Summary
Initial studies on the biology of IL-27 provided evidence of a role for this cytokine in the initiation of Th1 responses; however, subsequent work using models of pathogen-induced and autoimmune inflammation have indicated that IL-27 has broad inhibitory effects on Th1, Th2 and Th17 subsets of T cells as well as the expansion of inducible regulatory T cells. While, the aim of this review is to highlight the functions of IL-27 in the context of inflammation it will also serve to elaborate on the molecular mechanisms involved in the production of this cytokine. The initial description of IL-27 indicated that classical antigen-presenting cells such as macrophages and dendritic cells produce IL-27, however, the agonists and signaling pathways involved in activating transcription of the two subunits of IL-27, p28 and EBV-induced gene 3 (EBI3) have only recently been described.
Keywords: inflammation, cytokines
Introduction
The IL-6 sub-family of type I cytokines, includes a number of immune modulators: IL-6, IL-12, IL-23 and IL-27 that are defined based on similarities in the structural motifs of the ligands, such as a common four-helix bundle, and their receptors, which contain a hematopoietin receptor domain[1]. These cytokines are involved in the development and regulation of immune responses, and they initiate their activity through membrane bound receptor complexes that include either IL-12Rβ1 or gp130[1]. One of the defining members of this sub-family is IL-6, which is a single subunit cytokine that binds a unique IL-6Rα chain and gp130. The IL-6Rα chain can be found as a membrane bound form or as a soluble version, which can bind IL-6 and this complex can signal in trans through gp130[2]. The existence of this additional pathway for IL-6 signaling provides evidence for how the heterodimeric cytokines of this sub-family may have evolved. The signature cytokine of this sub-family, IL-12, is a heterodimer composed of p40 and p35, which is secreted by cells of the innate immune system. It promotes the production of IFN-γ by CD4+ and CD8+ T cells, and NK cells. The biological effects of IL-12 are mediated through its binding of a high affinity receptor composed of IL-12Rβ1 and IL-12Rβ2, which activates the Jak/STAT signaling pathway, a common feature of this class of cytokine receptors[3]. Consistent with a role in promoting inflammation IL-23, which is composed of p40 and a p35-like subunit p19, has been linked to the maintenance of a population of T cells that secrete IL-17 and which have a protective role against a number of extracellular pathogens[4–8]. Additionally, this Th17 population of T cells has been associated with the development of a number of autoimmune diseases in mice and humans[9–13].
Similar to IL-12 and IL-23, IL-27 is a heterodimeric cytokine consisting of EBI3 (an IL-12p40 homologue originally described to be secreted by Epstein-Barr-Virus-transformed B cells[14]) and p28, an IL-6 and p35 homologue[15]. IL-27 employs a unique receptor subunit IL-27ra (also known as WSX-1 or TCCR) paired with gp130 for signaling[15–17]. While activated T cells and NK cells express the highest levels of the IL-27R[18] there are a range of cells that co-express IL-27ra and gp130, including naïve T cells, mast cells, endothelial cells, activated B cells, monocytes, Langerhan’s cells and activated dendritic cells[15–17,19]. The expression of the full IL-27 receptor complex by these cell types indicate that they should be fully responsive to the effects of IL-27 as several studies have shown[20–22].
Produced primarily by antigen presenting cells (APCs) as well as neutrophils, IL-27 signaling results in the phosphorylation of a number of Jak and STAT proteins including Jak1, Jak2, Tyk2, STAT1, STAT3, STAT4 and STAT5 in T cells[23–26]. Other members of this cytokine family activate many of these same proteins, yet each of these interleukins displays unique properties with little functional overlap. Given the similarities in structure between this family of cytokines and their receptors it is possible that additional heterodimeric complexes can form. For example, an association between EBI3 and p35 has been documented[27], but until recently no distinct function had been assigned to this cytokine pairing. However, two recently published studies have provided evidence that CD4+CD25+Foxp3+ regulatory T cells secrete this heterodimer of EBI3 and p35, which has been designated IL-35, and may mediate the suppressive activity of this subset of T cells[28,29].
Regulation of IL-27 production
An understanding of the factors that trigger when, where and what cell types produce IL-27 is critical to understanding when this cytokine is available to modulate T cell responses. As mentioned previously, IL-27 is produced by APCs including dendritic cells (DCs) and macrophages, which monitor their local environment for invading pathogens. Increased expression of IL-27 has been associated with sites of inflammation during infection with Mycobacteria tuberculosis, Trichuris muris, and Toxoplasma gondii[20,26,30,31]. Similarly IL-27 expression is linked to auto-immune diseases such as patients with Crohn’s disease[32] and in mouse models of uveitis and multiple sclerosis[13,33–36]. In addition, increases in IL-27 production observed during experimental autoimmune encephalomyelitis (EAE) and chronic T. gondii infection have been associated with CNS resident cells such as microglia and astrocytes[31,34,35].
