Abstract
Surprisingly little is known about the role of acoustic cues in mammal female mate choice. Here, we examine the response of female red deer (Cervus elaphus) to male roars in which an acoustic cue to body size, the formants, has been re-scaled to simulate different size callers. Our results show that oestrous red deer hinds prefer roars simulating larger callers and constitute the first evidence that female mammals use an acoustic cue to body size in a mate choice context. We go on to suggest that sexual selection through female mating preferences may have provided an additional selection pressure along with male–male competition for broadcasting size-related information in red deer and other mammals.
Keywords: red deer, female mating preferences, vocal communication, formant frequencies, acoustic cues to body size
1. Introduction
Sexual selection theory predicts that females should mate selectively with high-quality males (Darwin 1871), and that they should choose their mates according to signals that reliably indicate male quality (Maynard Smith 1991). Numerous studies of animal vocal communication have confirmed that acoustic signals conveying some aspect of male quality influence female choice (for a review, see Andersson (1994)). However, the role of vocal signals in mammal female mate choice has received comparatively little attention. Whereas previous work on mammals has often suggested that male calls function to advertise male quality and directly attract females (McElligott et al. 1999; Craul et al. 2004), very few have been able to show conclusively, using systematic playback experiments, whether or not this is the case (but see McComb 1991).
The roaring of red deer (Cervus elaphus) stags is known to affect the outcome of male–male interactions, advance female ovulation and influence mate attraction (for a review on red deer roaring, see Reby & McComb (2003b)). Whereas earlier studies concentrated on more conspicuous features of the male call such as roaring rate, more recent work shows that hinds are able to discriminate between the roars of their harem holder and other stags (Reby et al. 2001), and suggests that females have the ability to attend to the finer acoustic structure of male roars. In the current study, we use re-synthesized calls and playback experiments to examine the behavioural responses of oestrous red deer hinds to male roars in which a specific acoustic cue to body size, the formant frequencies, has been modified to simulate callers of different body sizes.
Formants are the resonance frequencies of the vocal tract and are visualized as broadband frequency peaks in the spectral acoustic structure of vocalizations. While formants are the key acoustic parameters underlying phonemic variation and vowel identity in human speech (Titze 1994), the ability to use formants to discriminate between different human speech sounds is not exclusive to humans, but also present in a range of non-human animals (for a review, see Fitch (2002)). In addition, formants are ubiquitous in the vocalizations of animals lacking speech (Riede & Zuberbühler 2003; Rendall et al. 2004), suggesting that they may have functional significance in many non-human animal species-specific communication systems. In particular, formants have the potential to provide reliable information on the callers' body size in several mammals (Fitch 1997; Riede & Fitch 1999; Harris et al. 2006) including red deer (Reby & McComb 2003a), due to a close relationship between formant spacing, the caller's vocal tract length and overall body size. Recent work has demonstrated that red deer stags use formants in the roars of simulated rivals as acoustic cues to body size (Reby et al. 2005) and that red deer hinds perceive formant shifts (Charlton et al. in press); this raises the possibility that female red deer may make use of size-related formant information in a mate choice context.
Here we directly investigate whether oestrous adult red deer hinds use formants as acoustic cues to body size during mate assessment. We hypothesize that when presented with re-synthesized roar bouts originating from four stags, and simulating adult size variants representative of the natural population, oestrus hinds will prefer roars in which the formants indicate larger males. In addition, to determine whether any directional preferences existed for relatively larger males, we presented oestrous hinds with large and small size variants outside the natural adult range. Again, we suggest that oestrous adult hinds will prefer roars in which the formants indicate the larger size variant when presented with these supernormal stimuli.
