 0 |
| February | 23 | 3 | 0 | 2 | 0 |
| March | 11 | 0 | 1 | 1 | 0 |
| April | 6 | 1 | 0 | 2 | 0 |
| recovered | 61 | 5 | 7 | 0 | |
| alive summer 2003 | 1085 | 79 | 764 | 10 | |
| percentage recovered | 5.6 | 6.3 | 0.9 | 0.0 |
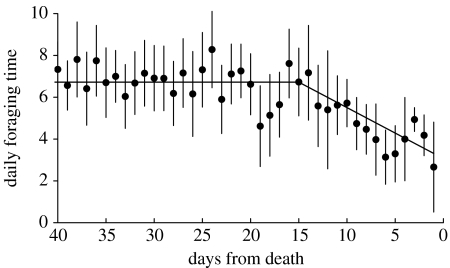
Logger data from the juveniles that died revealed a marked change in activity in the two weeks prior to death with birds spending progressively less time foraging (figure 2; REML on the 15 days prior to death, W=16.71; p<0.001).
Figure 2.
Mean (±s.e.) daily foraging time in relation to days before death. The best fitting model was a broken stick with flat asymptote (intercept 3.1; slope before break 0.24; break at 15.0 days and 6.7 h; r2=0.671; other significant candidate models: asymptotic exponential: r2=0.668; smoothing spline with 2 d.f.: r2=0.663).
4. Discussion
We collected novel data on juvenile foraging activity during two critical but poorly studied periods: post-fledging, and midwinter when peak mortality occurs. Crucially, we were able to show that the patterns observed reflected those shown by the population since timing and magnitude of mortality of instrumented birds was almost identical to the population as a whole.
Fledging heralds a period of increased energy requirements in juveniles as they become more active, and in species which exhibit post-fledging parental care, this may result in increased energy expenditure for parents, since prey capture rates of juveniles are likely to be low. Furthermore, adults may need to regain body condition once freed from the constraints of attending young at the nest. Our results are consistent with juveniles investing heavily in developing foraging skills while parents increased effort, although at present we cannot partition the latter into regaining condition and juvenile provisioning. The data suggest that juveniles are not fully independent until approximately 40 days after fledging, when adult foraging times returned to pre-fledging levels.
Neither adult nor juvenile shags carry substantial body reserves, and both overwinter in the same general area (Wanless & Harris 1997). Hence, differences in daily foraging times of the two age groups following independence reflect intrinsic differences in ability. The marked decrease in juvenile foraging times during August when adult values were increasing therefore suggests an improvement in juvenile proficiency. However, the decrease ceased after 20 days and over the following two months there was no further improvement, and young birds compensated for their lower proficiency by allocating 3 h more time per day to foraging than adults. Over this period, foraging times in both groups increased progressively indicating worsening environmental conditions. However, day length was also decreasing and by early November juveniles no longer had sufficient hours of daylight available and foraging time declined, closely tracking shortening days. We consider it unlikely that the decline reflected improved skills, rather, juveniles became limited by day length and were unable to compensate for poorer proficiency by increasing foraging time. Thus, foraging time was no longer a reflection of foraging success.
Daily foraging times of adults and juveniles converged in early December and tracked day length until early February. In the weeks following the winter solstice, five of the juveniles carrying loggers were found dead, and a similar peak in juvenile mortality was recorded in the much larger colour-ringed sample. The linear decline in foraging time in the last two weeks of life indicated that birds were unable to exploit increasing day length for foraging, but instead became increasingly moribund, spending on average just 3 h foraging immediately prior to death.
Our results suggest that juvenile foraging proficiency fell well short of adult levels during the period following independence. Reduced proficiency may be mediated through a variety of factors that we were unable to measure such as prey capture and handling skills, parasite loads and immunocompetence (Goss-Custard & Durell 1987; Stearns 1992; Gasparini et al. 2006). The limitation placed on juvenile foraging time by day length in winter, followed by the coincident moribund decline in foraging time among study juveniles and substantial mortality in the juvenile population, provides compelling evidence that foraging proficiency of juveniles is a key determinant of survival.
Acknowledgments
We thank Dave Stevens, Morten Frederiksen, Mike Harris, Mark Grantham, Jez Blackburn, Dirk Briggs, Mick Marquiss and Sue Lewis for logistics and discussions, Scottish Natural Heritage for access to the Isle of May and the British Ecological Society, Glasgow Natural History Society and The Seabird Group for funding.