Consistent with the idea that the innate immune system regulates many aspects of the adaptive immune system, EBI3 expression can be upregulated by pathogen and host-derived inflammatory stimuli including LPS, CD40 ligation or exposure to inflammatory cytokines[15,23,37–40]. Similar to IL-12p35, IL-27p28 is poorly secreted unless it is co-expressed with its partner EBI3, thus creating a situation where expression of IL-27 can be tightly controlled during an immune response. Enhanced expression of the subunits of IL-12 (p40 and p35) and IL-23 (p40 and p19) are induced by binding of Toll-like receptor agonists (such as CpG, PolyI:C and LPS) on APCs. Likewise, stimulation of human monocyte derived DCs (MoDC) as well as murine bone marrow derived dendritic cells (BMDCs) and macrophages with TLR agonists for TLR3 or TLR4, resulted in increased expression of the two subunits of IL-27[15,23,40–46]. Moreover, binding of double-stranded DNA to TLR9 on murine BMDCs also induced IL-27[47]. In contrast, stimulation with TLR2 and TLR7/8 agonists had only moderate effects on the expression of p28 and EBI3[43,45–47]. Additionally, human DCs upregulated IL-27p28 following priming with commensal gram negative but not gram positive bacteria[48]. However, incubation of human DCs with a pathogenic strain of gram positive bacteria, Streptococcus pyogenes, did increase the expression of p28 and EBI3, while the non-pathogenic gram positive Lactobacillus rhamnosus did not upregulate IL-27 mRNA. An important question that remains is whether p35, p19 and p28 can be coexpressed by the same APC or if the signaling events following TLR ligation lead to the expression of only one particular cytokine whilst repressing transcription of the other cytokine subunits within the same cell.
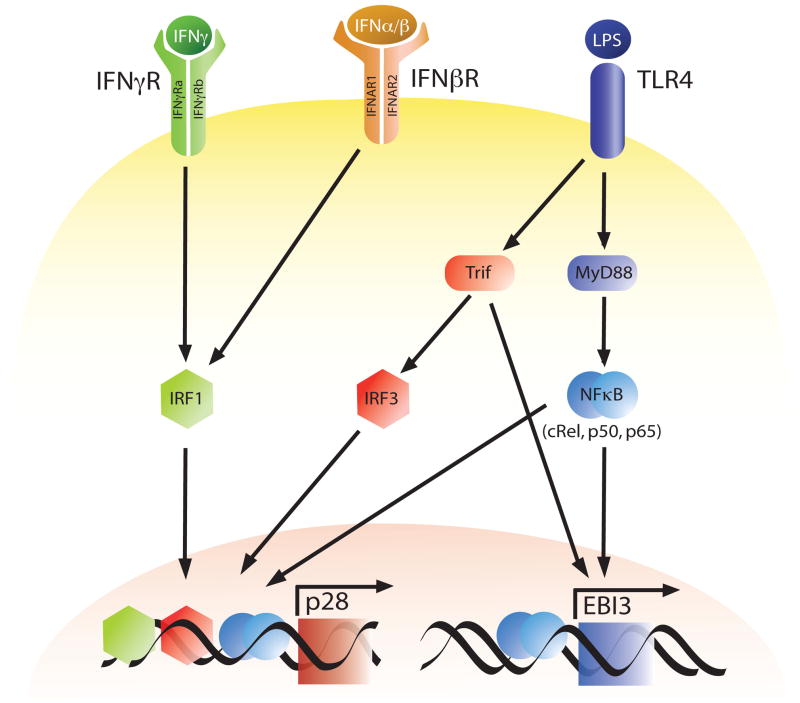
While the transcriptional regulation of IL-12 p40/p35 has been well studied, the signaling pathways and transcription factors involved in the regulation of IL-27 gene expression have only recently been defined. Thus, several groups have examined the molecular mechanisms involved in the production of the individual subunits of IL-27 in macrophages or DCs[40,42,43,45,46] (Figure 1). Consistent with the finding that the TLR4 agonist LPS upregulates transcripts for p28 and EBI3, the adapter protein MyD88 was found to be required for these events[40,42,43]. Similarly, the NF-κB family of transcription factors have been shown to play a vital role in LPS-mediated production of pro-inflammatory cytokines, and c-Rel is required for the transcription of IL-12 p35[49]. Liu and colleagues have also shown that c-Rel is required for optimal LPS-induced IL-27 p28 protein and mRNA expression[42]. In addition, the NF-κB family members p50 and p65 have also been linked to the expression of EBI3 as these transcription factors bind the EBI3 promoter in BMDCs, and the absence of p50 results in decreased EBI3 gene expression in response to LPS[40]. Moreover, Wirtz et al., showed that NF-κB p50/p65 was able to synergize with the Ets transcription factor PU.1 to increase EBI3 transcription[40].
