Abstract
Volatile secondary metabolites emitted by plants contribute to plant–plant, plant–fungus, and plant–insect interactions. The C16-homoterpene TMTT (for 4,8,12-trimethyltrideca-1,3,7,11-tetraene) is emitted after herbivore attack by a wide variety of plant species, including Arabidopsis thaliana, and is assumed to play a role in attracting predators or parasitoids of herbivores. TMTT has been suggested to be formed as a degradation product of the diterpene alcohol (E,E)-geranyllinalool. Here, we report the identification of Terpene Synthase 04 (TPS04; At1g61120) as a geranyllinalool synthase (GES). Recombinant TPS04/GES protein expressed in Escherichia coli catalyzes the formation of (E,E)-geranyllinalool from the substrate geranylgeranyl diphosphate. Transgenic Arabidopsis lines carrying T-DNA insertions in the TPS04 locus are deficient in (E,E)-geranyllinalool and TMTT synthesis, a phenotype that can be complemented by expressing the GES gene under the control of a heterologous promoter. GES transcription is upregulated under conditions that induce (E,E)-geranyllinalool and TMTT synthesis, including infestation of plants with larvae of the moth Plutella xylostella and treatment with the fungal peptide alamethicin or the octadecanoid mimic coronalon. Induction requires jasmonic acid but is independent from salicylic acid or ethylene. This study paves the ground to address the contribution of TMTT in ecological interactions and to elucidate the signaling network that regulates TMTT synthesis.
INTRODUCTION
The majority of the millions of insect species on Earth feed on plants to obtain their nutrients. Consequently, plants have evolved efficient direct and indirect defense mechanisms to protect themselves against herbivorous insects (Baldwin and Preston, 1999; Sabelis et al., 2001; Ament et al., 2004). In direct defense responses, herbivore performance is affected by toxic or antinutritional plant metabolites. In indirect defense responses, plants attract natural enemies of herbivores, most commonly by the release of volatiles upon herbivore damage. These tritrophic interactions increase the plant's fitness and therefore are evolutionarily advantageous (Dicke and Sabelis, 1989; Van Loon et al., 2000; Fritzsche-Hoballah and Turlings, 2001; Kessler and Baldwin, 2001).
The C16-homoterpene TMTT (for 4,8,12-trimethyltrideca-1,3,7,11-tetraene) is an herbivore-induced volatile that is emitted from a number of plants, including maize (Zea mays), lima bean (Phaseolus lunatus), and tomato (Solanum lycopersicum) (Hopke et al., 1994; Ament et al., 2004; Williams et al., 2005). Several studies suggest an ecological role of TMTT with respect to the attraction of herbivore enemies. For instance, carnivorous predatory mites (Phytoseiulus persimilis) preferred the odor source of lima bean plants attacked by spider mites (Tetranychus urticae) to the odor of plants damaged by beet armyworm (Spodoptera exigua) larvae (de Boer et al., 2004). Two pieces of evidence suggest that TMTT influenced the foraging behavior of the predatory mites. First, volatiles released by the spider mite–infested plants contained larger amounts of TMTT than volatiles from plants damaged by beet armyworm larvae. Second, when TMTT was added to the odor of beet armyworm–attacked plants, predatory mites preferred this odor to that of spider mite–infested plants. Likewise, tomato plants released larger amounts of TMTT after spider mite infestation than control plants (Kant et al., 2004). This increase in volatile production coincided with the increased olfactory preference of predatory mites for infested plants, suggesting that TMTT emission in tomato is likely to play a similar role in the attraction of predatory mites as in lima bean. In addition, TMTT induces the expression of defense genes in lima bean, indicating that it also might play a role in plant–plant interactions (Arimura et al., 2000).
In recent years, Arabidopsis thaliana was shown to be a suitable model plant for investigating tritrophic interactions between plants, herbivores, and their enemies. For example, the feeding of caterpillars of the crucifer pest Pieris rapae resulted in the emission of TMTT and other volatiles by Arabidopsis that attracted the parasitoid wasp Cotesia rubecula (Van Poecke et al., 2001). The parasitization of P. rapae caterpillars by C. rubecula resulted in an increase in plant fitness in terms of seed production (Van Loon et al., 2000). Olfactometer experiments with transgenic Arabidopsis constitutively emitting the C11-homoterpene 4,8-dimethyl-1,3,7-nonatriene (DMNT), a TMTT-related compound, showed attraction of the predatory mite P. persimilis (Kappers et al., 2005).
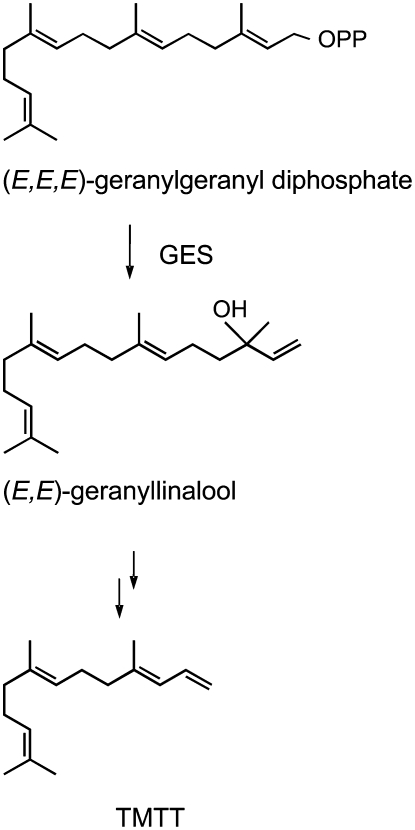
Previous studies by Boland and coworkers (1998) have shown that the C16-homoterpene TMTT is derived from the diterpene (C20) alcohol (E,E)-geranyllinalool. Feeding of deuterium-labeled (E,E)-geranyllinalool to lima bean leaves resulted in the conversion of this precursor to TMTT, suggesting a sequential enzymatic oxidative degradation of (E,E)-geranyllinalool to TMTT (Figure 1) (Gäbler et al., 1991). Recently, Ament et al. (2006) reported a stringent correlation between the synthesis of (E,E)-geranyllinalool and TMTT in tomato leaves in response to treatment with jasmonic acid (JA). It can be proposed that (E,E)-geranyllinalool is formed from geranylgeranyl diphosphate (GGPP), the central prenyl diphosphate intermediate in diterpene synthesis, via the activity of a diterpene synthase (geranyllinalool synthase [GES]), which catalyzes the hydrolysis of the diphosphate moiety and allylic rearrangement to give a tertiary alcohol with a terminal olefin (Figure 1). Terpene synthase (TPS) reactions analogous to the formation of (E,E)-geranyllinalool are mediated by linalool synthase (LIS) from Clarkia breweri (Pichersky et al., 1994, 1995) and (E)-nerolidol synthases (NES) from maize (Degenhardt and Gershenzon, 2000), cucumber (Cucumis sativus), and lima bean (Bouwmeester et al., 1999). Rather than converting the GES substrate, GGPP, these enzymes carry out the analogous conversion of the C10- and the C15-prenyl diphosphate intermediates, geranyl diphosphate (GPP) and farnesyl diphosphate (FPP), into linalool and (E)-nerolidol, respectively, of which nerolidol is further degraded into DMNT. Whereas several sequences for LISs and NESs are available from different species (Dudareva et al., 1996; Schnee et al., 2002; Aharoni et al., 2004; Martin et al., 2004), a gene encoding GES has not yet been identified.
Figure 1.
Proposed Biosynthesis of the Volatile C16-Homoterpene TMTT.
GES is proposed to catalyze the formation of (E,E)-geranyllinalool from the substrate all-trans-GGPP, the central precursor in diterpene biosynthesis. (E,E)-Geranyllinalool is further converted into TMTT by uncharacterized sequential steps of oxidative degradation (Boland et al., 1998).
Here, we present biochemical and genetic evidence that At1g61120 encodes a GES gene in Arabidopsis, thus specifying an important genetic component of herbivore-induced indirect defense systems. Regulation of GES transcription relies on the octadecanoid-dependent signaling pathway and is not modified by salicylic acid (SA) or ethylene.
RESULTS
TMTT Synthesis after Treatment of Arabidopsis Plants with Alamethicin
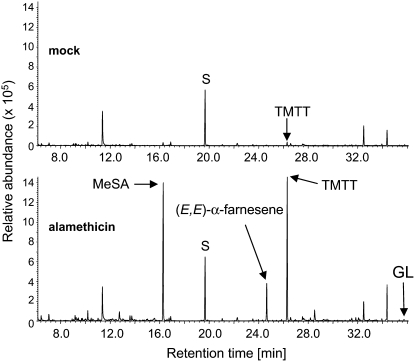
Our strategy to identify the GES gene from Arabidopsis was to determine conditions that would yield relatively high levels of TMTT and to screen the 32 potential TPS genes annotated in the Arabidopsis genome (Aubourg et al., 2002) for inducible expression. In lima bean, TMTT synthesis can be induced efficiently by alamethicin, a peptide mixture from the fungus Trichoderma viride that forms ion channels in membranes (Engelberth et al., 2001). To test whether alamethicin induces a similar response in Arabidopsis leaves, the 12 most expanded rosette leaves were detached from 5- to 6-week-old plants and incubated for 30 h with their petioles submerged in an aqueous solution of alamethicin. Volatiles were continuously collected during the first and second light periods (0 to 9 h and 21 to 30 h after the beginning of alamethicin treatment, respectively) using a closed-loop stripping method as described previously (Boland et al., 1984; Donath and Boland, 1995; Tholl et al., 2005). Three major induced volatile constituents were detected by gas chromatography–mass spectrometry (GC-MS) in both light periods: methyl salicylate (MeSA), the sesquiterpene (E,E)-α-farnesene, and the C16-homoterpene TMTT (Figure 2; see Supplemental Figure 1 online). Also, minor amounts of the presumed TMTT precursor (E,E)-geranyllinalool were emitted from induced leaves, as confirmed by mass spectrometry using a synthetic (E,E)-geranyllinalool standard (see Supplemental Figure 2A online).
