SUMMARY
Purpose
Since the ketogenic diet is effective in drug-resistant epilepsies, we sought to determine whether it is active in the 6-Hz seizure test, which identifies agents with a broader spectrum of activity than conventional antiepileptic screening tests.
Methods
Male (3–4 week old) NIH Swiss mice were fed a normal or ketogenic diet ad libitum for 2–21 days. The intensity of the corneal stimulation current required to elicit seizures in the 6-Hz test was measured. Blood glucose and β-hydroxybutyrate were measured on the day of seizure testing.
Results
CC50 (current intensity producing seizures in 50% of mice tested) was 50.6 mA and 15 mA in mice fed for 12 days with a ketogenic or normal diet, respectively (p < 0.001). CC50 was elevated in separate experiments after 16, but not 2, 5, and 21 days of ketogenic diet exposure. CC50 values of growing mice fed the normal diet does not differ, indicating CC50 does not vary with mouse weight during a rapid growth phase. β-Hydroxybutyrate was significantly higher, and glucose was significantly lower in mice fed the ketogenic diet than those fed the normal diet. Blood glucose and β-hydroxybutyrate levels did not correlate with CC50.
Discussion
The ketogenic diet significantly elevates the seizure threshold in the 6-Hz test in a time-specific manner. Protection from seizures in this model was not related to level of ketosis. CC50 was insensitive to body weight in mice fed the normal diet, demonstrating that the 6-Hz model can assess anticonvulsant regimens where weight is a confounding factor.
Keywords: Ketogenic diet, 6-Hz seizure model, Seizure, Intractable epilepsy
A wide variety of animal models are used to assess the potential clinical efficacy of antiseizure therapies. Among the most common is the maximal electroshock (MES) test, which employs a high-frequency (60 Hz), short-duration (0.2 s), suprathreshold electrical stimulus to induce a tonic hindlimb extensor response (Swinyard, 1972). An alternative electroshock paradigm described by Brown et al. (1953) uses low-frequency (6 Hz), long-duration (3 s) electrical stimulation to induce a “psychomotor” seizure involving forelimb clonus and stereotyped behaviors that are reminiscent of those exhibited by human patients with complex partial seizures (Toman, 1951; Toman et al., 1952; Brown et al., 1953). The 6-Hz model was not used widely because it was believed to lack clinical validity since the hydantoins phenytoin and thiantoin failed to show protective activity. However, Barton et al. (2001) observed that the clinically effective antiepileptic drug levetiracetam, which is not active in the conventional MES test, does exhibit protective activity in the 6-Hz model. This suggested that the 6-Hz model might be capable of identifying anti-seizure agents with a novel spectrum of activity.
The high-fat, low-carbohydrate ketogenic diet has been successfully used to treat epilepsy for nearly 90 years (Freeman et al., 2006). It is generally believed that the diet confers seizure protection for some patients with pharmacoresistant epilepsies. The diet is recognized to have protective activity in diverse rodent seizure models (Hartman et al., 2007). However, its pattern of protection is distinct from that of the major clinically used antiepileptic drugs, suggesting that it has a unique mechanism of action that does not overlap with that of therapeutic drugs. In particular, the diet only provides partial protection in the mouse MES test (Uhlemann & Neims, 1972), and it is inactive in the rat MES test (Likhodii et al., 2000; Thavendiranathan et al., 2003). Given the broad-spectrum efficacy of the diet, we sought to determine whether it would confer protection in the 6-Hz model. Here we describe experiments demonstrating the diet is highly protective in the 6-Hz model, but more than 5 days of treatment is required for protective activity to develop and efficacy is lost at 3 weeks.
Materials and Methods
Animals
Male postweaning NIH Swiss mice (3–4 weeks old) were kept in a vivarium under controlled laboratory conditions (temperature, 22–26°C; humidity, 40–50%) with an artificial 12-h light/dark cycle. Wood chips were used in all cages. Experiments were performed during the light phase of the light/dark cycle after a minimum 30-min period of acclimation to the experimental room. The animal facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All studies were performed under protocols approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke (NINDS) in strict compliance with the Guide for the Care and Use of Laboratory Animals of the National Research Council (National Academy Press, Washington, D.C.; http://www.nap.edu/readingroom/books/labrats/).
