Abstract
Testosterone (T) is hypothesized to be an important honesty reinforcer of animal sexual signals. Owing to its immunosuppressive effects, only those individuals that can immunologically withstand high T levels can develop the most exaggerated traits. To date, few studies have isolated phenotypic or genotypic buffers that provide ‘high-quality’ animals with such an advantage. Dietary carotenoid pigments may in fact confer such a benefit because when in high supply carotenoids boost immunocompetence and coloration in animals like birds and fishes. We examined the experimental effect of T elevation on carotenoid and immune status in male and female zebra finches (Taeniopygia guttata) and found that T was immunostimulatory in a generalized cell-mediated challenge. We also detected a significant interaction between T treatment and the change in plasma carotenoids that occurred during the immune challenge; the relationship between blood carotenoid change and immunity was positive in controls and negative in T-implanted birds. This suggests that, while correlationally birds with high carotenoid stores were inherently better at mounting strong immune responses, experimentally administered T induced birds to deplete carotenoids for maximizing their health. Our findings highlight a nutrient-specific mechanism by which animals escape high immune costs of T elevation and thus can still elevate ornamentation.
Keywords: carotenoid coloration, hormones, immunity, Taeniopygia guttata, zebra finch
1. Introduction
The immunocompetence handicap hypothesis (ICHH) was advanced to explain how hormonal effects on the immune system can influence the costliness of sexual ornamentation in animals (Folstad & Karter 1992). According to this idea, androgens like testosterone (T) not only stimulate the development of secondary sexual traits, but also compromise immunity. Thus, individuals are faced with a physiological trade-off, and must either limit T elevation, hence improving health but at the cost of ornament expression, or have some means by which they can counteract T-induced immunosuppression and still develop or maintain exaggerated ornamentation. To date, such escape mechanisms have been thought to have genetic (Verhulst et al. 1999) or endocrine bases (Wingfield et al. 2001), whereby individuals of the highest quality either are genetically more resistant to parasites and thus can elevate T at a lower immunological cost, or can better regulate T physiologically (e.g. using binding proteins to render T inert, enzymatically converting T to less harmful steroids), elevating levels only when needed and hence minimizing its costs.
However, there are other types of molecules (e.g. nutrients, stress hormones like corticosterone; Poiani et al. 2000; Owen-Ashley et al. 2004) that can be associated with sexual ornament expression and that may affect the relationship between T and immunity. Such a group of candidate biochemicals is the carotenoids, which are diet-derived pigments that generate bright coloration in a variety of animals. Carotenoid pigmentation is commonly under androgenic control in animals such as fishes and birds (Jayasooriya et al. 2002; McGraw et al. 2006), but carotenoids themselves act as potent antioxidants and immunomodulators in these same vertebrate groups (Blount et al. 2003; Grether et al. 2004). This raises the possibility that animals with high carotenoid levels may be able to physiologically offset any immune costs suffered by elevating T to become more colourful.
We studied how T treatment influenced carotenoid circulation and immune performance in the zebra finch (Taeniopygia guttata). Beak colour in this species is a carotenoid-derived (McGraw et al. 2002), sexually selected trait (Burley & Coopersmith 1987). In previous studies, we showed that T elevates carotenoid levels in the bloodstream via lipoprotein upregulation, thus making birds more colourful (McGraw et al. 2006), and that dietary supplementation with carotenoids elevates beak pigmentation and multiple measures of immunity (McGraw & Ardia 2003). This led us to suspect in this species that, unlike typical T-induced immunosuppression, the high carotenoid levels circulated by high T birds might dampen, negate or even override the immunosuppressive effects of T (McGraw & Ardia 2005). We implanted a group of males and females with crystalline T and compared their response to a generalized cutaneous cell-mediated immune challenge (mitogen injection), as well as their changes in carotenoid levels that occurred during that challenge, to unimplanted control birds.
2. Material and methods
Thirty-five adult zebra finches were randomly selected from our captive breeding colony (McGraw et al. 2003), housed in male–female pairs under pre-breeding conditions (without nest cups or material) for at least a month prior to the study, and randomly assigned to either a sham-control or T-implanted treatment group (see electronic supplementary material for additional information). After the experiment, we administered a commonly used immune challenge—the phytohaemagglutinin (PHA) wing-web-swelling test (Martin et al. 2006)—which in previous studies was enhanced by an abundant carotenoid supply in zebra finches (see McGraw & Ardia (2003) for methods). Blood was drawn from the wing vein at the time of PHA injection and at the 24 h wing-web measurement time point for plasma carotenoid analysis via high-performance liquid chromatography (sensu McGraw et al. 2003).