Figure 2.
Induced Emission of (E,E)-Geranyllinalool and TMTT from Arabidopsis Leaves in Response to Treatment with the Fungal Elicitor Alamethicin.
Total ion GC-MS chromatograms of volatiles emitted from mock-treated (0.1% ethanol; top panel) and alamethicin-treated (in 0.1% ethanol; bottom panel) Arabidopsis leaves. Volatiles were collected from detached rosette leaves during 0 to 9 h and 21 to 30 h of treatment by a closed-loop stripping procedure. Results are shown for the second interval of volatile collection. Mock-treated leaves emitted trace amounts of TMTT but no MeSA, (E,E)-α-farnesene, or (E,E)-geranyllinalool (GL). S, nonyl acetate standard.
Identification of Inducible TPS Transcripts
To identify candidate GES genes, RT-PCR analysis was performed on RNA of plants induced for 30 h with alamethicin using primers against 32 of the putative TPS cDNAs of the Arabidopsis genome (Chen et al., 2003). As described previously, most of the TPS genes were not transcribed in leaves (Ro et al., 2006). Four transcripts were inducible upon alamethicin treatment: At2g24210, At4g16740, At4g16730, and At1g61120 (see Supplemental Figure 3 online). At2g24210 has already been characterized as a myrcene/ocimene synthase and At4g16740 as an ocimene synthase (Bohlmann et al., 2000; Fäldt et al., 2003). Both genes and the closely related gene At4g16730 cluster in the TPS-b subfamily, of which all gene members characterized to date have been identified as monoterpene synthases (Aubourg et al., 2002; Chen et al., 2003, 2004). Thus, only At1g61120, which was annotated previously as TPS04 (Aubourg et al., 2002), remained as a potential candidate for encoding the diterpene synthase GES. TPS04 clusters along with the two characterized Arabidopsis diterpene synthases involved in gibberellin biosynthesis and thus is likely to convert the 20-carbon substrate GGPP. In addition, it is 43% similar to Clarkia-type linalool synthases (Aubourg et al., 2002) that catalyze a similar reaction as GES but convert the 10-carbon substrate GPP.
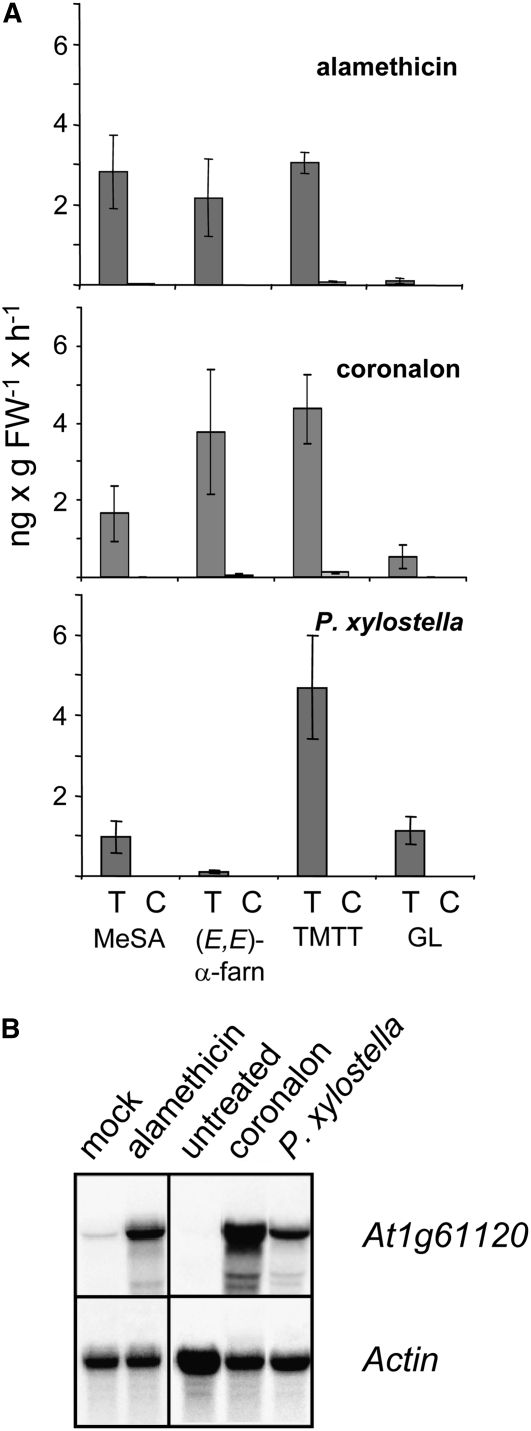
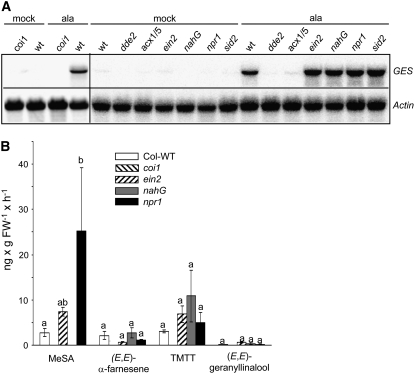
Our assumption that At1g61120 might encode a GES was corroborated by the observed correlation of At1g61120 transcription with the emission of TMTT and (E,E)-geranyllinalool under different stress conditions (Figure 3). When treating intact plants with the octadecanoid mimic coronalon or when infesting them with larvae of the moth Plutella xylostella, both (E,E)-geranyllinalool and TMTT as well as At1g61120 transcripts accumulated to higher levels compared with control plants. By contrast, the sesquiterpene α-farnesene was not emitted after P. xylostella feeding. Mechanical wounding by scratching the leaf surface with a razor blade and pinching leaves with forceps did not induce any of the four volatiles, either between 0 and 8 h after wounding or between 21 and 31 h. Consistently, no At1g61120 mRNA was detectable by RNA gel blot analysis after mechanical wounding (data not shown).
Figure 3.
Comparison of Volatile Emissions and Expression of At1g61120 in Arabidopsis Leaves upon Elicitor Treatment and Herbivore Challenge.
(A) Quantitative analysis of the four major volatiles MeSA, (E,E)-α-farnesene, TMTT, and (E,E)-geranyllinalool (GL). As highest emission rates in response to most treatments were observed in the second light phase, only results obtained during this period (21 to 30 h of treatment) are reported. Alamethicin (5 μg/mL) was applied to detached leaves, and coronalon (100 μM) was added to the medium of hydroponically grown plants. Continuous insect-feeding experiments were conducted by the application of two P. xylostella larvae in the third to fourth instar on each medium-sized and fully expanded rosette leaf. The results represent means ± SE of three replicates. Experiments were repeated at least once with similar results. C, control; FW, fresh weight; T, treatment.
(B) RNA gel blot analysis of At1g61120 transcription in Arabidopsis leaves after alamethicin treatment (5 μg/mL) applied through petioles of cut leaves, coronalon treatment (100 μM; applied to roots of intact plants for 31 h), and P. xylostella feeding for 31 h. Control RNA was collected from leaves treated with 0.1% ethanol through petioles (mock) or from intact hydroponically grown plants. The blot was rehybridized with a probe for Actin2 to document equal loading.
Functional Characterization of At1g61120 as GES
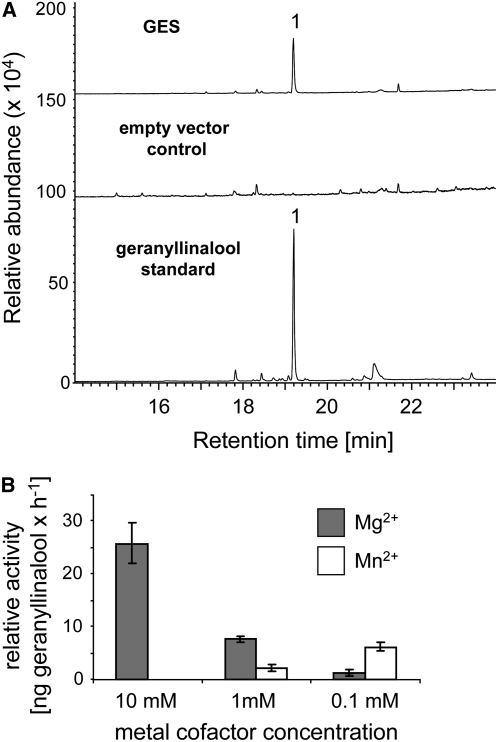
To confirm the catalytic activity of the At1g61120-encoded enzyme, the complete open reading frame was cloned into the Escherichia coli expression vector pASK-IBA7. In the presence of the affinity-purified recombinant enzyme, GGPP was converted into a single product (Figure 4A), which was identified as (E,E)-geranyllinalool by comparison of its retention time and mass spectrum with those of an authentic standard (see Supplemental Figure 2B online). No product was obtained after incubation of the enzyme with GPP and FPP as potential substrates. Based on this biochemical evidence, we call the At1g61120-encoded gene product GES from here on. Determination of GES activity in the presence of three different concentrations of Mg2+ and Mn2+ ions revealed the highest activity at 10 mM Mg2+ (Figure 4B), which is consistent with the ion preference of other plant TPSs (Davis and Croteau, 2000).
Figure 4.
GC-MS Analysis of Products Formed from GGPP by Recombinant GES Enzyme.
GES was expressed in E. coli, extracted, purified, and incubated with the substrate all-trans-GGPP. The resulting terpene products were separated by GC-MS.
(A) MS detector traces are shown for the products obtained from assays with the purified GES enzyme. An extract from E. coli carrying the empty expression vector was subjected to the same purification procedure and served as a negative control. The major peak was identified as (E,E)-geranyllinalool (1) via comparison with an authentic standard, as shown in the bottom chromatogram.