Diets and monitoring
Following acclimatization to the animal care facility for 1–3 days, mice were fasted overnight (12–18 h) and then had their diets randomly replaced by either a normal diet (NIH-07 Rodent Formula Diet) or a ketogenic diet (Bioserv F3666, Frenchtown, NJ, U.S.A.). All mice were allowed free access to water and food at all times before seizure tests were undertaken. The ketogenic diet used in the present study (6.3:1 ratio of fat to proteins + carbohydrates) has been described elsewhere (Bough et al., 2000b). Mice were weighed twice per week. To compare calorie intake between mice fed ketogenic and control diets, food consumption was calculated based on cage weight changes and the caloric content of the food. β-Hydroxybutyrate and glucose levels were measured in tail vein blood samples collected on the day of seizure testing from littermates randomly selected from each diet group that had not undergone seizure testing. Mice were anesthetized briefly with isofluorane (1.5–3%) in a small Plexiglas chamber for tail vein sampling, which was carried out at least 2 h before seizure testing. Once they appeared unconscious, approximately 1 mm of their distal tail was shaved with a razor blade, causing a small amount of bleeding, which was cauterized with silver nitrate. In some animals, trunk blood was sampled following CO2 narcosis and decapitation. With either sampling method, blood glucose (in mg/dl) and β-hydroxybutyrate (in mmol/ml) were determined immediately following sampling using a test strip system and reader (Precision Xtra Advanced Diabetes Management System with Precision Xtra blood ketone test strips and blood glucose test strips; Abbott Diabetes Care Inc., Alameda, CA, U.S.A.).
6-Hz seizure model
The protocol for the 6-Hz seizure model was as previously described (Brown et al., 1953; Kaminski et al., 2004). Topical anesthetic (0.5% tetracaine hydrochloride ophthalmic solution) was applied to the cornea 30 min before corneal stimulation (0.2-ms duration pulses at 6 Hz for 3 s) administered by a constant-current device (ECT Unit 57800; Ugo Basile, Comerio, Italy). Saline (0.9%) was used to wet the electrodes immediately before testing to ensure good electrical contact. Each animal was stimulated once at a selected current intensity in the range of 6–64 mA to determine the convulsive threshold (CC50) for mice in each group. The current intensity values were chosen using the modified staircase technique in which the stimulation intensity for a group of animals was based on whether the preceding group of animals did or did not exhibit a seizure (Barton et al., 2001). Mice were manually restrained during stimulation. Immediately following stimulation, mice were placed in a Plexiglas arena (27.5 cm × 20 cm × 15 cm) for behavioral observation. Seizures were characterized by a stunned or fixed posture often accompanied by rearing, forelimb clonus, and twitching. After the seizures, mice resumed normal exploratory behavior within 45 s. Mice not experiencing seizures exhibited normal exploratory behavior when placed in the arena (Brown et al., 1953). All mice were euthanized at the end of the experiment by CO2 narcosis.
Data analysis
In each experiment, groups of 6–8 mice were tested at three or four stimulation intensities per treatment and the percent of mice exhibiting seizures was calculated. Values of current intensity producing seizures in 50% of mice tested (CC50) with their 95% confidence limits (95% C.L.) were estimated by the log-probit method (Litchfield & Wilcoxon, 1949). The statistical significance of the relationship between the CC50 values and glucose, ketone levels, or weight was assessed with the Spearman coefficient. Blood β-hydroxybutyrate and glucose levels were compared in mice fed the ketogenic and normal diets at each time point using Student’s t-test. Differences were considered statistically significant when the probability of error was less than 0.05 (p < 0.05).
Results
Effect of the ketogenic diet in the 6 Hz seizure model
In mice fed the normal diet, 6-Hz stimulation currents of 10 mA and greater induced limbic seizures in some mice with the fraction of mice responding with seizures rising in a monotonic fashion with increasing current intensity to a level of 32 mA where all mice tested exhibited seizures (Fig. 1). In contrast, none of the mice fed the ketogenic diet for 12 days exhibited seizures with a 32-mA stimulus. Greater stimulation intensities were required to induce seizures in these animals, but with currents of 44 mA and higher, seizures were elicited in the diet-fed animals. There was a monotonic rise in the fraction of animals responding that paralleled that of the normal diet groups, indicating the threshold for seizure activation was elevated by the diet, but diet-fed animals still exhibited full seizure responsiveness. The CC50 values derived from the data presented in Fig. 1 are 50.6 mA (95% C.L., 46.3–55.3) for animals fed the ketogenic diet, and 15.0 mA (95% CL, 14.0–16.1) in mice fed the normal diet (p < 0.001).