We used two-way analysis of covariance (ANCOVA) to investigate the effects of T treatment, sex, change in plasma carotenoid concentration (as a covariate) and all interactions on cutaneous cell-mediated immune response (see electronic supplementary material for additional information).
3. Results
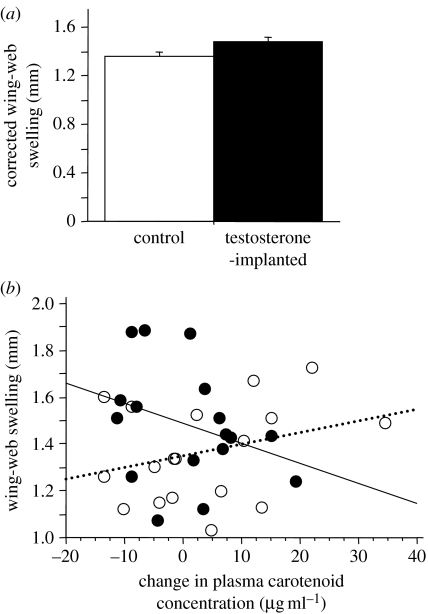
We found a significant effect of T treatment on cutaneous cell-mediated immunity (table 1). T-implanted birds mounted a stronger response (thicker wing-web swelling) to PHA injection than did control birds (figure 1a). The interaction between T treatment and change in plasma carotenoid concentration also had a significant effect on immunoresponsiveness (table 1). Control birds that had thicker wing-web swellings increased in plasma carotenoid concentration over the course of the experiment, whereas T-implanted birds with thicker wing-web swellings decreased in plasma carotenoid concentration during the study (figure 1b). We found no significant effect of sex or change in plasma carotenoid levels on wing-web swelling, though females tended to mount a stronger immune response to this challenge than did males (table 1).
Table 1.
ANCOVA table showing the effect of testosterone (T) treatment, sex, change in plasma carotenoid levels during the immune challenge and all possible interactions on cell-mediated immune response in captive zebra finches. (Significant terms are in bold. n=10 control females, 8 control males, 7 implanted females and 10 implanted males.)
| source of variation | d.f. | MS | F | p |
|---|---|---|---|---|
| T treatment | 1 | 0.304 | 71.6 | 0.013 |
| sex | 1 | 0.157 | 3.69 | 0.065 |
| change in plasma carotenoid levels | 1 | 0.0007 | 0.16 | 0.693 |
| T treatment×sex | 1 | 0.035 | 0.82 | 0.372 |
| T treatment×plasma carotenoid change | 1 | 0.188 | 4.42 | 0.045 |
| sex×plasma carotenoid change | 1 | 0.065 | 1.53 | 0.226 |
| T treatment×sex×plasma carotenoid change | 1 | 0.064 | 1.51 | 0.229 |
| residual | 27 | 0.042 |
Figure 1.
(a) Bar chart showing mean+s.e. differences between T-implanted and control zebra finches in ANCOVA-adjusted thicknesses of the wing web in response to a PHA injection. Sexes are not depicted separately here or in (b) because no significant sex effect was found. (b) Scattergram illustrating the significant interaction term (treatment×plasma carotenoid change) in our ANCOVA model; the relationship between wing-web swelling and change in carotenoid levels was positive for control birds (open circles, dashed line; r=0.31, 95% confidence interval for r=−0.19–0.68, p=0.22) and negative for T-implanted birds (filled circles, solid line; r=−0.32, 95% confidence interval for r=−0.70–0.19, p=0.21).