(B) The catalytic activity of the partially purified enzyme was measured in the presence of the divalent metal ions Mg2+ and Mn2+ at different concentrations. Means ± SE of triplicate assays are shown.
Analysis and Complementation of GES Loss-of-Function Plants
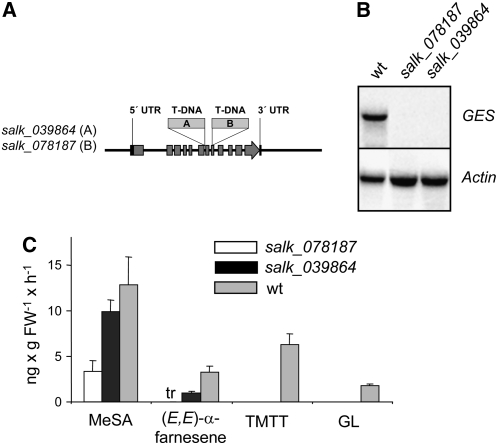
To prove the in planta function of the GES gene, corresponding knockout lines were analyzed. In lines salk_039864 and salk_078187, the T-DNAs are located in intron 6 and exon 8, respectively (Figure 5A). In contrast with wild-type plants, neither mutant line accumulated any detectable GES mRNA after coronalon treatment (Figure 5B). Consistently, no (E,E)-geranyllinalool and TMTT were detected in the head space of the mutants (Figure 5C). Although reduced emission of MeSA and (E,E)-α-farnesene upon coronalon treatment was observed in the mutants in this experiment, their appearance indicated that the treatment was effective.
Figure 5.
Coronalon-Induced Volatile Emission and GES Expression in Leaves of Arabidopsis Wild-Type and GES T-DNA Insertion Lines.
(A) Positions of T-DNA insertions in At1g61120 (GES). The exons are represented by the gray boxes, and flanking regions and introns are represented by the black line. Black boxes symbolize untranslated regions (UTR) of the first and last exons. The two independent T-DNA insertions are indicated as boxes A and B.
(B) RNA gel blot analysis of GES transcript levels in leaves of wild-type and T-DNA insertion lines after application of 100 μM coronalon for 30 h to roots of hydroponically grown plants. The blot was rehybridized with a probe for Actin2 to document equal loading.
(C) Quantitative analysis of volatiles emitted between 21 and 30 h after the beginning of coronalon treatment of single hydroponically grown wild-type and mutant plants. The results represent means + se of three replicates. FW, fresh weight; tr, trace.
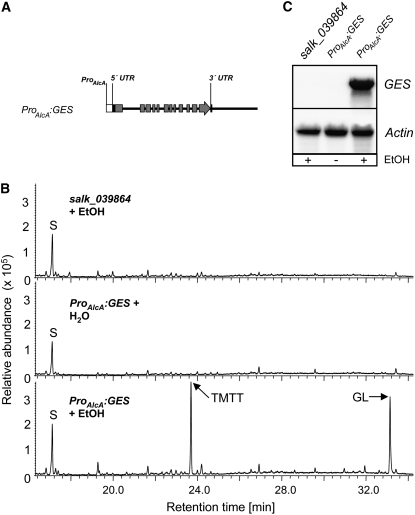
To complement the phenotype, the Arabidopsis knockout line salk_039864 was transformed with the GES gene under the control of heterologous promoters. To avoid possible secondary effects caused by constitutive (E,E)-geranyllinalool or TMTT production, we first expressed the GES gene under the control of the alcohol-inducible AlcA promoter (ProAlcA; Figure 6A). For reasons yet unknown, a 6-kb genomic fragment starting with the presumed transcriptional start site had to be used rather than the cDNA to obtain stable mRNA accumulation. As shown in Figure 6B, knockout mutants expressing the GES gene under the AlcA promoter emitted TMTT and (E,E)-geranyllinalool only after ethanol induction, which correlates with GES transcript accumulation under these conditions (Figure 6C).
Figure 6.
Complementation Analysis of the GES Insertion Line salk_039864.
(A) Schematic drawing of the chimeric gene used to complement the Arabidopsis insertion line salk_039864. The transgene (ProAlcA:GES) consists of a 6-kb genomic fragment starting with the presumed transcriptional start site (as inferred from the cDNA) under the control of the alcohol-inducible AlcA promoter. The exons are represented by the gray boxes, and flanking regions and introns are represented by the black line. UTR, untranslated region.
(B) GC-MS analysis of volatiles emitted from leaves of untransformed salk_039864 and of ProAlcA:GES transformants. Twenty 3-week-old plants grown on soil under long-day conditions were sprayed with 4.7% ethanol prior to continuous volatile collection for 31 h (control plants were sprayed with water only). Chromatograms selected for 69 m/z are shown. GL, (E,E)-geranyllinalool; S, nonyl acetate standard.
(C) GES transcript analysis in leaves of mutant lines. Twenty 3-week-old plants grown on soil under long-day conditions were sprayed with 4.7% ethanol (EtOH; control plants were sprayed with water only). Material for RNA analysis was harvested after 31 h. The blot was rehybridized with a probe for Actin2 to document equal loading.
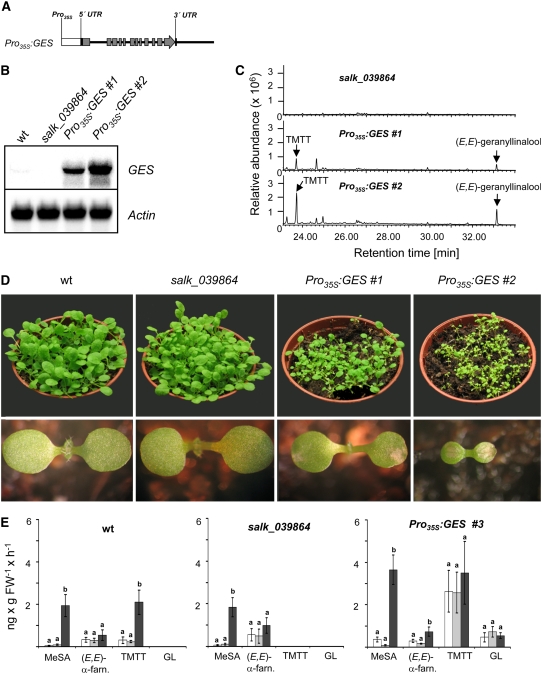
Transgenic plants expressing the GES genomic fragment under the control of the constitutive cauliflower mosaic virus (CaMV) 35S promoter (Pro35S) showed constitutive emission of TMTT and (E,E)-geranyllinalool (Figures 7A and 7C). These plants also showed reduced growth and the appearance of lesions on the cotyledons, depending on the degree of the expression of the transgene (Figures 7B and 7D). However, this phenotype, which was expressed only under long-day conditions, vanished when plants matured.
Figure 7.
Phenotype and Volatile Analysis of Seedlings with Constitutive GES Expression.
(A) Schematic drawing of the chimeric gene used to complement the Arabidopsis insertion line salk_039864. The transgene consists of a 6-kb genomic fragment starting with the presumed transcriptional start site (as inferred from the cDNA) under the control of the constitutive CaMV 35S promoter. The exons are represented by the gray boxes, and flanking regions and introns are represented by the black line. UTR, untranslated region.
(B) RNA gel blot analysis of GES transcripts in leaves of two Pro35S:GES lines (1 and 2) compared with the wild type and the knockout line salk_039864. Leaf material was harvested from 20 3-week-old untreated plants grown under long-day conditions. The blot was rehybridized with a probe for Actin2 to document equal loading.
(C) GC-MS analysis of volatiles emitted from leaves of salk_039864 transformed with the Pro35S:GES gene. Volatiles were collected continuously for 31 h from 20 plants grown as described in (B). Total ion chromatograms are shown.
(D) Top row, phenotype of 3-week-old plants grown under long-day conditions. Bottom row, phenotype of 10-d-old seedlings. The phenotype is representative for at least six independent lines showing expression of the GES transcript.
(E) Emission rates of MeSA, (E,E)-α-farnesene, TMTT, and (E,E)-geranyllinalool (GL) from leaves of wild-type, salk_039864, and Pro35S:GES lines. Plants were first grown for 3 weeks under long-day conditions, which allows the selection of strong expressors (deduced from the phenotype; see [D]). Subsequently, they were cultivated for 4 weeks under short-day conditions. Volatiles were collected from 16 to 20 detached leaves during 0 to 7 h of treatment in 20 mL of tap water (white bars), 0.1% ethanol (mock; gray bars), and alamethicin (5 μg/mL 0.1% ethanol; black bars). Volatile collection and analysis were performed as described in Methods. The results represent means ± SE of six replicates (wild type and Pro35S:GES) or four replicates (salk_039864; n = 3 for mock treatment). Statistical analysis (one-way analysis of variance) was performed for each compound on log-transformed data. Letters above each bar indicate significant differences among each set of volatiles after Tukey's test (P < 0.05). For statistical values, see Supplemental Table 1 online. FW, fresh weight.
Quantitative analysis (Figure 7E) of volatiles from detached leaves of 7-week-old Pro35S:GES plants revealed that TMTT is emitted at levels (means of 2.6 ng·g−1 fresh weight−1) comparable to those of wild-type plants after alamethicin treatment (Figures 3A [means of 3.1 ng·g−1 fresh weight·h−1] and 7E [means of 2.1 ng·g−1 fresh weight·h−1]). Emission rates of (E,E)-geranyllinalool (means of 0.47 ng·g−1 fresh weight·h−1) were similar to those of wild-type plants induced with coronalon or by P. xylostella (Figures 3A and 7E).