Figure 1.
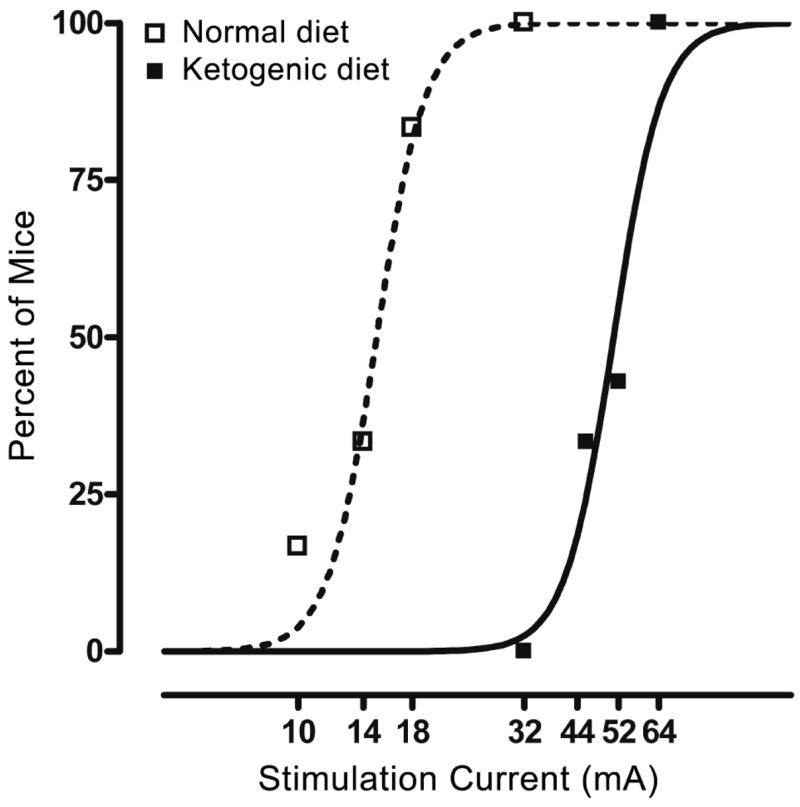
Incidence of seizures at different 6-Hz stimulation intensities in mice fasted overnight and then fed a normal or a ketogenic diet for 12 days. Datapoints indicate the percentage of animals exhibiting seizures when tested at the stimulation current value shown on the abscissa. Mice fed the ketogenic diet required higher stimulation currents to induce seizures than those fed the control diet. Testing was carried out as described in the text. Animals were stimulated only once. Each data-point represents six to eight mice. Epilepsia © ILAE
Time course
We next sought to determine the time course of seizure protection by conducting experiments similar to that presented in Fig. 1 except that the duration of exposure to respective diets was 2, 5, 16, or 21 days. A separate experiment with new animals was conducted at each of the time points. Each experiment included a control group of mice fed the normal diet. CC50 values were determined by testing subgroups of animals with different stimulation currents as in the experiment of Fig. 1. The CC50 values from these additional experiments are presented in Fig. 2. Significant differences between the ketogenic and normal diet groups were obtained with 12- and 16-day feeding but not with 2-, 5-, and 21-day feeding.
Figure 2.