Supplementary Material
In this section we explain why time in water is an accurate reflection of foraging time and foraging success because of two features of the biology of European shags: their wettable plumage and habit of not storing body reserves. We also describe the two situations relevant to this study in which foraging time does not reflect foraging success
In this section we describe how magnitude and timing of mortality was quantified in the two age classes from reported deaths in the winter 2003–2004 of birds recorded alive on the Isle of May the previous breeding season
References
- Aebischer N.J. Retrospective investigation of an ecological disaster in the shag, Phalacrocorax aristotelis: a general method based on long-term marking. J. Anim. Ecol. 1986;55:613–629. doi:10.2307/4743 [Google Scholar]
- Aebischer N.J. Philopatry and colony fidelity of shags Phalacrocorax aristotelis on the east coast of Britain. Ibis. 1995;137:11–18. [Google Scholar]
- Charlesworth B. Cambridge University Press; Cambridge, UK: 1980. Evolution in age-structured populations. [Google Scholar]
- Clutton-Brock T.H, Russell A.F, Sharpe L.L, Brotherton P.N.M, McIlrath G.M, White S, Cameron E.Z. Effects of helpers on juvenile development and survival and in meerkats. Science. 2001;293:2446–2449. doi: 10.1126/science.1061274. doi:10.1126/science.1061274 [DOI] [PubMed] [Google Scholar]
- Daunt F, Monaghan P, Wanless S, Harris M.P, Griffiths R. Sons and daughters: age-specific differences in parental rearing capacities. Funct. Ecol. 2001;15:211–216. doi:10.1046/j.1365-2435.2001.00515.x [Google Scholar]
- Daunt F, Afanasyev V, Silk J.R.D, Wanless S. Extrinsic and intrinsic determinants of winter foraging and breeding phenology in a temperate seabird. Behav. Ecol. Sociobiol. 2006;59:381–388. doi:10.1007/s00265-005-0061-4 [Google Scholar]
- Gasparini J, McKoy K.D, Tveraa T, Boulinier T. Related concentrations of specific immunoglobulins against the Lyme disease agent Borrelia burgdorferi sensu lato in eggs, young and adults of the kittiwake (Rissa tridatyla) Ecol. Lett. 2006;5:519–524. doi:10.1046/j.1461-0248.2002.00345.x [Google Scholar]
- Goss-Custard J.D, Durell S.E.A.le V.dit. Age-related effects in oystercatchers Haematopus ostralegus, feeding on mussels, Mytilus edulis. I Foraging efficiency and interference. J. Anim. Ecol. 1987;56:521–536. doi:10.2307/5065 [Google Scholar]
- Grémillet D, Tuschy I, Kierspel M. Body temperature and insulation in diving great cormorants and European shags. Funct. Ecol. 1998;12:386–394. doi:10.1046/j.1365-2435.1998.00199.x [Google Scholar]
- Grémillet D, Wright G, Lauder A, Carss D.N, Wanless S. Modelling the daily food requirements of wintering great cormorants: a bioenergetics tool for wildlife management. J. Appl. Ecol. 2003;40:266–277. [Google Scholar]
- Griffiths R, Daan S, Dijkstra C. Sex identification in birds using two CHD genes. Proc. R. Soc. B. 1996;263:1251–1256. doi: 10.1098/rspb.1996.0184. doi:10.1098/rspb.1996.0184 [DOI] [PubMed] [Google Scholar]
- Harris M.P, Buckland S.T, Russell S.M, Wanless S. Year-related and age-related variation in the survival of adult European shags over a 24-year period. Condor. 1994;96:600–605. doi:10.2307/1369462 [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Sutherland W.J, Jones D.W.F, Hadfield R.W. Age differences in the feeding ability of moorhens Gallinulla chloropus. Ibis. 1986;128:414–418. [Google Scholar]
- Wanless S, Harris M.P. Phalacrocorax aristotelis Shag. BWP Update. 1997;1:3–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this section we explain why time in water is an accurate reflection of foraging time and foraging success because of two features of the biology of European shags: their wettable plumage and habit of not storing body reserves. We also describe the two situations relevant to this study in which foraging time does not reflect foraging success
In this section we describe how magnitude and timing of mortality was quantified in the two age classes from reported deaths in the winter 2003–2004 of birds recorded alive on the Isle of May the previous breeding season