4. Discussion
We show that T did not depress cell-mediated immunity in zebra finches. The conserved immunosuppressive effects of T in male animals have been hotly debated, and the lack of T-induced immunosuppression is fairly commonly reported in a number of taxa (Roberts et al. 2004). However, we found evidence here that T treatment was immunoenhancing in zebra finches, and this was true in both sexes (T-mediated immunomodulation is rarely studied in females; Ketterson et al. 2005). Our previous work in zebra finches suggested that carotenoids may be available in sufficient amounts in T-implanted birds to offset any immunocompromising effects that elevated T might have. In fact, in the present experiment we found that the relationship between T and immunity was tied to carotenoid allocation in the body. T-implanted birds that mounted stronger immune responses decreased more in blood carotenoid supplies, implicating T in the active mobilization of circulating carotenoids for an immune boost (probably by offering antioxidant protection, which destroys carotenoid molecules, to active immune cells; Alonso-Alvarez et al. 2004). This suggests, unlike previously proposed genetic or endocrine mechanisms, that a nutrient serves as an overriding buffer for T-mediated immunosuppression. Put another way, the elevation of T appears to promote carotenoid accumulation that combats its immunosuppressive effects. Blas et al. (2006) recently demonstrated a similar relationship in red-legged partridges (Alectoris rufa), for which T implants increased plasma carotenoid levels and such high plasma carotenoid levels were associated with an increase in the same measure of cell-mediated immunity as ours.
Carotenoids also directly create sexual ornamentation in male and female zebra finches, however, and if they are depleted substantially by high circulating T in the most colourful birds, then this would break down the honesty of the signalling system (e.g. birds with faded beaks would have either low T and inherently fewer carotenoids or high T and depleted carotenoids). However, there are several pieces of evidence from this study and others in the literature that support the notion that more colourful birds do in fact have higher carotenoid stores, are healthier and can circulate higher T levels. For example, more colourful house finches (Carpodacus mexicanus) in the wild accumulate more carotenoids, carry fewer and better resist parasitic infections and circulate higher T levels (Hill 2002). In addition, control zebra finches in our study, unlike our T-implanted birds, showed a positive relationship between blood carotenoid changes and immunity, which probably reflects the true superiority of some birds at accumulating carotenoids while maintaining good health (as has been found in many single-time-point correlational studies of carotenoid status and health in birds; Hill 2006). Altogether, these and our results are consistent with the ICHH for sexual ornaments, such that birds using high T to elevate carotenoid coloration can in fact still maintain superior health status and ornamentation.
The fact that we found rare T-induced immunoenhancement that is putatively linked to carotenoid availability suggests that other animals with elevated carotenoid supplies or coloration should also experience such an advantage. On the contrary, a review of the literature in birds demonstrates that four out of the five carotenoid-coloured species studied to date in this context all experience some form of T-driven immunosuppression under laboratory experimental conditions (see electronic supplementary material for this review). However, in all of these studies, systemic carotenoid levels have been drained to very low levels (via the diet) in all animals, thus potentially leaving birds with few carotenoids in the body to combat infectious challenges. Zebra finches, on the other hand, are ideal models for such work because they maintain physiologically relevant carotenoid levels when fed the same seed diets (Hill 2006). Overall, future studies examining the interactions between carotenoids, androgens and disease should more carefully consider carotenoid variability statistically and experimentally (perhaps co-manipulating dietary carotenoid availability and T treatment) so that we can continue to improve our understanding of how these three traits covary.
Acknowledgments
Procedures in this study were approved by the Institutional Animal Care and Use Committee at Cornell University.
We thank D. Sheils, T. van Deusen and P. Smith for animal husbandry, E. Adkins-Regan for use of animals and facilities, and P. Deviche and three anonymous referees for their helpful comments on the manuscript. Support was provided by the Environmental Protection Agency (STAR fellowships to K.J.M. and D.R.A.) and the School of Life Sciences and the College of Liberal Arts and Sciences at Arizona State University (to K.J.M.).