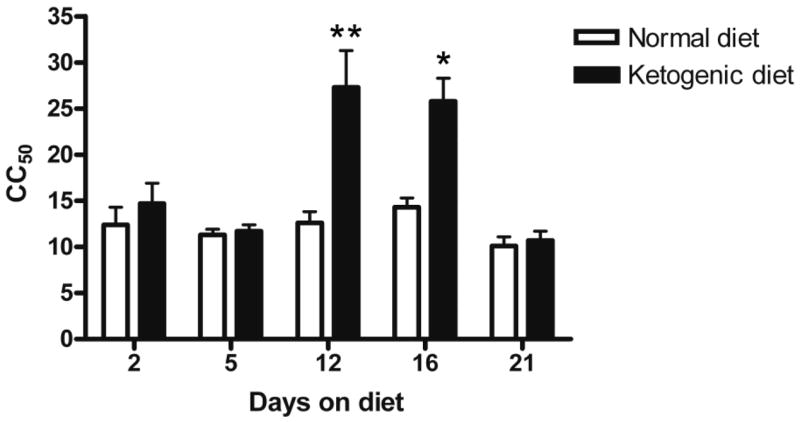
Time course of the effects of the ketogenic diet on CC50 values in the 6-Hz model. Significant increases in the ketogenic diet-fed groups were obtained in experiments where the animals fed the diet for 12 and 16 days, but not with feeding for 2, 5, and 21 days. Each bar indicates the CC50 (and 95% C.L.) values in mA derived from experiments carried out as in Fig. 1; the 12-day CC50 values are from a separate experiment. *p < 0.05; **p < 0.01.
Epilepsia © ILAE
Effects of body weight on CC50 values
To determine whether seizure threshold in the 6-Hz test was dependent on body weight, we compared the CC50 values among the groups of mice fed the normal diet that had markedly different body weights because of age differences. The mean ± SE weights were 12.7 ± 0.3 g for the 2-day group, 18.7 ± 0.4 g for the 5-day group, 23.9 ± 0.4 g for the 12-day group, 23.0 ± 0.4 g for the 16-day group, and 25.6 ± 0.3 g for the 21-day group. There was no correlation between mean body weight and CC50 value as given in Fig. 2 (Spearman coefficient, −0.36; p = 0.52), indicating that the 6-Hz test was insensitive to differences in weight during the period of rapid growth studied here. The mean weight value for the 21-day group (6–7 weeks of age) is 70% of the predicted adult body weight at 33 weeks of age (the age of retirement as breeders).
Body weight was significantly lower for the ketogenic diet-fed groups at all time points (p < 0.01). The mean ± SE weights were 11.4 ± 0.3 g for the 2-day group, 13.1 ± 0.3 g for the 5-day group, 16.0 ± 0.4 g for the 12-day group, 20.3 ± 0.2 for the 16-day group, and 18.0 ± 0.5 g for the 21-day group. In pilot data, mice fed the ketogenic diet consumed ~85–90% of the calories consumed by their normal diet littermates.
Blood β-hydroxybutyrate levels and glucose levels
Fig. 3 shows the mean blood β-hydroxybutyrate and glucose levels in the different control and ketogenic diet groups. With all durations of feeding, mean β-hydroxybutyrate levels were elevated in the ketogenic diet group compared with the normal diet group. In contrast, blood glucose levels were reduced with all diet durations. There was no correlation between the levels of either ketones or glucose and CC50 (β -hydroxybutyrate: Spearman coefficient = 0.2, p = 0.92; glucose: Spearman coefficient = −0.5, p = 1).
Figure 3.
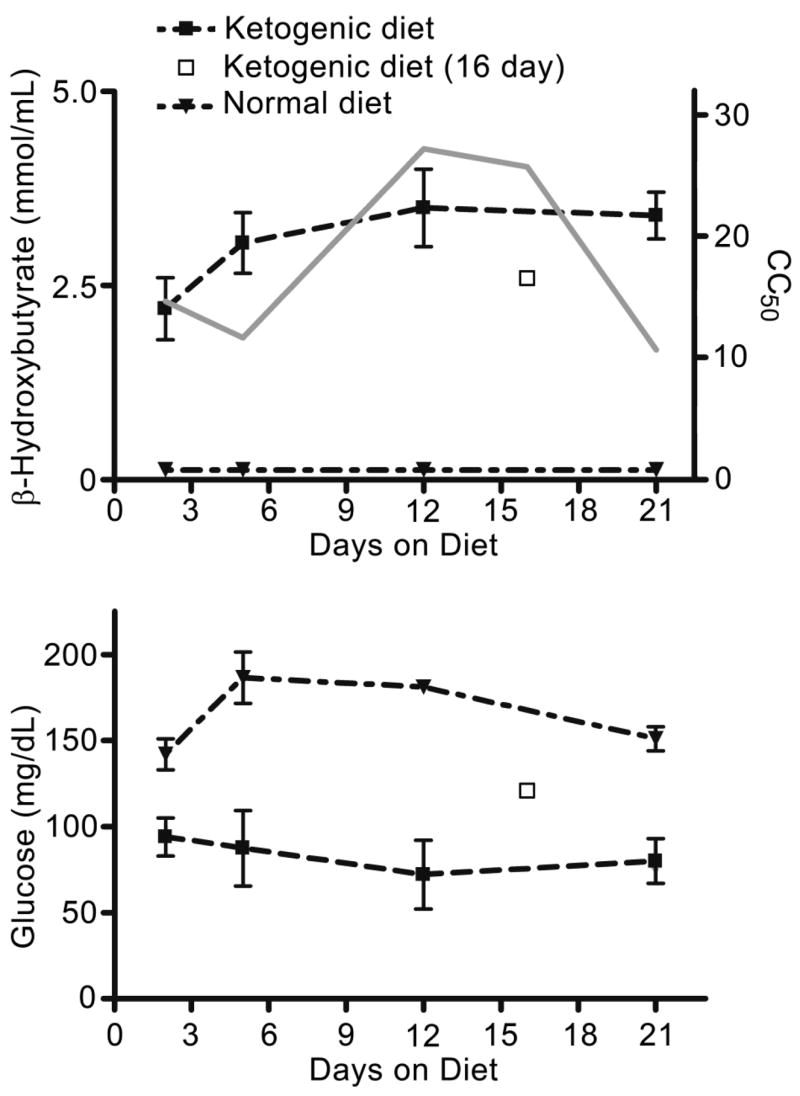
Mean blood β-hydroxybutyrate and glucose levels in mice selected randomly from the ketogenic diet and the normal diet groups. All differences between the mean values for ketogenic diet and corresponding normal diet groups were significant at the p < 0.001 level except for the blood glucose values after 2 days of feeding that were significant at p < 0.05. For comparison, the CC50 values for the ketogenic diet groups from Fig. 2 are depicted by the grey line. There is no correlation between the β-hydroxybutyrate or glucose levels and the CC50 values. Measurements in the 16-day groups were made using trunk blood and are not directly comparable with the values in other groups that were derived from blood collected from the tail.