Fig. 1.
Transcriptional Regulation of IL-27p28 and EBI3. Expression of IL-27 p28 and EBI3 are upregulated through a MyD88 or TRIF dependent signaling cascade in antigen presenting cells following stimulation with the TLR4 agonist LPS. Additionally, type I and II interferons can induce p28 transcription in an IRF1 dependent manner.
In addition to MyD88 there are other adaptor proteins linked to TLR signaling such as TRIF, which is associated with TLR4 and TLR3, and serves to activate IFN regulatory factor (IRF) 3, an important transcription factor for the expression of IFN-β[50]. In the absence of TRIF, Molle and colleagues showed that murine BMDCs stimulated with LPS are deficient in their ability to upregulate p28 and EBI3 gene expression[43]. Similarly, LPS induced p28 synthesis, but not EBI3 was significantly diminished in IRF3−/− BMDCs, thus, establishing a role for the MyD88-independent TRIF/IRF3 signaling cascade in p28 expression[43]. Additionally, since IRF3 activation leads to expression of IFN-β following TLR4 stimulation this suggested the possibility that IFNs may mediate some of these effects. Consistent with this idea IFN-β was found to upregulate IL-27 p28 synthesis by BMDCs. However, the addition of exogenous IFN-β to LPS-stimulated IRF3−/− BMDCs did not restore the levels of p28 mRNA to those observed in wild-type BMDCs stimulated with LPS alone. Furthermore, IFN-α and IFN-γ have also been shown to enhance TLR-dependent expression of IL-27 p28 by APCs[23,42,45,46]. One of the proteins that is activated by interferon signaling in APCs is IRF1, which is essential for many IFN-γ mediated responses including upregulation of IL-12 p35 gene expression in macrophages in response to IFN-γ [51,52]. Similar to IL-12 p35, IL-27 p28 expression can be regulated by IRF1, as p28 protein secretion and mRNA synthesis are completely absent in IRF1−/− macrophages stimulated with IFN-γ [42]. Furthermore, IRF1 has been shown to bind an ISRE in the p28 promoter both in vitro and in vivo in response to not only IFN-α, IFN-β and IFN-γ but also LPS[42,43,45,46] providing further evidence that IRF1 is important in the transcriptional regulation of p28.
Anti-inflammatory properties of IL-27
Inhibition of Th1 responses
While IL-27 has also been ascribed pro-inflammatory activity, see references[53–57], the remainder of this article will highlight the anti-inflammatory properties of IL-27. The initial data implicating IL-27 in the regulation of inflammation came from in vivo models of parasitic infection using IL-27ra−/− mice. For example, studies from this laboratory revealed that IL-27ra−/− mice infected with Toxoplasma gondii effectively generated an IFN-γ response to control parasite replication, but subsequently developed a lethal CD4+ T cell mediated inflammatory disease[26]. This pathological response was characterized by enhanced T cell proliferation, increased production of IL-2 and IFN-γ and the maintenance of a population of activated (CD25+, CD62Llow) CD4+ and CD8+ T cells. Similarly, additional groups have found that infection of IL-27ra−/− mice with Trypanosoma cruzi or Leishmania donovani resulted in exaggerated T cell responses coupled with enhanced pro-inflammatory cytokine production[58,59]. This phenotype of immune-mediated pathology is not restricted to models of parasitic infection as IL-27ra−/− mice infected with Mycobacterium tuberculosis displayed lower bacterial burden, but more severe lung pathology and they succumbed to this inflammatory disease[20,60]. Moreover, Yamanaka and colleagues have made a similar observation in a noninfectious model in which the absence of IL-27 led to increased susceptibility to concanavalin A-induced hepatitis characterized by elevated production of IFN-γ and IL-4 by NKT cells[61]. Together, these findings redefined the role of IL-27 and identified it as a key antagonist of T cell mediated inflammation.