The ratios of TMTT to (E,E)-geranyllinalool emitted from 3-week-old plantlets of the alcohol-inducible and constitutive GES-expressing lines were in the range of 1 to 1.5, respectively (Figures 6C and 7C), which were lower than those observed in wild-type plants treated with alamethicin (∼30:1), coronalon (∼9:1), or P. xylostella (∼4:1) (Figure 3), indicating less efficient oxidative degradation of (E,E)-geranyllinalool. However, in mock-treated or untreated leaves of 7-week-old Pro35S:GES plants (Figure 7E), the TMTT:(E,E)-geranyllinalool ratio was higher (3 to 5.5:1) than in the younger transgenic plants, indicating that the steps leading to oxidative degradation might already be induced under these conditions. Levels of TMTT and (E,E)-geranyllinalool in Pro35S:GES plants did not change significantly after induction with alamethicin (Figure 7E), indicating that the amounts of precursor metabolites and/or the activity of the enzymes catalyzing the oxidative degradation could not be enhanced further in this experiment.
Subcellular Localization of GES
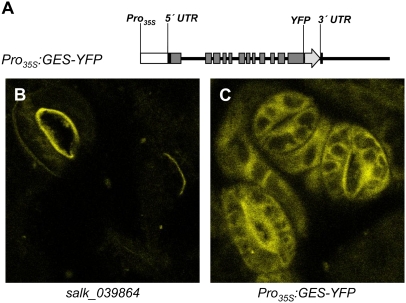
To determine the subcellular localization of GES, transgenic GES knockout plants expressing a GES-YFP (for yellow fluorescent protein) fusion under the control of the CaMV 35S promoter were established. Analysis of leaf epidermal cells from five Pro35S:GES-YFP plant lines revealed yellow fluorescence outside the plastids (Figure 8). Volatile analysis of the transgenic plants documented the emission of (E,E)-geranyllinalool, confirming the functionality of the expressed protein as GES (see Supplemental Figure 4 online). Pro35S:GES-YFP plants had no visual phenotype, which might be due to lower TMTT and/or (E,E)-geranyllinalool synthesis compared with Pro35S:GES plant lines.
Figure 8.
Subcellular Localization of GES-YFP Fusion Proteins in Arabidopsis.
(A) Schematic drawing of the GES-YFP fusion gene used to transform the Arabidopsis insertion line salk_039864. The transgene consists of a 6-kb genomic fragment starting with the presumed transcriptional start site (as inferred from the cDNA) under the control of the constitutive CaMV 35S promoter. The YFP tag was integrated upstream of the GES stop codon. The exons are represented by gray boxes, and flanking regions and introns are represented by the black line. UTR, untranslated region.
(B) and (C) Epidermal peels from true leaves of 10-d-old plants of the salk_039864 line (B) and the same knockout line transformed with Pro35S:GES-YFP (C) were used for fluorescence microscopy.
Induction of GES Transcription and TMTT Synthesis in Mutants Deficient in Stress-Related Signaling Pathways
Next, we tested the possible roles of major stress-related signaling pathways for the induction of GES transcription and TMTT synthesis. Respective Arabidopsis mutants were treated with alamethicin and RNA was extracted for RNA gel blot analysis (Figure 9A). The dde2-2 mutant (von Malek et al., 2002), which is defective in the biosynthesis of the major octadecanoid phytohormones 12-oxo-phytodienoic acid (OPDA) and JA (Park et al., 2002), as well as the acx1/5 double mutant, which is unable to convert OPDA into JA (Schilmiller et al., 2007), did not respond to alamethicin. Thus, JA or its derivatives are essential for induction by alamethicin. Also, the F-box protein COI1, which is a central regulator of most JA-mediated responses (Xie et al., 1998; Devoto et al., 2005), is required for GES transcription. JA signaling can be influenced by two other stress hormones: ethylene (Penninckx et al., 1998) and SA (Spoel et al., 2003). Ethylene-insensitive ein2 (Guzman and Ecker, 1990), SA-degrading nahG (Gaffney et al., 1993), and SA synthesis–deficient sid2 (Nawrath and Metraux, 1999; Wildermuth et al., 2001) plants were equally efficient in GES induction as wild-type plants. Plants compromised in a major branch of the SA signal transduction network (npr1; Cao et al., 1994) also did not show an altered response with regard to GES induction.
Figure 9.
GES Transcript Analysis and Leaf Volatile Emission in Different Genotypes Impaired in SA, JA, and Ethylene Signaling/Biosynthesis after Induction with Alamethicin.
(A) RNA gel blot analysis of GES transcript levels in detached leaves of wild-type plants and mutants deficient in SA, JA, and ethylene abundance or signal transduction. Alamethicin (ala; 5 μg/mL) was applied through petioles of cut leaves; for mock treatment, a 0.1% ethanol solution was used. RNA was harvested after 24 h. The blots were rehybridized with a probe for Actin2 to document equal loading. Induction of the coi1 mutant was done in intact hydroponically grown plants as described in Methods. The experiment was repeated twice with similar results.
(B) Volatiles were collected during 21 to 30 h after the beginning of alamethicin treatment from detached leaves of wild-type plants and mutants deficient in SA, JA, and ethylene abundance or signal transduction. Treatments and volatile analysis were conducted as described in Methods. The results represent means ± SE of three replicates. Letters above each bar indicate significant differences for each set of volatiles after Tukey's test (P < 0.05). For statistical values, see Supplemental Table 2 online. FW, fresh weight.
Consistent with the results at the GES transcript level, no significant differences in the amount of (E,E)-geranyllinalool and TMTT synthesis were detected when comparing the volatile profiles of selected SA- and ethylene-deficient/insensitive mutants with the profile of wild-type plants (Figure 9B). As expected, nahG plants failed to emit MeSA. The npr1 mutant, which lacks the autoregulatory negative feedback loop for SA synthesis (Cao et al., 1994), emitted ∼10-fold higher amounts of MeSA than wild-type plants. Emission of all the volatiles, including TMTT and (E,E)-geranyllinalool, depended on COI1.
Expression Profile of the GES Promoter
To assess whether the steady state levels of the GES mRNA are determined by the activity of the GES promoter, a 1.62-kb fragment upstream of the annotated transcriptional start site was inserted 5′ to the β-glucuronidase (GUS) reporter gene, and the resulting chimeric gene (ProGES:GUS) was stably transformed into Arabidopsis plants (Figure 10A). RNA gel blot analysis revealed GUS transcription upon alamethicin treatment (Figure 10B). However, in contrast with the endogenous GES mRNA, some residual GUS transcript levels were detected in the uninduced state, indicating that the GES promoter might retain some activity in the absence of inducing conditions.
Figure 10.
GUS Transcript Levels and GUS Activity in ProGES:GUS Plants.
(A) Schematic drawing of the ProGES:GUS construct. The GES promoter (−1162 to +3) was cloned upstream of the GUS reporter gene using Gateway technology. The attB2 site is part of the 5′ untranslated region of the GUS gene.
(B) RNA gel blot analysis of GUS and GES transcript levels in hydroponically grown transgenic ProGES:GUS plants after induction with alamethicin (5 μg/mL) or ethanol (0.1%) for 31 h.
(C) Histochemical GUS staining of a silique (1), a flower (2), an inflorescence (3), and a wounded leaf (4) from ProGES:GUS plants. The results are representative for at least 10 independent transgenic lines.
(D) GES expression in wild-type plants in response to wounding (1 to 12 h) and treatment with alamethicin (ala; 24 h). Six-week-old hydroponically grown plants were wounded with forceps. Leaf material within ∼3 mm adjacent to the wound marks was harvested. Material from five leaves was combined before extraction. Relative levels of GES and VSP2 (a wound-inducible control gene) were determined by real-time RT-PCR using SYBR Green I chemistry. Values were normalized to the expression of At1g13320 (protein phosphatase type 2). As a control, plants were treated with 5 μg/mL alamethicin through the roots, and RNA was harvested after 24 h. The results represent means + SE of three technical replicates. The experiment was repeated with similar results (see Supplemental Figure 5 online).
In developing or mature flowers, GUS activity was detected in the stigma, anthers, filaments, and sepals but not in petals (Figure 10C). GUS activity occurred also in the abscission zone of floral organs (Figure 10C). In leaves, GUS activity was detected at wound sites (Figure 10C), which was somewhat unexpected given that no GES mRNA was detected by RNA gel blot analysis, at least at 31 h after wounding (see above). This inconsistency was resolved by a time course analysis of transcript levels in leaf material adjacent to the wound site (2 to 3 mm). Real-time RT-PCR revealed that GES transcripts accumulated transiently up to 2 h after wounding, albeit at approximately threefold lower levels than after treatment with alamethicin (Figure 10D). Transcript levels were down to background levels at 4 h. The observed GUS staining is likely to result from this early and transient increase of promoter activity. However, this increase does not seem to be sufficient for sustained TMTT emission after wounding (see above). The wound-inducible VSP2 gene showed a longer period of transcript accumulation, indicating that different mechanisms of wound-induced activation act on the two genes analyzed here.
DISCUSSION
Herbivore-induced release of volatiles is a common plant defense mechanism that serves to attract predators or parasitoids of the attacking insects. The composition of the herbivore blend can vary depending on the type of the insect pest, raising questions concerning the roles of the individual components and the mechanisms that regulate their synthesis. A prerequisite for this analysis is the knowledge of the corresponding biosynthetic genes. Here, we report the identification of a TPS that is required for the synthesis of the TMTT precursor (E,E)-geranyllinalool in Arabidopsis. Transgenic/mutant plants with reduced or increased GES expression and promoter:reporter gene fusions provide valuable tools for elucidating the function, biosynthesis, and regulated synthesis of TMTT in Arabidopsis.