Supplementary Material
Explanations of various procedures in our study
References
- Alonso-Alvarez C, Bertrand S, Devevey G, Gaillard M, Prost J, Faivre B, Sorci G. An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am. Nat. 2004;164:651–659. doi: 10.1086/424971. doi:10.1086/424971 [DOI] [PubMed] [Google Scholar]
- Blas J, Perez-Rodriguez L, Bortolotti G.R, Vinuela J, Marchant T.A. Testosterone increases bioavailability of carotenoids: insights into the honesty of sexual signaling. Proc. Natl Acad. Sci. USA. 2006;103:18 633–18 637. doi: 10.1073/pnas.0609189103. doi:10.1073/pnas.0609189103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount J.D, Metcalfe N.B, Birkhead T.R, Surai P.F. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300:125–127. doi: 10.1126/science.1082142. doi:10.1126/science.1082142 [DOI] [PubMed] [Google Scholar]
- Burley N, Coopersmith C. Bill colour preferences of zebra finches. Ethology. 1987;76:133–151. [Google Scholar]
- Folstad I, Karter A.J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi:10.1086/285346 [Google Scholar]
- Grether G.F, Kasahara S, Kolluru G.R, Cooper E.L. Sex-specific effects of carotenoid intake on the immunological response to allografts in guppies (Poecilia reticulata) Proc. R. Soc. B. 2004;271:45–49. doi: 10.1098/rspb.2003.2526. doi:10.1098/rspb.2003.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G.E. Oxford University Press; New York, NY: 2002. A red bird in a brown bag: the function and evolution of colorful plumage in the house finch. [Google Scholar]
- Hill G.E. Environmental regulation of ornamental coloration. In: Hill G.E, McGraw K.J, editors. Bird coloration. Mechanisms and measurements. vol. 1. Harvard University Press; Cambridge, MA: 2006. pp. 507–560. [Google Scholar]
- Jayasooriya A.P, Weisinger R.S, Weisinger H.S, Mathai M.L, Sinclair A.J. Attraction to orange: sexiness, not gluttony. Science. 2002;296:847–848. doi: 10.1126/science.296.5569.847b. doi:10.1126/science.296.5569.847b [DOI] [PubMed] [Google Scholar]
- Ketterson E.D, Nolan V, Jr, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on the evolution of sexual dimorphism, or both? Am. Nat. 2005;166:S85–S98. doi: 10.1086/444602. doi:10.1086/444602 [DOI] [PubMed] [Google Scholar]
- Martin L.B, II, Han P, Lewittes J, Kuhlman J.R, Klasing K.C, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct. Ecol. 2006;20:290–299. doi:10.1111/j.1365-2435.2006.01094.x [Google Scholar]
- McGraw K.J, Ardia D.R. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 2003;162:704–712. doi: 10.1086/378904. doi:10.1086/378904 [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Ardia D.R. Sex differences in carotenoid status and immune performance in zebra finches. Evol. Ecol. Res. 2005;7:251–262. [Google Scholar]
- McGraw K.J, Adkins-Regan E, Parker R.S. Anhydrolutein in the zebra finch: a new, metabolically derived carotenoid in birds. Comp. Biochem. Physiol. B. 2002;132:811–818. doi: 10.1016/s1096-4959(02)00100-8. doi:10.1016/S1096-4959(02)00100-8 [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Gregory A.J, Parker R.S, Adkins-Regan E. Diet, plasma carotenoids, and sexual coloration in the zebra finch (Taeniopygia guttata) Auk. 2003;120:400–410. doi:10.1642/0004-8038(2003)120[0400:DPCASC]2.0.CO;2 [Google Scholar]
- McGraw K.J, Correa S.M, Adkins-Regan E. Testosterone upregulates lipoprotein status to control sexual attractiveness in a colorful songbird. Behav. Ecol. Sociobiol. 2006;60:117–122. doi:10.1007/s00265-005-0135-3 [Google Scholar]
- Owen-Ashley N.T, Hasselquist D, Wingfield J.C. Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am. Nat. 2004;164:490–505. doi: 10.1086/423714. doi:10.1086/423714 [DOI] [PubMed] [Google Scholar]
- Poiani A, Goldsmith A.R, Evans M.R. Ectoparasites of house sparrows (Passer domesticus): an experimental test of the immunocompetence handicap hypothesis and a new model. Behav. Ecol. Sociobiol. 2000;47:230–242. doi:10.1007/s002650050660 [Google Scholar]
- Roberts M.L, Buchanan K.L, Evans M.R. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. doi:10.1016/j.anbehav.2004.05.001 [Google Scholar]
- Verhulst S, Dieleman S.J, Parmentier H.K. A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Proc. Natl Acad. Sci. USA. 1999;96:4478–4481. doi: 10.1073/pnas.96.8.4478. doi:10.1073/pnas.96.8.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J.C, Lynn S, Soma K.K. Avoiding the ‘costs’ of testosterone: ecological bases of hormone–behavior interactions. Brain Behav. Evol. 2001;57:239–251. doi: 10.1159/000047243. doi:10.1159/000047243 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Explanations of various procedures in our study