Epilepsia © ILAE
Two-day feeding of 5-week-old mice
Although it is plausible that the lack of efficacy of the ketogenic diet in the 6-Hz model with 2 and 5 days of feeding is due to the delayed onset of the ketogenic diet’s antiseizure activity, an alternative explanation is that young mice respond less well to the diet than older mice inasmuch as the mice in the 12- and 16-day experiments are older because of the duration of feeding. To exclude this latter possibility, we studied older mice at an age (5 weeks old) found to be responsive to the diet but who were exposed to the diet for only 2 days. As with the previously described 2-day feeding experiment, there was no significant difference in CC50 values between ketogenic diet fed and control mice (11.0 vs. 11.6 mA; p = 0.84). Indeed, the CC50 values in this experiment were comparable and not significantly different from the corresponding values in the previously described 2-day feeding experiment. Mean β-hydroxybutyrate levels in the ketogenic diet group in this experiment (2.4 ± 0.2 mmol/ml) were also not significantly different from the values in the 2-day experiment shown in Fig. 2 (2.2 ± 0.4 mmol/ml; p = 0.69).
Discussion
The ketogenic diet protects against seizures in many but not all rodent seizure models (Stafstrom, 1999; Hartman et al., 2007). Notably, the diet is weakly active in the mouse MES test and inactive in the rat MES test (Thavendiranathan et al., 2003). The diet also is inactive in the mouse pentylenetetrazol model although it has been reported to have activity in this model in the rat. Here we have found the diet to be highly active in the mouse 6-Hz model, at least when tested after 12 and 16 days of feeding. The ketogenic diet is most commonly used clinically in young, growing children with medically intractable seizures. To simulate this situation, in the present study we used rapidly growing juvenile mice. In addition to demonstrating a high degree of efficacy of the diet in the 6-Hz model, our study demonstrates that the activity of the diet is dissociated from the level of ketosis as assessed by measurements of blood β-hydroxybutyrate. The lack of relationship between the level of ketosis and anticonvulsant efficacy is consistent with other studies in rodents that have failed to find ketosis to be a reliable biomarker of efficacy (Bough et al., 1999; Bough et al., 2000a; Todorova et al., 2000). In clinical practice, elevated β-hydroxybutyrate levels appear to be necessary for seizure control but, as in the animal studies, do not ensure that seizure protection will be obtained (Gilbert et al., 2000). We also failed to find a correlation between the reduction in blood glucose levels obtained during ketogenic diet treatment and anticonvulsant efficacy, as has been the case in clinical studies (Bergqvist et al., 2006).