The Primary Structure of GES Is Consistent with its Presumed Function as a Diterpene Synthase
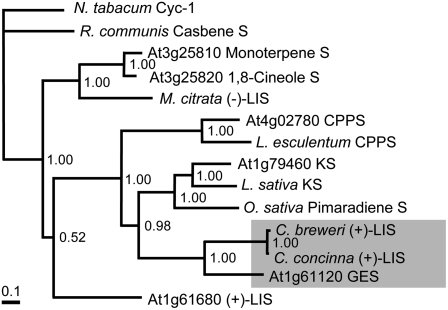
Compared with known annotated TPSs from Arabidopsis and other species, GES shares highest similarity (43%) to LISs from C. breweri and Clarkia concinna (Cseke et al., 1998; Aubourg et al., 2002) (Figure 11). As the formation of (E,E)-geranyllinalool from the C20 substrate GGPP is analogous to the synthesis of the C10-monoterpene alcohol linalool from the precursor GPP, the functional characterization of At GES is consistent with its phylogenetic position close to Cb LIS in the TPS-f subfamily (Aubourg et al., 2002). Clarkia-type LISs form an odd group within the TPS superfamily, as they are the only known monoterpene synthases of angiosperm origin that contain a conserved N-terminal 200–amino acid domain of unknown function and that exhibit a gene structure of 12 exons, which has been suggested to be similar to that of the presumed progenitor gene of all plant TPSs (Bohlmann et al., 1998; Trapp and Croteau, 2001; Aubourg et al., 2002). All of the other known monoterpene synthases of angiosperm origin, for instance the Arabidopsis LIS, consist of only seven exons and lack the conserved 200–amino acid domain. It seems attractive to explain the unusual position of Clarkia-type LISs by assuming that they have evolved from a GES-type diterpene synthase by mutations limiting the size of the substrate binding pocket at the catalytically active site of the enzyme. Analysis of the volatile bouquet of Clarkia leaves after treatment with one of the elicitors of TMTT emission could clarify whether Clarkia expresses a GES.
Figure 11.
Sequence Comparison of Arabidopsis GES with Additional Arabidopsis and Other Plant TPSs.
Bayesian tree generated from an alignment of 14 TPS proteins, including Arabidopsis GES (At1g61120), C. breweri LIS, C. concinna LIS, and selected Arabidopsis and other angiosperm monoterpene and diterpene synthases of primary and secondary metabolism. Arabidopsis genes used for the analysis are as follows: GES (At1g61120), LIS (At1g61680), myrcene/(E)-β-ocimene synthase (At3g25810), 1,8-cineole synthase (At3g25820), CPPS (At4g02780), and KS (At1g79460). Other monoterpene and diterpene synthase genes come from Nicotiana tabacum, Rhizinus communis, Mentha citrata, Solanum lycopersicum, Lactuca sativa, and Oryza sativa. Numbers above nodes represent Bayesian posterior probabilities.
Compared with the other annotated TPSs of the Arabidopsis genome (Aubourg et al., 2002), GES is most similar to the two characterized diterpene synthases copalyl diphosphate synthase (CPPS) and ent-kaurene synthase (KS) (Figure 11). Thus, the primary structure of GES is consistent with its function as a diterpene synthase. CPPS, KS, and GES differ from the other Arabidopsis TPSs by the N-terminal conserved 200–amino acid motif and the ancestral exon/intron structure (12 to 15 exons) mentioned above. Like KS, GES contains the highly conserved amino acid sequence motif DDXXD that is required for the ionization-initiated reaction mechanism of most TPSs (Davis and Croteau, 2000; Tholl, 2006).
Biotic stress–induced TMTT emissions have been described in several plant species (Hopke et al., 1994; Ament et al., 2004; Williams et al., 2005), which suggested that this defense reaction might have widespread importance. Therefore, it is likely that genes encoding GES enzymes are present in many other plant species. An herbivore-inducible GES activity that correlated with induced TMTT emission was found in tomato (Ament et al., 2006), but database searches do not provide evidence for the existence of Arabidopsis-type GES genes in the tomato genome or other genomes. This might be due to variations in the nucleotide sequence hampering a straightforward identification of the gene by sequence similarity. Alternatively, GES genes might have arisen independently in other species as a product of convergent gene evolution. Comprehensive phylogenetic analysis of plant TPSs, including angiosperm and gymnosperm LIS, showed that convergent evolution is a typical feature of the TPS gene family (Martin et al., 2004).
GES Is Not Localized to the Plastids
While diterpene synthases are primarily located in the plastid, no obvious plastid transit peptide sequence could be identified for GES with the TargetP and ChloroP algorithms. Indeed, compared with the other ancestral TPS genes CPPS and KS, GES has lost three N-terminal exons that are still present in the plastid-localized proteins CPPS and KS (Trapp and Croteau, 2001). Protein-YFP fusion studies confirmed that the GES enzyme is not targeted to the chloroplast (Figure 8) and indicated that it resides in the cytosol or the endoplasmic reticulum It is likely that the GES substrate GGPP is present in these compartments, since previous GFP fusion studies of Arabidopsis GGPP synthases identified two enzymes with a localization pattern similar to that observed for GES (Okada et al., 2000).
Expression of GES under Heterologous Promoters Allows the Uncoupling of (E,E)-Geranyllinalool and TMTT Synthesis from the Emission of Other Volatiles
In plants expressing GES under the promoters ProAlcA and Pro35S, emission of (E,E)-geranyllinalool and TMTT was detected, indicating that GES expression is sufficient to generate the products of the pathway (Figures 6 and 7). TMTT and (E,E)-geranyllinalool levels in these transgenic plants did not exceed the values observed after P. xylostella infestation and could not be further elevated by alamethicin. This could be due to either the limited availability of precursor metabolites or the negative feedback mechanisms of the products TMTT and (E,E)-geranyllinalool on the respective biosynthetic enzymes.
In this work, we noticed lower TMTT:(E,E)-geranyllinalool ratios (1- to 1.5-fold; Figures 6C and 7C) when volatiles were measured from 3-week-old intact transgenic plantlets compared with measurements performed with detached leaves of 7-week-old plants (3.7- to 5.5-fold; Figure 7E). In addition, TMTT emission levels of younger untreated transgenic plants (Figure 6C) were ∼10-fold lower compared with those of detached leaves of older plants. As the background levels of α-farnesene and TMTT were exceptionally high in the experiments shown in Figure 7E, we suspect that precursor synthesis and oxidative degradation might have been preinduced. Further work on the regulation of these potentially rate-limiting steps of TMTT synthesis is needed to understand the pathway comprehensively. The relatively high levels of (E,E)-geranyllinalool in alamethicin-treated Pro35S:GES plants (Figure 7E) compared with alamethicin-treated wild-type plants suggest that under conditions of high GES activity, the oxidative degradation can become rate-limiting.
The Arabidopsis plants ectopically expressing At GES can be compared with plants expressing strawberry (Fragaria sp) NES targeted to mitochondria (Kappers et al., 2005). Analogous to the oxidative degradation of TMTT to (E,E)-geranyllinalool, nerolidol is converted to the C11-homoterpene DMNT, presumably by the same enzymes. The conversion of nerolidol to DMNT in uninduced plants was variable and could be stimulated by JA. However, as these processes might depend on the environmental conditions, experiments with plants encoding both transgenes, Pro35S:GES and Pro35S:FaNES, would have to be performed to determine whether the efficiency of oxidative degradation of nerolidol and (E,E)-geranyllinalool follows the same pattern.
At the seedling stage, constitutive GES expression leads to growth retardation, which was also observed for DMNT-emitting plants (Kappers et al., 2005). Moreover, plants expressing GES developed lesions on the cotyledons (Figure 7D). One potential explanation is that the GGPP pool in the cytosol/endoplasmic reticulum compartment, which is needed for protein prenylation, is depleted by the constitutive activity of GES. Constitutive expression of the plastidic diterpene synthase taxadiene synthase leads to a pale bleached phenotype because of the depletion of precursors for chlorophyll biosynthesis (Besumbes et al., 2004). The different phenotypes—lesions versus bleaching—support our finding that GES is not localized in the plastid (Figure 8). Apart from metabolic disturbances caused by constitutive GES activity, the lesion phenotype could be elicited by TMTT and/or (E,E)-geranyllinalool acting as plant signals. This notion is supported by the findings of Arimura et al. (2000), who reported TMTT-induced transcription of defense genes in lima bean.
GES Transcription Requires a Functional Octadecanoid Pathway and Is Not Affected by SA and Ethylene
Mutant analysis proved that JA or its derivatives and the F-box protein COI1 are necessary for alamethicin-induced GES transcription and the emission of (E,E)-geranyllinalool and TMTT (Figure 9). In addition, GES transcription and the emission of (E,E)-geranyllinalool and TMTT were induced by the octadecanoid mimic coronalon (Figure 3). Coronalon has structural resemblance to the phytotoxin coronatine and the JA-Ile conjugate, which was recently shown to interact with COI1 (Thines et al., 2007). Therefore, we postulate that JA-Ile or a structurally related conjugate represents the signal that induces GES transcription.
Many JA-dependent responses are modulated by other phytohormones like SA, ethylene, and abscisic acid, allowing plants to integrate different external stimuli into their defense responses. The induced emission of MeSA in response to herbivore feeding and alamethicin treatment (Figures 2 and 3) suggested that SA might be a positively modulating hormone, as described for lima bean and tomato (Koch et al., 1999; Ament et al., 2004). However, GES transcript levels and emission of (E,E)-geranyllinalool and TMTT were not altered in nahG plants, which are characterized by reduced SA levels (Figure 9). In lima bean, SA directly affects JA biosynthesis by blocking the conversion of OPDA to JA. This mechanism leads to a different composition of the volatile blend depending on the internal SA levels: in the absence of SA, JA-inducible monoterpenes and sesquiterpenes are synthesized; in the presence of SA, OPDA or other octadacenaoids, which accumulate as a result of the SA-mediated inhibition of the last steps of JA biosynthesis, induce mostly the homoterpenes DMNT and TMTT (Koch et al., 1999). In tomato, JA is sufficient to induce TMTT release, but SA is required after spider mite infection, which indicates that SA also plays a role in modulating octadecanoid regulation in this species (Ament et al., 2006). At the same time, our results show that GES transcription and (E,E)-geranyllinalool/TMTT synthesis are not subject to the antagonistic effect of SA that was demonstrated for PDF1.2 and other JA-inducible genes (Glazebrook et al., 2003; Spoel et al., 2003). As revealed by the ein2 mutant (Figure 9), ethylene also does not influence the response.