2. Material and methods
To test our hypothesis, two independent playback experiments using re-synthesized roar stimuli were conducted at Redon Experimental Deer Farm, France; each with two conditions simulating stags of different apparent vocal tract lengths and body sizes played from one of two speakers (for details of acoustic analysis and roar re-synthesis, see electronic supplementary material). In experiment 1, the playback stimuli simulated stags with vocal tract lengths of 85.7 and 77.5 cm, corresponding to males of large and small body sizes representative of the natural population. The playback stimuli for experiment 2 simulated stags with vocal tract lengths of 101.7 and 67.8 cm, corresponding to adults of abnormally large and small body sizes outside the natural range. In both experiments, the size variant played from each speaker and the exemplar used to simulate the size variants were alternated to control for any preferences for areas of the experimental site or particular exemplars. Moreover, by balancing the presentation of each of the four original stag exemplars across subjects, we could assess whether the origin of the re-synthesized playback stimuli affected hind response.
Each of the playback sequences consisted of six roar bouts from each of two different stag exemplars representing each size variant and were broadcast in a manner that simulated a natural vocal interaction between two rutting males. A total of four unique combinations of exemplar pairings were presented to the hinds and their movement responses quantified from a position equidistant from both speakers (figure 1). One hour later, each of the hinds was returned to the experimental enclosure, this time with exit gates leading towards either speaker open, and re-exposed to the same playback trial that they had been presented in the initial part of the experiment (with the order of the roar bout sequences A–F reversed to minimize habituation). This was done to determine the amount of time each hind spent within a 3 m radius of either speaker, termed the proximity zone (Clayton 1990), see figure 2.
Figure 1.
Experimental set-up and spectrograms of the large and small adult size variants inside the natural range. The arrow indicates the seventh formant (F7). VTL refers to apparent vocal tract length.
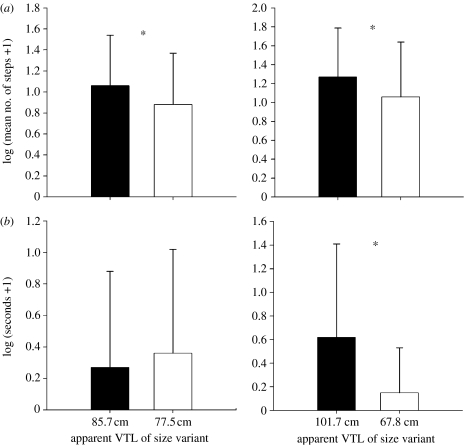
Figure 2.
Error bar charts showing log-transformed means+s.e. of hind behavioural responses (*p<0.05). (a) Movement towards either speaker and (b) time in proximity zones.
3. Results
When male size variants within the natural adult range were played back, hinds took significantly more steps towards the speaker playing the large size variant than the speaker playing the small size variant (F1,12=4.945, p=0.046). When presented with male roars simulating adult size variants outside the normal range, hinds took significantly more steps towards the speaker playing the abnormally large size variant than the speaker playing the abnormally small size variant (F1,17=9.893, p=0.006). The amount of time spent by the hinds within the proximity zone of both large and small adult size variants within the natural range was not significantly different (F1,12=0.164, p=0.693). However, hinds did spend significantly longer in the abnormally large size variants proximity zone than that of the abnormally small size variant (F1,17=4.972, p=0.040).
The combination of unique exemplar pairings used to create the playback sequences (exemplar pairing) had no effect on the number of steps taken by the hinds towards either speaker (experiment 1: F3,12=0.293, p=0.829; experiment 2: F3,17=0.582, p=0.635) or the amount of time spent in either proximity zone (experiment 1: F3,12=1.294, p=0.347; experiment 2: F3,17=0.806, p=0.508), indicating that the origin of our re-synthesized playback stimuli did not affect hind response. Additionally, there was no interaction between exemplar pairing and size variant condition for steps taken towards either speaker (experiment 1: F3,12=1.079, p=0.395; experiment 2: F3,17=0.640, p=0.599) or time spent in either proximity zone (experiment 1: F3,12=1.343, p=0.307; experiment 2: F3,17=0.559, p=0.650), indicating that the exemplar pairing had no effect on the behavioural response of the hinds to the different size variants.