Regulation of Th2 responses by IL-27
While the previous section highlights that IL-27 can regulate inflammation during a Th1 polarized response, it has also become evident that IL-27 can also influence additional T cell subsets. Initial interpretations of IL-27ra−/− mice infected with Leishmania major or the nematode parasite Trichuris muris indicated that these mice displayed a diminished Th1 response compared to wild-type mice leading to the conclusion that IL-27 was essential for the development of Th1 immunity[19,62]. However, other reports using these pathogens indicated that IL-27 was a potent inhibitor of Th2 responses. Thus, IL-27ra−/− mice challenged with T. muris developed an accelerated Th2 response characterized by increased production of Th2 associated cytokines, exaggerated goblet cell hyperplasia and mastocytosis in the gut, and early clearance of worm burden[30]. Furthermore, when the Th1 response was blocked in wild-type mice this hyper-resistant phenotype was not observed indicating that the increased production of Th2 cytokines was not the consequence of the inability of these mice to generate an effective IFN-γ response in the absence of IL-27R signaling.
Indeed studies with other experimental systems confirmed that when IL-27ra−/− mice are pretreated with antibody specific for IL-4 the early susceptibility to L. major is reversed[63]. In addition, even though IL-27ra−/− mice were susceptible to leishmaniasis in the early stages of infection, at later time points mice that lack the IL-27ra chain or EBI3 developed leishmania-specific Th1 cells and controlled the infection[63,64]. Moreover, in models of asthma and glomerulonephritis the absence of IL-27 signaling led to an exacerbated Th2 response in both cases[65,66]. The molecular basis for the ability of IL-27 to inhibit Th2 cell development may in part be due to its capacity to inhibit expression of the Th2 lineage-specific transcription factor GATA3[24].
Inhibitory effects of IL-27 on Th17 cells
The recent description of Th17 cells, which produce IL-17A, IL-17F, IL-6 and IL-22 upon differentiation, and contribute to the development of autoimmune inflammation in mouse models of multiple sclerosis and rheumatoid arthiritis[10,11], has sparked much attention in defining the ontogeny of these pathological CD4+ T cells. In addition, there have been multiple studies focused on determining the natural antagonists of Th17 cell activity. Two such reports focused on the development of enhanced CNS inflammation in IL-27ra−/− mice chronically infected with T. gondii or immunized with MOG peptide to induce EAE[31,33]. In these models the lack of IL-27 signaling resulted in an increase in the number of IL-17 producing CD4+ T cells in the CNS, associated with exacerbated clinical disease. Both groups went on to show that IL-27 was capable of inhibiting the development of Th17 cells in vitro, a result recently confirmed by others[67–69]. Consistent with the ability of IL-27 to decrease Th17 responses continual delivery of IL-27 significantly suppressed the establishment of clinical disease in EAE, associated with a decreased proportion of Th17 cells in the CNS[34]. In addition, upregulation of EBI3 and p28 transcripts have been observed in the retina of mice in an experimental autoimmune uveoretinitis (EAU) model with the highest levels of IL-27 expression coinciding with the peak of disease[13]. However, as seen in the latter study the endogenous production of IL-27 by cells in the retina served to inhibit Th17 cell expansion in a co-culture system and neutralization of endogenous IL-27 increased the level of IL-17 in this model. Together, these studies define a role for IL-27 in antagonizing the development of Th17 cells similar to its ability to modulate Th1 and Th2 differentiation.
IL-27 prevents the generation of inducible regulatory T cells (iTregs)
While the sections above highlight the inhibitory effects of IL-27 on Th cells, IL-27 can also suppress the induction of a subset of regulatory T cells (iTregs), which differentiate and expand upon stimulation with TGF-β[67,70]. Given the prominent role for Tregs in limiting inflammation, this observation seems inconsistent with the anti-inflammatory activity of IL-27. However, the significance of this suppression of iTreg development by IL-27 in the setting of an inflammatory response in vivo remains an open question. In addition, IL-27 may also influence the activity of other populations of Tregs as CD4+CD25+ “natural Tregs” express high levels of the IL-27ra subunit[18], but to date no studies have assessed whether IL-27 promotes or inhibits this cell-type. Nevertheless, it is unlikely that IL-27 is essential for the development of this subset of Tregs as IL-27ra−/− mice have normal numbers of CD4+CD25+ Tregs[18].