Our notion that compounds derived from the octadecanoid pathway are necessary, if not sufficient, for the induction of GES transcription is supported by public databases (http://www.genevestigator.ethz.ch/at [Zimmermann et al., 2004] and http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi) that report increased GES transcript levels after treatments that activate octadecanoid biosynthesis, like infection with Botrytis cinerea or Alternaria brassicicola (van Wees et al., 2003), and treatment with ozone. Furthermore, expression is induced by infection with the coronatine-synthesizing bacterium Pseudomonas syringae macilicola in wild-type, nahG, sid2, npr1, and ein2 genotypes (Glazebrook et al., 2003), confirming our results that SA and ethylene do not modify the response (Figure 9).
The GES Promoter Is Constitutively Active in Flowers and Reveals Local and Transient Activation upon Wounding
In general, steady state mRNA levels are determined by the activity of the promoter and the stability of the mRNA. To investigate the contribution of the GES promoter to the regulation of GES expression, transgenic plants carrying a chimeric ProGES:GUS gene were generated. The GUS transgene was inducible by alamethicin, indicating that regulatory trans factors bind to the 1162 promoter fragment. Therefore, the chimeric ProGES:GUS gene can be used to dissect the regulatory events that determine the activity of the promoter. However, elevated transcript levels of the reporter gene in the uninduced state were detected, hinting at a lower stability of the GES transcript compared with the GUS transcript.
GUS staining was observed in floral organs (specifically, the stigma, anthers, and sepals) (Figure 10), which correlates with the detection of trace amounts of TMTT from flowers (Tholl et al., 2005). Moreover, there is a strong correlation between genes expressed in sepals and MeJA-induced genes (www.genevestigator.ethz.ch/at; Zimmermann et al., 2004), supporting our notion that metabolites of the octadecanoid pathway play an important role in the regulation of GES transcription. The expression pattern of GES in the flower resembles those of the recently characterized Arabidopsis caryophyllene, linalool, and multiproduct monoterpene synthases. However, these genes are expressed exclusively in flowers (Tholl et al., 2005) and cannot be induced in leaves by treatment with alamethicin.
Real-time RT PCR analysis of plant material adjacent to wound sites indicated that GES mRNA (Figure 10D) accumulated transiently with kinetics that resemble the kinetics of JA accumulation (Stintzi et al., 2001). Apparently, this induction leads to strong local GUS staining at the wound sites (Figure 10C) but is not sufficient to yield TMTT levels that are detectable by the experimental setup used in this study. Transcript levels of the wound-inducible VSP2 gene were induced for a longer period. Whether the maintenance of GES transcription upon P. xylostella feeding is a consequence of continuous wounding or whether it is induced by specific insect-derived elicitors remains to be investigated.
In conclusion, the identification of GES provides a useful tool to address the contribution of TMTT in plant–plant, plant–fungus, and plant–insect interactions. Moreover, the signaling pathway that regulates TMTT synthesis can be further dissected using the GES gene.
METHODS
Growth Conditions and Plant Material
The Columbia (Col-0) ecotype of Arabidopsis thaliana was used as the wild type (obtained from Lehle Seeds). Wild-type (Col-0) and mutant/transgenic plants were grown either in soil in a growth chamber (22°C and ∼140 μmol·m−2·s−1 PAR) or in a nonsterile hydroponic culture (Gibeaut et al., 1997) on a rockwool support. If not indicated otherwise in the figure legends, plants were grown for 5 to 6 weeks under short-day conditions (8-h-light/16-h-dark photoperiod). Plants were used in the prebolting rosette stage with a total biomass of ∼5 g. Mutants were obtained from the following sources: coi1-1 (Xie et al., 1998) from J. Turner (University of East Anglia, Norwich, UK); dde2-2 (Park et al., 2002) from B. von Malek and B. Keller (University of Zurich, Switzerland); acx1/5 (Schilmiller et al., 2007) from G. Howe (Michigan State University, East Lansing); ein2-1 (Guzman and Ecker, 1990) and npr1-1 (Cao et al., 1994) from the Nottingham Arabidopsis Stock Center (Nottingham, UK); nahG (Gaffney et al., 1993; Lawton et al., 1995) from L. Friedrich (Syngenta Biotechnology, Research Triangle Park NC); and sid2-2 Nawrath and Metraux, 1999; Wildermuth et al., 2001) from F. Ausubel (Harvard University, Cambridge, MA). coi1-1 mutant plants, which are male-sterile, were screened from F2 seed pools as described (Reymond et al., 2000) and afterward transferred to hydroponic culture for further analysis.
Plant Treatments
For treatments with coronalon (or alamethicin for wild-type and coi1 plants [Figure 9]), hydroponically grown plants were transferred with their supports into individual falcon tubes (for gene expression analysis) or glass beakers (for volatile collection) containing 25 and 30 mL of hydroponic medium, respectively. Either alamethicin (Sigma-Aldrich) or coronalon (W. Boland, Max-Planck-Institute for Chemical Ecology) was added to a final concentration of 5 μg/mL, 0.1% ethanol or 33 μg/mL (100 μM), 0.1% ethanol, respectively. Control plants were treated with 0.1% ethanol only (mock). For alamethicin treatment of detached leaves, plants were grown on soil for 4 to 6 weeks as described above. If not stated otherwise in the figure legends, 14 rosette leaves were cut off from the base of the petiole and transferred into a small glass beaker filled with 10 mL of alamethicin solution (5 μg/mL, 0.1% ethanol) so that only the petiole of each leaf was submerged. For feeding experiments with Plutella xylostella (kindly provided by T. Mitchell-Olds, Duke University, Durham, NC), 6-week-old soil-grown rosette plants with their root balls wrapped in aluminum foil were placed in 3-liter bell jars. Two third- or fourth-instar larvae were applied on each medium-sized and fully expanded rosette leaf and allowed to feed for 30 h, leading to an estimated consumption of total leaf mass of ∼35%. For induction of the alcohol-inducible AlcA promoter, 20 3-week-old plants grown on soil were sprayed with 5 mL of 4.7% ethanol prior to volatile collection. As a control treatment, plants were sprayed with 5 mL of water only.
Volatile Collection and Analysis
For volatile collection in response to treatment with alamethicin and coronalon, detached rosette leaves or single intact hydroponically grown plants were placed in glass beakers containing the elicitor solution as described above and transferred into a 1-liter bell jar. If not indicated otherwise in the figure legends, emitted volatiles were collected during 0 to 9 h and 21 to 30 h from the beginning of the treatment period in a controlled-climate chamber (23°C, 150 μmol·m−2·s−1 PAR) by a closed-loop stripping method (Boland et al., 1984; Donath and Boland, 1995) as described previously (Chen et al., 2003; Tholl et al., 2005). Collection periods were interrupted by a 12-h dark period, and bell jars were vented between collection intervals. Volatiles from 3-week old soil-grown transgenic GES-expressing lines were sampled continuously for 31 h. For the P. xylostella treatment, plants were placed in 3-liter bell jars as described above and volatiles were collected during 0 to 9 h and 21 to 30 h from the start of larval feeding. Volatiles were routinely collected on Super-Q (25 mg) traps, but charcoal traps were used for volatile collection from intact soil-grown plants of the Pro35S:GES lines and from plants in response to mechanical wounding or insect feeding. Volatile compounds were eluted with 100 μL (Super-Q traps) or 40 μL (charcoal traps) of CH2Cl2, and 120 ng of nonyl acetate in 6 μL was added as a standard. No major differences in the volatile profiles were observed between the different trapping materials. Samples were analyzed on a Hewlett-Packard 6890 gas chromatograph coupled to a Hewlett-Packard 5973 quadrupole mass selective detector. Separation was performed on a 5% phenyl-methylpolysiloxane (DB5) column (J&W Scientific) of 30 m × 0.25 mm i.d. × 0.25 μm film thickness. Helium was the carrier gas (flow rate, 2 mL/min), a splitless injection (injection volume, 2 μL) was used, and a temperature gradient of 5°C/min from 40°C (3-min hold) to 220°C was applied.
The identities of (E,E)-α-farnesene, MeSA, TMTT, and (E,E)-geranyllinalool were determined by comparison of retention time and mass spectra with those of authentic standards and with mass spectra in the National Institute of Standards and Technology and Wiley libraries. For identification of (E,E)-geranyllinalool, a commercial standard (Acros Organics) and (E,E)-geranyllinalool derived from acid hydrolysis of all-trans-GGPP were used. Treatment of all-trans-GGPP with 3 M HCl leads to the formation of (E,E)-geranyllinalool as the primary product via allylic rearrangement and all-trans-geranylgeraniol as a minor product (Hefner et al., 1998). For quantification, representative selected ion peaks of each compound were integrated (single ion method) and the amounts were calculated in relation to the response of nonyl acetate at m/z 69. Response curves for the quantified compounds relative to nonyl acetate were generated by injecting a mixture of equal amounts of authentic standards and nonyl acetate.
Heterologous Expression of GES in Escherichia coli
For expression in E. coli, the open reading frame of GES was amplified with the primer pair PI/PII and cloned as a BsaI fragment into the expression vector pASK-IBA7 (IBA). The construct was introduced into the E. coli strain TOP10 (Invitrogen). Sequence analysis confirmed that no errors were introduced by DNA amplification. Liquid cultures of the bacteria harboring the expression construct were grown at 18°C for 8 h. Expression was induced by the addition of anhydrotetracycline (IBA) to a final concentration of 200 μg/L. After 20 h of incubation at 18°C, the cells were collected by centrifugation and disrupted by a 4 × 30-s treatment with a sonicator (Bandelin UW2070) in chilled extraction buffer (50 mM MOPSO, pH 7.0, 5 mM MgCl2, 5 mM sodium ascorbate, 0.5 mM phenylmethylsulfonyl fluoride 5 mM DTT, and 10% [v/v] glycerol). The cell fragments were removed by centrifugation at 14,000g, and the supernatant was desalted into assay buffer (10 mM MOPSO, pH 7.0, 1 mM DTT, and 10% [v/v] glycerol) by passage through an Econopac 10DG column (Bio-Rad), yielding the partially purified enzyme analyzed in Figure 4B. The Strep-tagged enzyme was further purified on a Strep-Tactine affinity column (IBA) according to the manufacturer's instructions.
