Abstract
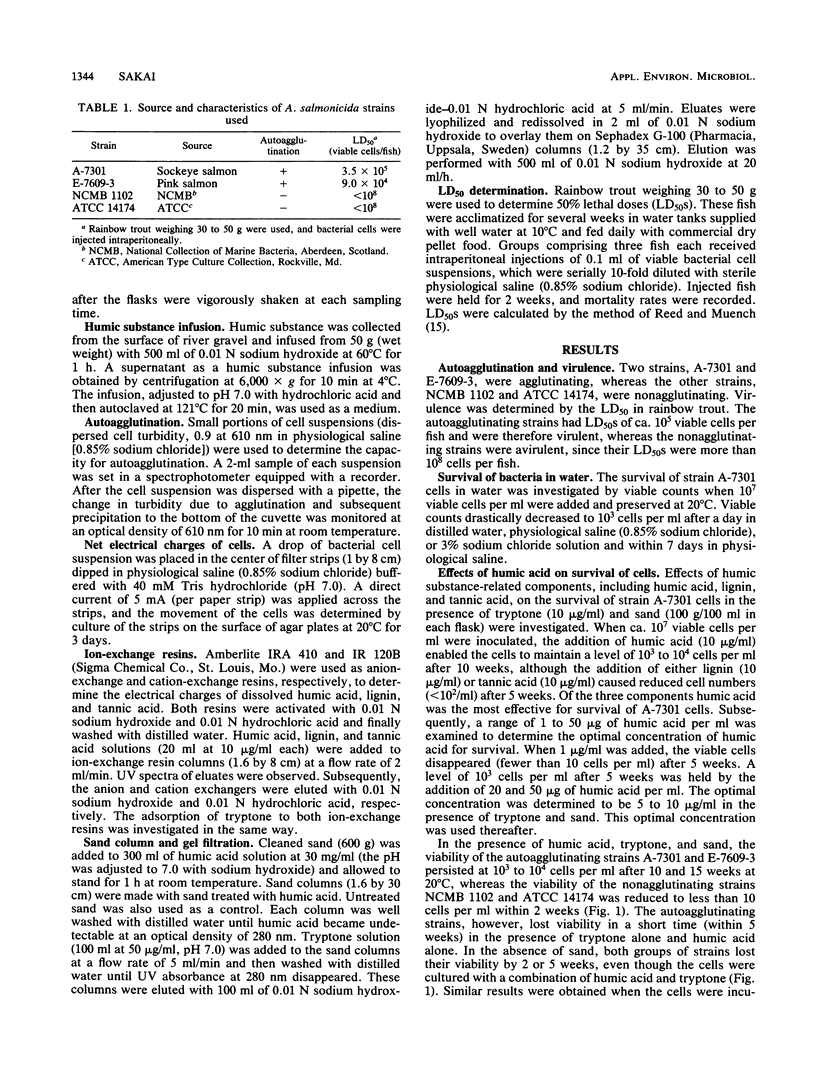
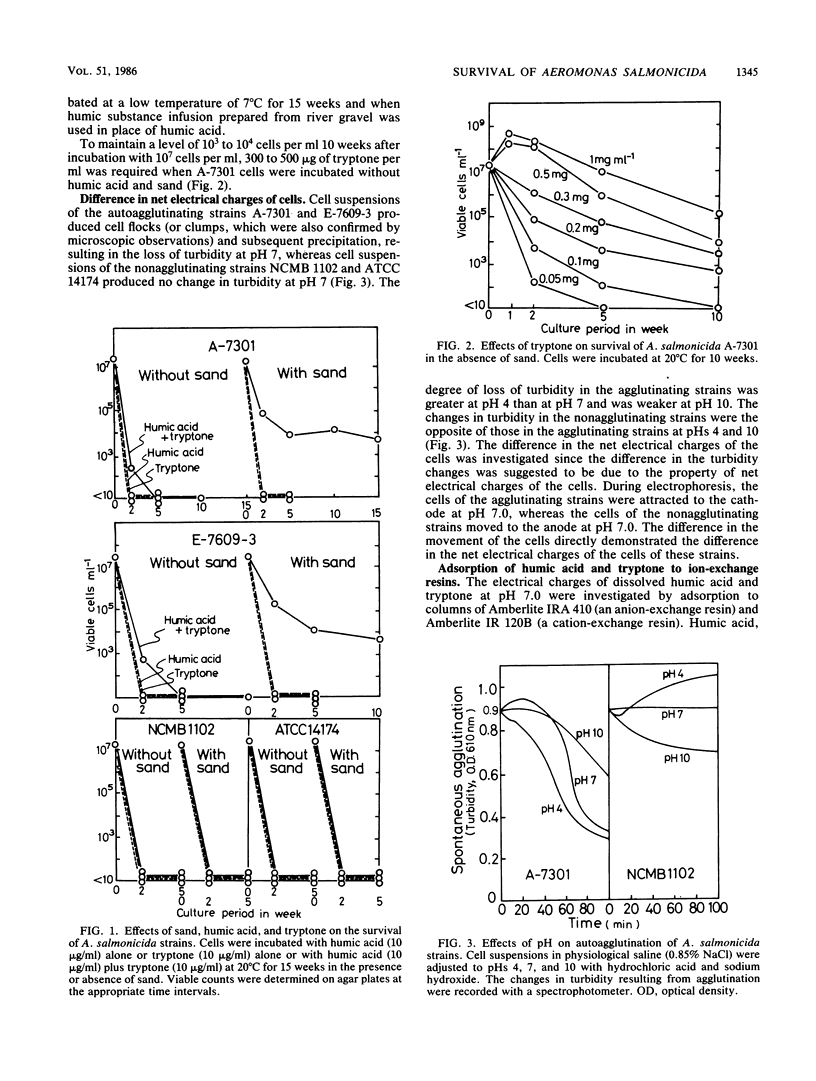
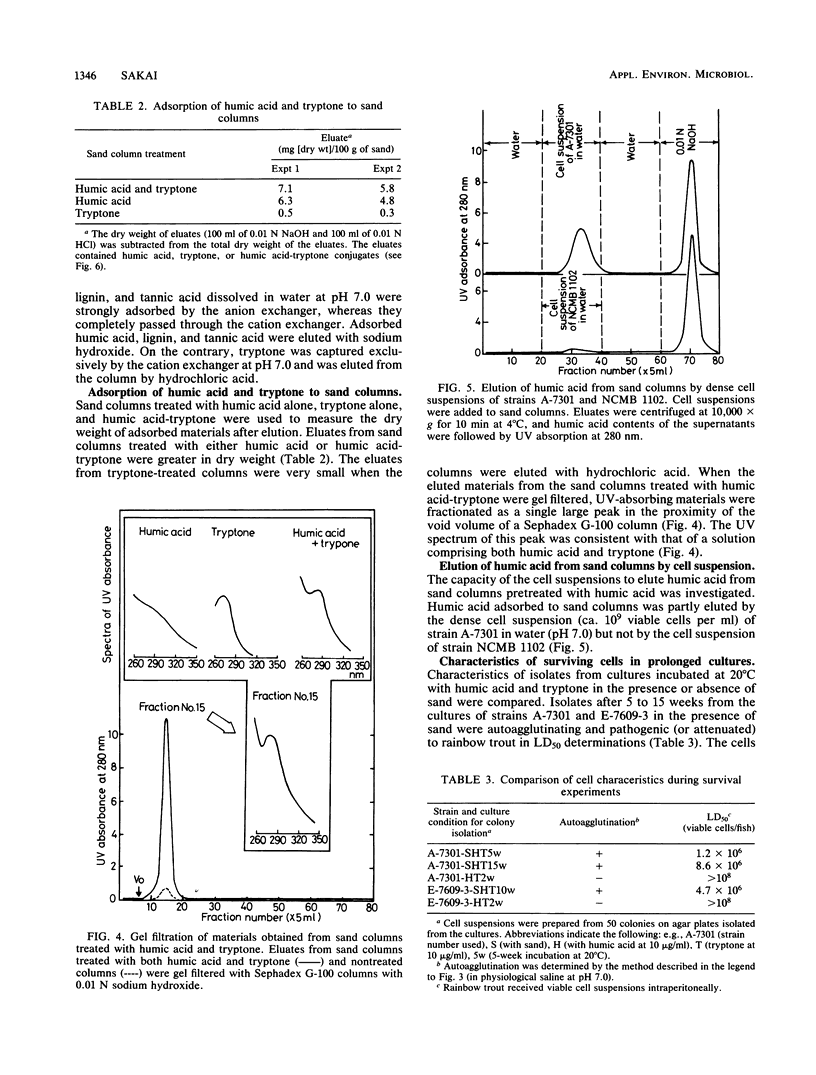
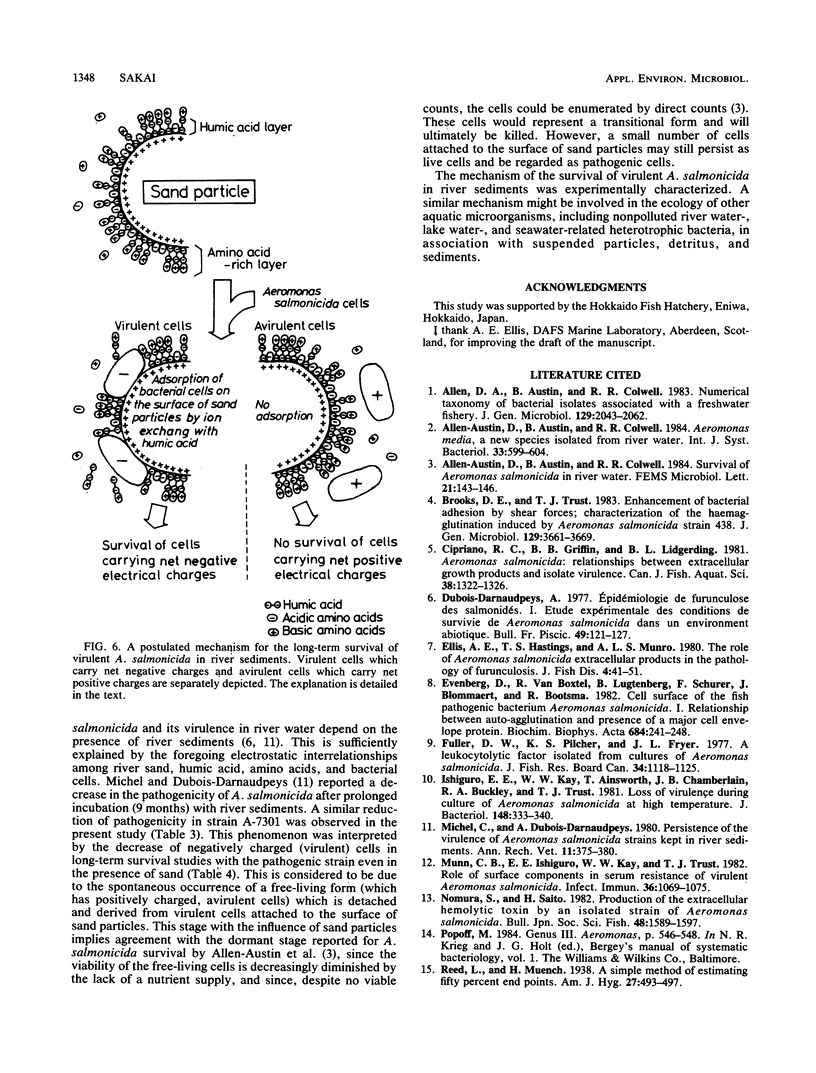
The ecological mechanism of survival of Aeromonas salmonicida, the bacterial pathogen of fish furunculosis, in river water was investigated by laboratory-based experiments with two virulent strains (which were autoagglutinating) and two virulent strains (which were nonagglutinating). A difference in net electrical charge of A. salmonicida cells was detected by electrophoresis; cells of the virulent strains were negative, whereas cells of the avirulent strains were positive. Despite the loss of viable cells within a week in distilled water and physiological saline (0.85% sodium chloride), the cells of the virulent strains survived for more than 15 weeks in the presence of diluted humic acid (10 micrograms/ml), tryptone (10 micrograms/ml), and cleaned river sand (100 g/100 ml of medium), but loss of viable cells occurred within 5 weeks in the absence of sand. The cells of the avirulent strains lost viability within 2 weeks with no relation to the presence of sand. Using