Effects of IL-27 on T cell proliferation
It is apparent from the reports described above that IL-27 has a broad effect on the kinetics of T cell responses that are not limited to a particular Th cell subset. One explanation for this wide range of activity could be attributed to the effect of IL-27 on the regulation of T cell proliferation. Specifically, in the absence of the IL-27R CD4+ T cells displayed an enhanced proliferative capacity following activation in vitro[16,19,71]. Furthermore, as previously stated, increased expansion of CD4+ T cells has been observed during acute infection with T. gondii, which was also associated with exaggerated production of IL-2[26] suggesting a potential role for IL-27 in limiting IL-2 production and thereby T cell proliferation. Support for this idea come from a study in which survival of IL-27ra−/− mice infected with T. gondii was improved upon neutralization of IL-2, which was associated with a reduction in IFN-γ[71]. In addition, production of IL-2 by CD4+ T cells was shown to be directly regulated by IL-27 in vitro[68,71,72]. In terms of CD4+ T cell expansion it was determined that neutralization of IL-2 in vitro resulted in a modest but significant decrease in the proliferation of CD4+ T cells from IL-27ra−/− and WT mice; however, the proliferative capacity of the IL-27R deficient CD4+ T cells was still higher than that of the wild-type cells indicating that enhanced IL-2 production was not solely responsible for the hyperproliferative phenotype displayed by the IL-27ra−/− T cells[71]. Therefore, identification of additional mechanisms that lead to the enhanced expansion of T cells in IL-27ra−/− mice remains an area that requires further investigation.
Additional anti-inflammatory properties of IL-27
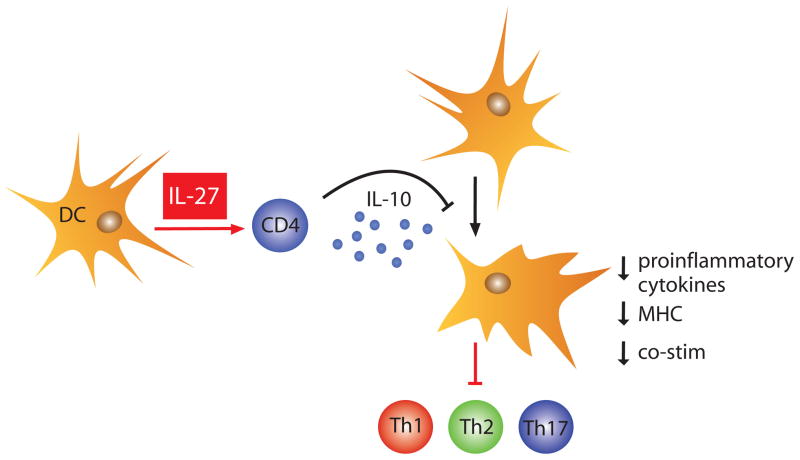
In the previous sections the anti-inflammatory activity of IL-27 has been attributed to its ability to inhibit cytokine production by specific Th subsets. However based on the findings of three recent reports, it has become apparent that IL-27 can also promote IL-10 synthesis by CD4+ and CD8+ T cells[73–75]. In fact the most striking finding from a multiple analyte profile for secreted immune products using supernatant from CD4+ T cells activated under non-polarizing conditions in the presence or absence of IL-27, was that IL-27 induced a 1000-fold increase in IL-10. This result was corroborated in vivo as T cells from IL-27ra−/− mice chronically infected with T. gondii displayed a reduced capacity to produce IL-10[73]. Additionally, IL-27 was able to suppress IL-2 production in the absence of IL-10[74], however, there were discrepancies in the ability of IL-27 to inhibit IL-17 in the absence of IL-10. For example, using splenocytes from IL-10−/− mice IL-27 was able to prevent Th17 differentiation driven by TGF-β and IL-6[73] while it displayed a reduced ability to suppress IL-17 production under IL-23 containing Th17 growth conditions indicating a partial role for IL-10 mediated suppression of IL-17[74]. Despite these discrepancies, Fitzgerald et al. provided evidence that IL-27-mediated inhibition of EAE was dependent on IL-10[74]. Thus, the capacity of IL-27 to induce IL-10 provides a new pathway that leads to the production of this key inhibitor of accessory cell functions, which are required for T cell activation of inflammatory responses (Figure 2). Additionally, the enhanced inflammation observed in IL-27ra−/− mice under various infectious and inflammatory settings may, at least in part, be the consequence of defective IL-10 responses.
Fig. 2.
IL-27 induces CD4+ T cells to produce IL-10. The release of IL-10 by T cells subsequently serves as a broad inhibitor of accessory cell function by downregulating expression of MHC and co-stimulatory molecules, and inhibiting the release of pro-inflammatory cytokines; thus, diminishing T cell activation and an ongoing inflammatory response.