Although the ketogenic diet shows weak or no activity in maximal stimulation seizure tests, such as the MES test, Thavendiranathan et al. (2003) proposed that “threshold” electroconvulsant (or chemoconvulsant) models may be more sensitive to the diet. However, using conventional 60-Hz electroshock stimulation in the rat electroconvulsive shock (ECS) threshold test, these authors obtained only relatively small (15–20%) threshold elevations. This compares with the 237% increase in CC50 value after 12 days on the diet obtained using 6-Hz stimulation in the present series of experiments. Therefore, the 6-Hz model is exceptionally sensitive to the ketogenic diet. It is noteworthy that we determined the activity of the diet by examining its activity on seizure threshold in response to graded stimulation intensities. Conventionally, the protective activity of anticonvulsant drugs is assessed in the 6-Hz model with supramaximal stimulation (Barton et al., 2001). Thus, a quantitative comparison between the activity of the diet and anticonvulsant drugs is limited.
It has been noted that the antiepileptic drug levetiracetam, which is effective in the treatment of partial seizures, is highly active in the 6-Hz model, whereas it is not protective in other antiepileptic drug screening models when carried out according to standard protocols (Barton et al., 2001). Therefore, the 6-Hz seizure model may be useful for identifying antiseizure agents with unique anticonvulsant profiles and that act by novel mechanisms, as does levetiracetam (Lynch et al., 2004). In this regard, it is interesting to note that the ketogenic diet has a distinct profile of activity from commonly used antiepileptic drugs, suggesting that it may act in a mechanistically distinct way from these medications (Hartman et al., 2007). In line with its possible unique mechanism of action, it is believed that the diet may have efficacy in pharmacoresistant epilepsies. The high activity of the ketogenic diet in the 6-Hz model is compatible with this possibility.
Animals fed a ketogenic diet may gain weight less well than those fed a normal diet, and it has been suggested that the mismatch in weights between the diet and control groups skews results obtained in seizure models, such as the pentylenetetrazol infusion test, where threshold doses are calculated on a per body weight basis (Likhodii & Burnham, 2003). Because the 6-Hz model does not require administering a per body weight dose, it is not subject to this concern. Moreover, we found that the 6-Hz threshold was not influenced by body weight in animals fed a normal diet, supporting the view that the 6-Hz model is applicable in situations where there are differences in these factors between treatment groups.
A surprising result in the present study is that activity of the ketogenic diet in the 6-Hz model was not obtained at 3 weeks, despite maintained ketosis and reduced blood glucose levels. Such a loss of protection by the ketogenic diet over time in the face of maintained ketosis was noted previously in studies with the adult EL mouse (Todorova et al., 2000). In these animals, seizure protection was not obtained until 3 weeks after feeding of the diet was begun and seizure protection was no longer evident after 6 weeks. Tolerance to the ketogenic diet could be due to resolution of unidentified biochemical factors responsible for the activity of the diet or to compensatory phenomena that override the anticonvulsant effect, including changes in targets responsible for the anticonvulsant effect.
We observed that animals fed the ketogenic diet gained slightly less weight than those on a control diet. In addition, despite free access to the ketogenic diet, they consumed 10–15% less calories in our pilot studies. Interestingly, this self-imposed calorie restriction is of a similar magnitude to the degree of calorie restriction (~10%) imposed during treatment of children as applied according to the Johns Hopkins ketogenic diet protocol (Z. Turner, personal communication). Calorie restriction itself can protect against seizures in animal models, such as the EL mouse (Mantis et al., 2004). However, the degree of calorie restriction required is far greater than in the present study so that calorie restriction alone is unlikely to account for the seizure protection, although it could be contributory.