ion-exchange columns, humic acid and the amino acids of tryptone were found to be anionic and cationic in water (pH 7.0), respectively. Sand particles had a high capacity to adsorb humic acid alone and amino acid-humic acid complexes. Thirty to fifty times the environmental concentration of amino acids (10 micrograms/ml) were accumulated on the surface of sand particles, thereby permitting only bacterial cells carrying net negative electrical charges (virulent cells) to survive for a long period on the surface of the sand particles. These electrostatic interrelationships among river sand, humic acid, and bacterial cells are closely implicated in the mechanism of long-term survival of virulent A. salmonicida in river sediments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks D. E., Trust T. J. Enhancement of bacterial adhesion by shear forces: characterization of the haemagglutination induced by Aeromonas salmonicida strain 438. J Gen Microbiol. 1983 Dec;129(12):3661–3669. doi: 10.1099/00221287-129-12-3661. [DOI] [PubMed] [Google Scholar]
- Evenberg D., Van Boxtel R., Lugtenberg B., Schurer F., Blommaert J., Bootsma R. Cell surface of the fish pathogenic bacterium Aeromonas salmonicida. I. Relationship between autoagglutination and the presence of a major cell envelope protein. Biochim Biophys Acta. 1982 Jan 22;684(2):241–248. doi: 10.1016/0005-2736(82)90012-8. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Kay W. W., Ainsworth T., Chamberlain J. B., Austen R. A., Buckley J. T., Trust T. J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J Bacteriol. 1981 Oct;148(1):333–340. doi: 10.1128/jb.148.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C., Dubois-Darnaudpeys A. Persistence of the virulence of Aeromonas salmonicida strains kept in river sediments. Ann Rech Vet. 1980;11(4):375–380. [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. K. Loss of virulence in a protease-deficient mutant of Aeromonas salmonicida. Infect Immun. 1985 Apr;48(1):146–152. doi: 10.1128/iai.48.1.146-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. K. Significance of Extracellular Protease for Growth of a Heterotrophic Bacterium, Aeromonas salmonicida. Appl Environ Microbiol. 1985 Oct;50(4):1031–1037. doi: 10.1128/aem.50.4.1031-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