Concluding Remarks
Major advances in understanding the biology of IL-27 have been achieved since its discovery, but many questions still remain in regards to its role in regulating immune responses. For example, it is still unclear as to whether IL-27 is effective only during the initial phases of T cell differentiation or if it can inhibit cytokine production by fully polarized Th cells. In a study conducted by Yoshimura and colleagues they addressed these questions using various protocols in which IL-27-containing medium was added to in vitro cultures of purified CD4+ T cells at different times after activation[68]. This study indicated that IL-27 was only able to suppress cytokine production (IL-4, IL-2, IL-17 and IFN-γ) when it was present from the start in the cultures. Thus, addition of IL-27 as early as one day after T cell activation limited the anti-inflammatory potential of this cytokine. These findings suggest a model in which IL-27 is only capable of inhibiting cytokine production when CD4+ T cells are in an uncommitted state after activation and before differentiation; however, this does not appear to be the case in vivo during an active immune response. For instance, when T cells isolated from the brains of mice chronically infected with T. gondii are restimulated in vitro with antigen the addition of IL-27 to these cultures results in a marked decrease in the production of pro-inflammatory cytokines (IL-17 and IFN-γ) and an increase in IL-10 production[31,73] (J.S. Stumhofer and C.A. Hunter unpublished findings). As all of the T cells isolated from the brain of infected mice displayed an activated phenotype (CD44highCD62Llow) this result indicated that fully differentiated T cells are responding to stimulation with IL-27 upon restimulation in vitro. Additionally, IL-27 when delivered continuously by a subcutaneous osmotic pump was shown to suppress active EAE in vivo[34]. The interpretation of these findings is further complicated by evidence showing that the IL-27ra subunit is upregulated upon T cell activation and T cells with a memory phenotype express higher levels of the IL-27ra subunit than naïve T cells[18]. However there is additional information indicating that gp130, the other subunit required for IL-27 signaling, is downregulated upon activation of T cells[68,76]. The impact of these opposing changes in receptor subunit expression levels have on IL-27 signaling remains unclear.
While the majority of the literature has focused on the effects of IL-27 on T cells, the IL-27 receptor complex is expressed by a number of innate immune cells including macrophages, dendritic cells, mast cells, neutrophils, NK cells and B cells[15,18,21,22,30,61,77–79]. However, most of these reports only provide limited details on the effects of IL-27 on these cell types, and a more in depth analysis on IL-27-signaling in these cell types is warranted. Specifically, the use of conditional knock mice in which the IL-27ra subunit or one of the subunits of IL-27 (EBI3 or p28) is deleted in one of these cell populations will further aid in delineating the cell specific effects of this cytokine during an immune response. Moreover, the advent of transgenic reporter mice has provided a tool that allows the identification of cells programmed for the expression of particular cytokines in the steady state and those that arise in the course of an infection or the development of an autoimmune disease without affecting the endogenous loci[80]. Thus, this technological approach offers a means to identify the specific subsets of cells that produce IL-27 p28 and EBI3, but also to visualize in real-time the spatial and temporal production of this cytokine in vivo.
Based on the pleiotropic nature of IL-27, the question that arises is whether IL-27 can be used therapeutically to antagonize ongoing inflammatory responses or if it can be blocked in order to augment anti-pathogen responses or vaccine-induced immunity. Indeed, a recent study indicated that neutralization of IL-27 may serve as a potential therapy to treat sepsis as administration of a soluble IL-27ra fusion protein to block IL-27 led to enhanced bacterial clearance and a significant increase in survival in a mouse model of sepsis[79]. However, this type of treatment may only be beneficial as a short-term option as neutralization of IL-27 for any extended period of time may facilitate the development of autoimmunity. Alternatively, treatment with IL-27 has the potential to suppress inappropriate inflammatory responses, prevent the growth and dissemination of tumors or serve as an adjuvant to promote a Th1 response. While administration of IL-12 systemically resulted is severe side effects in humans[81], IL-27 may prove to be safer than IL-12 as it induced less splenomegaly and lower liver toxicity than IL-12 upon systemic administration in a comparative study in mice[82]. Additionally, local expression of IL-27 by tumor cells has been shown to provide protection and in many cases resulted in immunological memory in the host[82–86], therefore, this approach to antitumor therapy may offer a safer and more effective delivery of IL-27 than systemic administration.