4. Discussion
Our results indicate that oestrous red deer hinds move preferentially towards male roars with lower formant values and spacing, simulating callers of larger body size, and constitute the first evidence that a non-human female mammal uses an acoustic cue to body size in a mate choice context. In the wild, the active approach to a stag by an oestrus hind invariably leads to mating (Clutton-Brock et al. 1982). In our experiment, the movement of the hinds towards the playback source simulating the presence of a stag is clearly equivalent to a mating choice/decision, and the preferential movement towards one type of stimulus can safely be assumed to reflect a mating preference. Furthermore, this preference was maintained when the size variants used for the roar stimuli fell outside the typical adult range. Extrapolating to mate choice in the wild, this response pattern suggests that when given a relative choice between different size callers hinds will mate preferentially with males producing roars with lower formant values, and may indicate the presence of directional selection on this feature of the male roar.
Hinds often switch harems and associate with more stags during oestrus (Clutton-Brock et al. 1982). On the basis of these results, stags producing roars with lower formant values and spacing would be more likely to attract oestrous hinds towards their harems and keep their own females. Indeed, such a female preference may, at least partly, explain the correlation that exists between male reproductive success and vocal tract length, and hence minimum formant values, in this species (Reby & McComb 2003a). Moreover, this female preference could evolve and be maintained because oestrous hinds choosing the harems of larger males are expected to gain the indirect benefits of larger, more competitive offspring when mated, and possibly the direct benefits of greater protection when mating.
The mobile larynx of red deer stags suggests the presence of especially strong selection pressures to exaggerate and broadcast body size through formant lowering (Fitch & Reby 2001). Red deer stags have recently been shown to use formants as acoustic cues to body size during agonistic interactions (Reby et al. 2005). The results of the current study suggest that female mating preferences, for male roars in which lower formant values and spacing indicate larger callers, may have provided an additional selection pressure alongside male–male competition for broadcasting size-related formant information in red deer. More generally, the laryngeal descent observed in other mammals, either permanently descended and/or descended during vocalizations (Fitch & Reby 2001; Weissengruber et al. 2002; Frey & Gebler 2003), may also be the result of evolutionary pressures to exaggerate size in a mate choice context.
Acknowledgments
This work was carried out under the Association for the study of Animal Behaviour/Animal Behaviour Society guidelines for the use of animals in research.
We thank INRA and all the staff at Redon. BBSRC provided financial support to B.C. and D.R. was supported by funding from the Nuffield Foundation.
Supplementary Material
Details on control of hind hormonal state; acoustic analysis and re-synthesis of male roar stimuli; experimental setup and equipment; structure of the playback trials; statistical analysis and origin of playback stimuli
Descriptive statistics of both the raw and log transformed data for hind behavioural responses to the different adult size variants
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Charlton, B. D., Reby, D. & McComb, K. In press. Female perception of size related formants in red deer (Cervus elaphus). Anim. Behav
- Clayton N.S. Mate choice and pair formation in Timor and Australian mainland zebra finches. Anim. Behav. 1990;39:474–480. doi:10.1016/S0003-3472(05)80411-7 [Google Scholar]
- Clutton-Brock T.H, Albon S, Guinness F.E. University of Chicago Press; Chicago, IL: 1982. Red deer: behavior and ecology of two sexes. [Google Scholar]
- Craul M, Zimmermann E, Radespiel U. First experimental evidence for female mate choice in a nocturnal primate. Primates. 2004;45:271–274. doi: 10.1007/s10329-004-0097-5. doi:10.1007/s10329-004-0097-5 [DOI] [PubMed] [Google Scholar]