To determine the catalytic activity of the recombinant protein, enzyme assays containing 89 μL of the protein extract, 10 μL of GGPP (440 ng/μL), and 1 μL of MgCl2 (1, 0.1, and 0.01 M, respectively) or 1 μL of MnCl2 (1, 0.1, and 0.01 M, respectively) were performed in a Teflon-sealed, screw-capped 1-mL GC glass vial. The assays were overlaid with 80 μL of hexane containing 10 ng/μL nonyl acetate as an internal standard and incubated for 2 h at 30°C. To extract the enzyme products, the assays were mixed for 60 s. The organic phase then was removed and analyzed by GC-MS as described above using a temperature program from 60°C (2-min hold) to 280°C at 8°C/min.
Genotyping of Plant Material
Mutants carrying a T-DNA insertion in the GES gene were identified in the insertional mutant population distributed by the Nottingham Arabidopsis Stock Center (ecotype Col-0) (Sessions et al., 2002). Genomic DNA was isolated using the method described (Fulton et al., 1995). The T-DNA insertion event in At1g61120 was confirmed by PCR and sequencing of the right and left border PCR products.
Complementation Analysis
The BAC clone JATY50A19 (pYLTAC17 with an ∼40-kb insert) containing the At1g61120 locus was identified from the Arabidopsis thaliana Integrated Database (http://atidb.org/cgi-perl/gbrowse/atibrowse) and obtained from the John Innes Centre Genome Laboratory (http://dev.jicgenomelab.co.uk/jgl/). JATY50A19 was cut with AatII, and resulting fragments were separated on a 0.7% agarose gel. A 13-kb fragment that contained the At1g61120 locus was gel-purified and cloned in the AatII site of pGEM-T (Promega), resulting in the plasmid pGEM-T/GES. The CaMV 35S promoter or the AlcA promoter (Caddick et al., 1998; Roslan et al., 2001) was fused to the 5′ untranslated region of GES as annotated by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) by recombinant PCR. For first-round PCR, the CaMV 35S promoter (−1011 to +3) was amplified from pB2GW7 (Karimi et al., 2005) using the primers P1 and P3 (see Supplemental Table 3 online for primer sequences). The AlcA promoter (−298 to +3) including 77 bp of vector sequences was amplified from pCAMBIA3300 (a kind gift of Andreas Hiltbrunner, Syngenta, Jealott's Hill International Research Centre) using the primers P2 and P3. P3 was extended by the first 36 bp of the GES 5′ untranslated region. The PCR products were gel-purified and used together with pGEM-T/GES in a second round of PCR using P1 or P2 and the primer P4 of the GES genomic fragment. The resulting PCR products contained the 5′ untranslated region and part of the first exon (up to +302) of GES downstream of the CaMV 35S or AlcA promoter. After subcloning in pSK (Stratagene) and sequencing, the GES promoter in pGEM-T/GES was replaced by the CaMV 35S or AlcA promoter using a Bsp120I/Esp3I restriction digest. The Pro35S:GES construct was cut with AatII/SacI and ligated in the AatII/SacI-cut binary vector pB2GW7 (Karimi et al., 2005). To combine the ProAlcA:GES gene with the Pro35S:AlcR construct (Caddick et al., 1998) in a binary vector, a synthetic HindIII double-stranded oligonucleotide (annealed P5 and P6) including a SdaI and a XmaJI site was first cloned in HindIII-cut pCAMBIA3300. The ProAlcA:GES construct was cut with NheI/PstI and cloned in the XmaJI/SdaI sites of the modified pCAMBIA3300.
For the generation of transgenic plants, binary plasmids were electroporated (GenePulser II; Bio-Rad) into Agrobacterium tumefaciens strain GV3101 (pMP90). The resulting agrobacteria were used to transform Col-0 plants by the floral dipping method (Clough and Bent, 1998). Transgenic seeds containing the Pro35S:GES or the ProAlcA:GES construct were selected by spraying a solution of BASTA (glufosinate) herbicide according to the manufacturer's instructions (AgrEvo).
C-Terminal Fusion of GES with YFP
Recombinant PCR was performed to integrate the restriction sites Cfr9I and AsiSI upstream of the GES stop codon. The PCR fragment was generated using pGEM-T/Pro35S:GES as template and the oligonucleotides P7, P8, and P9 as primers (see Supplemental Table 3 online for primer sequences). The PCR product was cut with BshTI and cloned in BshTI-cut pGEM-T/Pro35S:GES. The YFP gene was amplified using primers P10 and P11, which introduced Cfr9I and AsiSI sites at the two ends. The YFP PCR fragment was cut with Cfr9I and AsiSI and cloned in the Cfr9I/AsiSI sites of the construct created before. The transfer of the Pro35S:GES-YFP construct in the binary vector pB2GW7 (Karimi et al., 2005) and the subsequent generation of transgenic plants was done as described above. Transformants expressing the transgene were selected by RNA gel blot analysis. The functionality of the fusion protein was verified by volatile collection. For fluorescence imaging, 2-week-old plants grown on Murashige and Skoog medium, 2% sucrose, 0.64% agar were analyzed by laser scanning microscopy.
RNA Gel Blot Analysis
Total RNA was extracted using the Trizol method (Invitrogen) and analyzed by RNA gel blot analysis as described (Heinekamp et al., 2002). An 846-bp GES-specific probe was amplified by PCR using the primer combination P12 and P13 (see Supplemental Table 3 online for primer sequences). Generation of the Actin2 probe was done as described (An et al., 1996).
Construction and Analysis of the ProGES:GUS Reporter Gene Fusion
A 1.2-kb GES promoter fragment was amplified by PCR from genomic DNA using primers P14 and P15 (see Supplemental Table 3 online for primer sequences) that carried the recombination sequences for the Gateway technology. The GES promoter sequences were recombined into pDONR201 (Invitrogen) by a BP recombination reaction according to the manufacturer's instructions. The GES promoter fragment was subsequently recombined into the binary vector pGateGUS (kindly provided by S. Kushnir, Ghent University) by an LR recombination reaction. Transgenic plants were generated as described above. Histochemical GUS assays were done as described (Jefferson et al., 1987).
Quantification of GES Transcript Levels with Real-Time RT-PCR
RNA extraction of plant leaf material was performed as described above. cDNA synthesis was performed with 1 μg of total RNA, 20 pmol of oligo(dT) (18 dT), and 200 pmol of random nonamer oligonucleotides. Water was added to a final reaction volume of 12.5 μL. The mixture was heated to 70°C for 10 min, 20 nmol deoxynucleoside triphosphates (dNTPs), 4 μL of 5× reaction buffer (Fermentas), and 30 units of ribonuclease inhibitor (Eppendorf) were added (final volume, 20 μL), and the mixture was incubated at 37°C for 10 min. One hundred units of RevertAid H Minus M-MuLV reverse transcriptase (Fermentas) was added (final volume, 20 μL), and the mixture was incubated at 42°C for 70 min, then heated to 70°C for 10 min. The iCycler system (Bio-Rad) was used for the amplification and quantification of cDNA using primers for GES (P16 and P17), VSP2 (P18 and P19), and At1g13320 (protein phosphatase type 2; P20 and P21) as references (see Supplemental Table 3 online for primer sequences) (Czechowski et al., 2005). The amplification mix consisted of 1× NH4 reaction buffer (Bioline), 2 mM MgCl2, 100 μM dNTPs, 0.4 μM of primers, 0.25 units of BIOTaq DNA polymerase (Bioline), 10 nM fluorescein (Bio-Rad), 100,000× diluted SYBR Green I solution (Cambrex), 1 μL of a 1:10 dilution of cDNA as template, and double distilled water to a total volume of 25 μL. PCR consisted of a 3-min denaturation step at 95°C followed by 40 cycles of 20 s at 95°C, 20 s at 55°C, and 40 s at 72°C. Three technical replicates were done on the same cDNA during one run in the iCycler.
Determination of TPS Gene Transcription by RT-PCR
For expression analysis of the TPS genes At2g24210, At4g16730, At4g16740, and At1g61120 in detached rosette leaves after 30 h of treatment with alamethicin, semiquantitative RT-PCR was performed with primers P22 and P23 (At2g24210), P24 and P25 (At4g16730), P26 and P27 (At4g16740), and P28 and P29 (At1g61120) as listed in Supplemental Table 3 online. RNA extraction of plant leaf material was performed as described above. Two micrograms of RNA were reverse-transcribed into cDNA in a 20-μL reaction with 0.5 μg of poly(dT) primer, 0.2 mM of each dNTP, and 200 units of SuperScript II reverse transcriptase (Invitrogen). PCR was performed in 30 cycles with 0.2 μM of each primer, 0.2 mM of each dNTP, and 0.25 units of Platinum Taq polymerase (Invitrogen). Reactions with primers P30 and P31 for Actin8 (see Supplemental Table 3 online) were performed to judge the equality of cDNA template concentrations.