Whether these proposed therapies will have any efficacy in humans is dependent on the importance of IL-27 in this setting and determining the significance of this cytokine in humans may have to wait for the identification of individuals with primary genetic defects in the ability to make or respond to IL-27. Recently, polymorphisms in the IL-27p28 gene were shown to be associated with increased susceptibility to asthma in the Korean population; however, what effects these polymorphisms had on the activity of IL-27 remain undefined[87]. Nevertheless, this finding offers the first evidence that IL-27 may be important in limiting a Th2 response associated with asthma in humans, but additional studies will be required to assess its ability to inhibit other human T cell subsets.
Acknowledgments
We thank Fraser Marshall for his figure illustrations and our colleagues Alejandro Villarino, Christiaan J.M. Saris, Matthias Ernst, John O’Shea as well as the faculty and students of the Pathobiology Department and the Mari Lowe Center for Comparative Oncology. This work was supported by grants from the State of Pennsylvania and NIH (AI42334). Jason Stumhofer is supported by 1-T32-AI-055428.
Funding State of Pennsylvania, 1-T32-AI-055428, AI 42334
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boulay JL, O’Shea JJ, Paul WE. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity. 2003;19:159–163. doi: 10.1016/s1074-7613(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 2.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 4.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 5.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006 doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 7.Chung DR, Kasper DL, Panzo RJ, Chitnis T, Grusby MJ, Sayegh MH, Tzianabos AO. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170:1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 8.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 9.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 10.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 14.Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Ghilardi N, Wang HTBHXM, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 17.Sprecher CA, Grant FJ, Baumgartner JW, Presnell SR, Schrader SK, Yamagiwa T, Whitmore TE, O’Hara PJ, Foster DF. Cloning and characterization of a novel class I cytokine receptor. Biochem Biophys Res Commun. 1998;246:82–90. doi: 10.1006/bbrc.1998.8576. [DOI] [PubMed] [Google Scholar]
- 18.Villarino AV, Larkin J, 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, de Sauvage FJ, Hunter CA. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, Saris CJ, Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 20.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 21.Ruckerl D, Hessmann M, Yoshimoto T, Ehlers S, Holscher C. Alternatively activated macrophages express the IL-27 receptor alpha chain WSX-1. Immunobiology. 2006;211:427–436. doi: 10.1016/j.imbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of Antigen-Presenting and Th1-Promoting Functions of Dendritic Cells by WSX-1(IL-27R) Deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 23.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 24.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 26.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 27.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 29.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 30.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, Koretzky G, Goldschmidt M, Wu GD, de Sauvage F, Miller HR, Saris CJ, Scott P, Hunter CA. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 31.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer SC, Stallmach A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Sarma JD, Gran B, Zhang GX, Rostami A. Suppressive Effect of IL-27 on Encephalitogenic Th17 Cells and the Effector Phase of Experimental Autoimmune Encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Gran B, Zhang GX, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 37.Devergne O, Cahir McFarland ED, Mosialos G, Izumi KM, Ware CF, Kieff E. Role of the TRAF binding site and NF-kappaB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol. 1998;72:7900–7908. doi: 10.1128/jvi.72.10.7900-7908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashimoto K, Durbin JE, Zhou W, Collins RD, Ho SB, Kolls JK, Dubin PJ, Sheller JR, Goleniewska K, O’Neal JF, Olson SJ, Mitchell D, Graham BS, Peebles RS., Jr Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J Allergy Clin Immunol. 2005;116:550–557. doi: 10.1016/j.jaci.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 39.van Seventer JM, Nagai T, van Seventer GA. Interferon-beta differentially regulates expression of the IL-12 family members p35, p40, p19 and EBI3 in activated human dendritic cells. J Neuroimmunol. 2002;133:60–71. doi: 10.1016/s0165-5728(02)00362-4. [DOI] [PubMed] [Google Scholar]
- 40.Wirtz S, Becker C, Fantini MC, Nieuwenhuis EE, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J Immunol. 2005;174:2814–2824. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 41.Larousserie F, Pflanz S, Coulomb-L’Hermine A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202:164–171. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, Willems F, Goldman M, Goriely S. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol. 2007;178:7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 44.Schuetze N, Schoeneberger S, Mueller U, Freudenberg MA, Alber G, Straubinger RK. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella Enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int Immunol. 2005;17:649–659. doi: 10.1093/intimm/dxh247. [DOI] [PubMed] [Google Scholar]