- Darwin C. Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Fitch W.T. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 1997;102:1213–1222. doi: 10.1121/1.421048. doi:10.1121/1.421048 [DOI] [PubMed] [Google Scholar]
- Fitch W.T. Comparative vocal production and the evolution of speech: reinterpreting the descent of the larynx. In: Wray A, editor. The transition to language. Oxford University Press; Oxford, UK: 2002. pp. 21–45. [Google Scholar]
- Fitch W.T, Reby D. The descended larynx is not uniquely human. Proc. R. Soc. B. 2001;268:1669–1675. doi: 10.1098/rspb.2001.1704. doi:10.1098/rspb.2001.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey R, Gebler A. The highly specialized vocal tract of the male Mongolian gazelle (Procapra gutturosa Pallas, 1777—Mammalia, Bovidae) J. Anat. 2003;203:451–471. doi: 10.1046/j.1469-7580.2003.00232.x. doi:10.1046/j.1469-7580.2003.00232.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.R, Fitch W.T, Goldstein L.M, Fashing P.J. Black and white colobus monkey (Colobus guereza) roars as a source of both honest and exaggerated information about body mass. Ethology. 2006;112:911–920. doi:10.1111/j.1439-0310.2006.01247.x [Google Scholar]
- Maynard Smith J. Theories of sexual selection. Trends Ecol. Evol. 1991;6:146–151. doi: 10.1016/0169-5347(91)90055-3. doi:10.1016/0169-5347(91)90055-3 [DOI] [PubMed] [Google Scholar]
- McComb K.E. Female choice for high roaring rates in red deer, Cervus elaphus. Anim. Behav. 1991;41:79–88. doi:10.1016/S0003-3472(05)80504-4 [Google Scholar]
- McElligott A.G, O'Neill K.P, Hayden T.J. Cumulative long-term investment in vocalization and mating success of fallow bucks, Dama dama. Anim. Behav. 1999;57:1159–1167. doi: 10.1006/anbe.1999.1076. doi:10.1006/anbe.1999.1076 [DOI] [PubMed] [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim. Behav. 2003a;65:519–530. doi:10.1006/anbe.2003.2078 [Google Scholar]
- Reby D, McComb K. Advances in the study of behavior. vol. 33. Academic Press, Inc; San Diego, CA: 2003b. Vocal communication and reproduction in deer. pp. 231–264. [Google Scholar]
- Reby D, Hewison M, Izquierdo M, Pepin D. Red deer (Cervus elaphus) hinds discriminate between the roars of their current harem-holder stag and those of neighbouring stags. Ethology. 2001;107:951–959. doi:10.1046/j.1439-0310.2001.00732.x [Google Scholar]
- Reby D, McComb K, Cargnelutti B, Darwin C, Fitch W.T, Clutton-Brock T.H. Red deer stags use formants as assessment cues during intrasexual agonistic interactions. Proc. R. Soc. B. 2005;272:941–947. doi: 10.1098/rspb.2004.2954. doi:10.1098/rspb.2004.2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendall D, Owren M.J, Weerts E, Hienz R.D. Sex differences in the acoustic structure of vowel-like grunt vocalizations in baboons and their perceptual discrimination by baboon listeners. J. Acoust. Soc. Am. 2004;115:411–421. doi: 10.1121/1.1635838. doi:10.1121/1.1635838 [DOI] [PubMed] [Google Scholar]
- Riede T, Fitch W.T. Vocal tract length and acoustics of vocalization in the domestic dog (Canis familiaris) J. Exp. Biol. 1999;202:2859–2867. doi: 10.1242/jeb.202.20.2859. [DOI] [PubMed] [Google Scholar]
- Riede T, Zuberbühler K. The relationship between acoustic structure and semantic information in Diana monkey alarm vocalization. J. Acoust. Soc. Am. 2003;114:1132–1142. doi: 10.1121/1.1580812. doi:10.1121/1.1580812 [DOI] [PubMed] [Google Scholar]
- Titze I.R. Prentice Hall; Englewood Cliffs, NJ: 1994. Principles of voice production. [Google Scholar]
- Weissengruber G.E, Forstenpointner G, Peters G, Kubber-Heiss A, Fitch W.T. Hyoid apparatus and pharynx in the lion (Panthera leo), jaguar (Panthera onca), tiger (Panthera tigris), cheetah (Acinonyx jubatus) and domestic cat (Felis silvestris f. catus) J. Anat. 2002;201:195–209. doi: 10.1046/j.1469-7580.2002.00088.x. doi:10.1046/j.1469-7580.2002.00088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details on control of hind hormonal state; acoustic analysis and re-synthesis of male roar stimuli; experimental setup and equipment; structure of the playback trials; statistical analysis and origin of playback stimuli
Descriptive statistics of both the raw and log transformed data for hind behavioural responses to the different adult size variants