In conclusion, we found that the ketogenic diet strongly elevates the threshold for 6-Hz seizures in juvenile mice. We are not aware of an animal seizure model in which the ketogenic diet shows such a strong antiseizure response. Therefore, this model may be useful in mechanistic studies of the ketogenic diet. The efficacy of the diet waned at 3 weeks, despite persistent ketosis, indicating that factors other than ketosis must account for seizure protection, at least in this model. The 6-Hz model may therefore also be useful to assess factors responsible for the loss of efficacy that occurs in some patients who are treated with the ketogenic diet.
Acknowledgments
The authors gratefully acknowledge the guidance provided by Dr. Rafal Kaminski and the expert technical assistance of Ms. Karen Wayns. This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, NIH, and by the Epilepsy Foundation through the generous support of Pfizer, Inc. (ALH).
Footnotes
Conflict of interest: There are no conflicts of interest to report. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Bergqvist AGC, Schall JI, Richard EL, Gallagher PR, Stallings VA. Blood glucose does not predict response to the ketogenic diet (abstract) Epilepsia. 2006;47 (Suppl 4):334. [Google Scholar]
- Bough KJ, Chen RS, Eagles DA. Path analysis shows that increasing ketogenic ratio, but not beta-hydroxybutyrate, elevates seizure threshold in the rat. Dev Neurosci. 1999;21:400–406. doi: 10.1159/000017390. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Yao SG, Eagles DA. Higher ketogenic diet ratios confer protection from seizures without neurotoxicity. Epilepsy Res. 2000a;38:15–25. doi: 10.1016/s0920-1211(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Matthews PJ, Eagles DA. A ketogenic diet has different effects upon seizures induced by maximal electroshock and by pentylenetetrazole infusion. Epilepsy Res. 2000b;38:105–114. doi: 10.1016/s0920-1211(99)00079-0. [DOI] [PubMed] [Google Scholar]
- Brown WC, Schiffman DO, Swinyard EA, Goodman LS. Comparative assay of antiepileptic drugs by “psychomotor” seizure test and minimal electroshock threshold test. J Pharmacol Exp Ther. 1953;107:273–283. [PubMed] [Google Scholar]
- Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E for the Institute of Neurology IRCCS C. Mondino Foundation. The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Res. 2006;68:145–180. doi: 10.1016/j.eplepsyres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Pyzik PL, Freeman JM. The ketogenic diet: seizure control correlates better with serum β-hydroxybutyrate than with urine ketones. J Child Neurol. 2000;15:787–790. doi: 10.1177/088307380001501203. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EPG, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6 Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Likhodii SS, Musa K, Mendonca A, Dell C, Burnham WM, Cunnane SC. Dietary fat, ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia. 2000;41:1400–1410. doi: 10.1111/j.1528-1157.2000.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Likhodii SS, Burnham WM. Calorie restriction may not elevate the threshold of PTZ-induced seizures. Epilepsy Res. 2003;57:77–78. doi: 10.1016/j.eplepsyres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharm Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metab (Lond) 2004;1:11. doi: 10.1186/1743-7075-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE. Animal models of the ketogenic diet: what have we learned, what can we learn? Epilepsy Res. 1999;37:241–259. doi: 10.1016/s0920-1211(99)00067-4. [DOI] [PubMed] [Google Scholar]
- Swinyard EA. Electrically induced seizures. In: Purpura DP, Penry JK, Tower DB, Woodbury DM, Walter RD, editors. Experimental models of epilepsy: a manual for the laboratory worker. Raven Press; New York: 1972. pp. 433–458. [Google Scholar]
- Thavendiranathan P, Chow C, Cunnane S, McIntyre Burnham W. The effect of the ‘classic’ ketogenic diet on animal seizure models. Brain Res. 2003;959:206–213. doi: 10.1016/s0006-8993(02)03744-7. [DOI] [PubMed] [Google Scholar]
- Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–940. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Toman JEP. Neuropharmacologic considerations in psychic seizures. Neurology. 1951;1:444–460. doi: 10.1212/wnl.1.11-12.444. [DOI] [PubMed] [Google Scholar]
- Toman JEP, Everett GM, Richards RK. The search for new drugs against epilepsy. Tex Rep Biol Med. 1952;10:96–104. [PubMed] [Google Scholar]
- Uhlemann ER, Neims AH. Anticonvulsant properties of the ketogenic diet in mice. J Pharmacol Exp Ther. 1972;180:231–238. [PubMed] [Google Scholar]