- 45.Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN-{alpha} regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol. 2007;82:1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 46.Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN-beta modulates the response to TLR stimulation in human DC: Involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol. 2007 doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- 47.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 48.Smits HH, van Beelen AJ, Hessle C, Westland R, de Jong E, Soeteman E, Wold A, Wierenga EA, Kapsenberg ML. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34:1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 49.Grumont R, Hochrein H, O’Keeffe M, Gugasyan R, White C, Caminschi I, Cook W, Gerondakis S. c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J Exp Med. 2001;194:1021–1032. doi: 10.1084/jem.194.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Cao S, Herman LM, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J Exp Med. 2003;198:1265–1276. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Guan X, Tamura T, Ozato K, Ma X. Synergistic activation of interleukin-12 p35 gene transcription by interferon regulatory factor-1 and interferon consensus sequence-binding protein. J Biol Chem. 2004;279:55609–55617. doi: 10.1074/jbc.M406565200. [DOI] [PubMed] [Google Scholar]
- 53.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 54.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 55.Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol. 2004;173:715–720. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- 56.Villarino AV, Hunter CA. Biology of recently discovered cytokines: discerning the pro- and anti-inflammatory properties of interleukin-27. Arthritis Res Ther. 2004;6:225–233. doi: 10.1186/ar1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med. 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 58.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 59.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-Cell Cytokine Receptor) Gene-Deficient Mice Display Enhanced Resistance to Leishmania donovani Infection but Develop Severe Liver Immunopathology. Am J Pathol. 2006;168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 61.Yamanaka A, Hamano S, Miyazaki Y, Ishii K, Takeda A, Mak TW, Himeno K, Yoshimura A, Yoshida H. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J Immunol. 2004;172:3590–3596. doi: 10.4049/jimmunol.172.6.3590. [DOI] [PubMed] [Google Scholar]
- 62.Bancroft AJ, Humphreys NE, Worthington JJ, Yoshida H, Grencis RK. WSX-1: a key role in induction of chronic intestinal nematode infection. J Immunol. 2004;172:7635–7641. doi: 10.4049/jimmunol.172.12.7635. [DOI] [PubMed] [Google Scholar]
- 63.Artis D, Johnson LM, Joyce K, Saris C, Villarino A, Hunter CA, Scott P. Cutting edge: early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J Immunol. 2004;172:4672–4675. doi: 10.4049/jimmunol.172.8.4672. [DOI] [PubMed] [Google Scholar]
- 64.Zahn S, Wirtz S, Birkenbach M, Blumberg RS, Neurath MF, von Stebut E. Impaired Th1 responses in mice deficient in Epstein-Barr virus-induced gene 3 and challenged with physiological doses of Leishmania major. Eur J Immunol. 2005;35:1106–1112. doi: 10.1002/eji.200425926. [DOI] [PubMed] [Google Scholar]
- 65.Miyazaki Y, Inoue H, Matsumura M, Matsumoto K, Nakano T, Tsuda M, Hamano S, Yoshimura A, Yoshida H. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175:2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 66.Shimizu S, Sugiyama N, Masutani K, Sadanaga A, Miyazaki Y, Inoue Y, Akahoshi M, Katafuchi R, Hirakata H, Harada M, Hamano S, Nakashima H, Yoshida H. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) J Immunol. 2005;175:7185–7192. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- 67.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 68.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 69.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 72.Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 suppresses CD28-medicated IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 73.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007 doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 74.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007 doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 75.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007 doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 76.Betz UA, Muller W. Regulated expression of gp130 and IL-6 receptor alpha chain in T cell maturation and activation. Int Immunol. 1998;10:1175–1184. doi: 10.1093/intimm/10.8.1175. [DOI] [PubMed] [Google Scholar]
- 77.Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176:5890–5897. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- 78.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 79.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203:1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 81.Portielje JE, Gratama JW, van Ojik HH, Stoter G, Kruit WH. IL-12: a promising adjuvant for cancer vaccination. Cancer Immunol Immunother. 2003;52:133–144. doi: 10.1007/s00262-002-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oniki S, Nagai H, Horikawa T, Furukawa J, Belladonna ML, Yoshimoto T, Hara I, Nishigori C. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006;66:6395–6404. doi: 10.1158/0008-5472.CAN-05-4087. [DOI] [PubMed] [Google Scholar]
- 83.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 84.Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 85.Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 86.Shimizu M, Shimamura M, Owaki T, Asakawa M, Fujita K, Kudo M, Iwakura Y, Takeda Y, Luster AD, Mizuguchi J, Yoshimoto T. Antiangiogenic and antitumor activities of IL-27. J Immunol. 2006;176:7317–7324. doi: 10.4049/jimmunol.176.12.7317. [DOI] [PubMed] [Google Scholar]
- 87.Chae SC, Li CS, Kim KM, Yang JY, Zhang Q, Lee YC, Yang YS, Chung HT. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]