Phylogenetic Analysis
Amino acid sequence alignment (see Supplemental Data Set 1 online) was produced with ClustalX (http://bips.u-strasbg.fr/fr/Documentation/ClustalX) and exported as a Nexus file. A Bayesian tree was generated in MrBayes 3.1.2 (Huelsenbeck and Ronquist, 2001; Ronquist and Huelsenbeck, 2003), which uses the Markov chain Monte Carlo method. The program was run for 300,000 generations with sample frequency of 10, and the SD of split frequencies was stabilized at ∼0.002. When summarizing the substitution model parameters and trees, 7500 samples (i.e., the first 25% of all samples) were used for burn-in. Model jumping was used to estimate the appropriate amino acid fixed-rate model. The Wag model was selected with >99% posterior probability. Two independent runs converged to the same tree. Statistical support for nodes was assessed by Bayesian posterior probability. Treeview (Page, 1996) was used to visualize the tree. Bayesian analysis based on an alignment of truncated proteins lacking putative transit peptide sequences resulted in an almost identical phylogenetic tree with minor changes in Bayesian posterior probabilities.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: Arabidopsis 1,8-cineole synthase (At3g25820), AY691947; Arabidopsis ent-copalyl diphosphate synthase (At4g02780), U11034; Arabidopsis kaurene synthase (At1g79460), AF034774; Arabidopsis (+)-linalool synthase (At1g61680), AF497485; Arabidopsis multiproduct monoterpene synthase (At3g25810), AF497484; Clarkia breweri (+)-linalool synthase, U58314; Clarkia concinna (+)-linalool synthase, AF067602; Solanum lycopersicum copalyl diphosphate synthase, AB015675; Lactuca sativa KS, AB031205; Mentha citrata (−)-linalool synthase, AY083653; Nicotiana tabacum Cyc-1 diterpene synthase, AY049090; Oryza sativa 9β-pimara-7,15-dienesynthase, AB126934; Rhizinus communis casbene synthase, L32134.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Time Course of Volatile Emissions from Arabidopsis Rosette Leaves in Response to Treatment with Alamethicin.
Supplemental Figure 2. Mass Spectral Analysis of (E,E)-Geranyllinalool Emitted from Arabidopsis Leaves and Synthesized by the Recombinant GES Enzyme.
Supplemental Figure 3. Induction of TPS Gene Transcripts in Arabidopsis Rosette Leaves upon Treatment with Alamethicin.
Supplemental Figure 4. GC-MS Analysis of Volatiles Emitted from Leaves of Pro35S:GES-YFP and Pro35S:GES Transformants.
Supplemental Figure 5. Biological Replicate of Results Shown in Figure 10D: Wound-Induced Gene Expression of Arabidopsis GES as Determined by Real-Time RT-PCR.
Supplemental Table 1. Statistical Values for One-Way Analysis of Variance Performed for the Data Analysis Shown in Figure 7E.
Supplemental Table 2. Statistical Values for One-Way Analysis of Variance Performed for the Data Analysis Shown in Figure 9B.
Supplemental Table 3. List of Primers.
Supplemental Data Set 1. Protein Sequence Alignment of Arabidopsis GES with Other Arabidopsis and Plant TPSs Corresponding to Figure 11.
Supplementary Material
Acknowledgments
We thank Bettina Raguschke, Katrin Heisse, Anna Hermann, Annette Gunkel, and Roland Scholz for excellent technical assistance and G. Howe for providing the acx1 acx5 double mutant prior to publication. We thank Grit Kunert for help with the statistical analysis. We thank Shuangchun (Jemery) Yan and Boris Vinatzer for support with the molecular phylogenetic analysis. This work was supported by the Max Planck Society (D.T., J.G., and W.B.), by funds from Virginia Tech (D.T.), and by grants from the Alfried-Krupp-von-Bohlen-and-Halbach Foundation (M.H.) and the Deutsche Forschungsgemeinschaft (Grant GA330-16; K.G.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Christiane Gatz (cgatz@gwdg.de).
Online version contains Web-only data.
References
- Aharoni, A., Giri, A.P., Verstappen, F.W., Bertea, C.M., Sevenier, R., Sun, Z., Jongsma, M.A., Schwab, W., and Bouwmeester, H.J. (2004). Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16 3110–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament, K., Kant, M.R., Sabelis, M.W., Haring, M.A., and Schuurink, R.C. (2004). Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 135 2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament, K., Van Schie, C.C., Bouwmeester, H.J., Haring, M.A., and Schuurink, R.C. (2006). Induction of a leaf specific geranylgeranyl pyrophosphate synthase and emission of (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene in tomato are dependent on both jasmonic acid and salicylic acid signaling pathways. Planta 224 1197–1208. [DOI] [PubMed] [Google Scholar]
- An, Y.Q., McDowell, J.M., Huang, S., McKinney, E.C., Chambliss, S., and Meagher, R.B. (1996). Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 10 107–121. [DOI] [PubMed] [Google Scholar]
- Arimura, G., Ozawa, R., Shimoda, T., Nishioka, T., Boland, W., and Takabayashi, J. (2000). Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406 512–515. [DOI] [PubMed] [Google Scholar]
- Aubourg, S., Lecharny, A., and Bohlmann, J. (2002). Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol. Genet. Genomics 267 730–745. [DOI] [PubMed] [Google Scholar]
- Baldwin, I.T., and Preston, C.A. (1999). The eco-physiological complexity of plant responses to insect herbivores. Planta 208 137–145. [Google Scholar]
- Besumbes, O., Sauret-Gueto, S., Phillips, M.A., Imperial, S., Rodriguez-Concepcion, M., and Boronat, A. (2004). Metabolic engineering of isoprenoid biosynthesis in Arabidopsis for the production of taxadiene, the first committed precursor of Taxol. Biotechnol. Bioeng. 88 168–175. [DOI] [PubMed] [Google Scholar]
- Bohlmann, J., Martin, D., Oldham, N.J., and Gershenzon, J. (2000). Terpenoid secondary metabolism in Arabidopsis thaliana: cDNA cloning, characterization, and functional expression of a myrcene/(E)-beta-ocimene synthase. Arch. Biochem. Biophys. 375 261–269. [DOI] [PubMed] [Google Scholar]
- Bohlmann, J., Meyer-Gauen, G., and Croteau, R. (1998). Plant terpenoid synthases: Molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA 95 4126–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland, W., Gäbler, A., Gilbert, M., and Feng, Z. (1998). Biosynthesis of C11 and C16 homoterpenes in higher plants: Stereochemistry of the C–C-bond cleavage reaction. Tetrahedron 54 14725–14736. [Google Scholar]
- Boland, W., Ney, P., Jaenicke, L., and Gassmann, G. (1984). A ‘closed-loop-stripping’ technique as a versatile tool for metabolic studies of volatiles. In Analysis of Volatiles, P. Schreier, ed (Berlin: Walter de Gruyter), pp. 371–373.
- Bouwmeester, H.J., Verstappen, F.W., Posthumus, M.A., and Dicke, M. (1999). Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol. 121 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddick, M.X., Greenland, A.J., Jepson, I., Krause, K.P., Qu, N., Riddell, K.V., Salter, M.G., Schuch, W., Sonnewald, U., and Tomsett, A.B. (1998). An ethanol inducible gene switch for plants used to manipulate carbon metabolism. Nat. Biotechnol. 16 177–180. [DOI] [PubMed] [Google Scholar]
- Cao, H., Bowling, S.A., Gordon, A.S., and Dong, X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F., Ro, D.K., Petri, J., Gershenzon, J., Bohlmann, J., Pichersky, E., and Tholl, D. (2004). Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole. Plant Physiol. 135 1956–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F., Tholl, D., D'Auria, J.C., Farooq, A., Pichersky, E., and Gershenzon, J. (2003). Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 15 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Cseke, L., Dudareva, N., and Pichersky, E. (1998). Structure and evolution of linalool synthase. Mol. Biol. Evol. 15 1491–1498. [DOI] [PubMed] [Google Scholar]
- Czechowski, T., Stitt, M., Altmann, T., Udvardi, M.K., and Scheible, W.R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, E.M., and Croteau, R. (2000). Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. In Topics in Current Chemistry: Biosynthesis—Aromatic Polyketides, Isoprenoids, Alkaloids, F.J. Leeper and J.C. Vederas, eds (Heidelberg, Germany: Springer-Verlag), pp. 53–95.
- de Boer, J.G., Posthumus, M.A., an d Dicke, M. (2004). Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J. Chem. Ecol. 30 2215–2230. [DOI] [PubMed] [Google Scholar]
- Degenhardt, J., and Gershenzon, J. (2000). Demonstration and characterization of (E)-nerolidol synthase from maize: A herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210 815–822. [DOI] [PubMed] [Google Scholar]
- Devoto, A., Ellis, C., Magusin, A., Chang, H.S., Chilcott, C., Zhu, T., and Turner, J.G. (2005). Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 58 497–513. [DOI] [PubMed] [Google Scholar]
- Dicke, M., and Sabelis, M.W. (1989). Does it pay plants to advertise for bodyguards? In Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants, H. Lambers, M.L. Cambridge, H. Konings, and T.L. Pons, eds (The Hague, The Netherlands: SPB), pp. 341–358.
- Donath, J., and Boland, W. (1995). Biosynthesis of acyclic homoterpenes: Enzyme selectivity and absolute configuration of the nerolidol percursor. Phytochemistry 39 785–790. [Google Scholar]
- Dudareva, N., Cseke, L., Blanc, V.M., and Pichersky, E. (1996). Evolution of floral scent in Clarkia: Novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell 8 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth, J., Koch, T., Schuler, G., Bachmann, N., Rechtenbach, J., and Boland, W. (2001). Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol. 125 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäldt, J., Arimura, G., Gershenzon, J., Takabayashi, J., and Bohlmann, J. (2003). Functional identification of AtTPS03 as (E)-beta-ocimene synthase: A monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta 216 745–751. [DOI] [PubMed] [Google Scholar]
- Fritzsche-Hoballah, M.E., and Turlings, T.C.J. (2001). Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evol. Ecol. Res. 3 553–565. [Google Scholar]
- Fulton, T.M., Chunwongse, J., and Tanksley, S.D. (1995). Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol. Biol. Rep. 13 207–209. [Google Scholar]
- Gäbler, A., Boland, W., Preiss, H., and Simon, H. (1991). Stereochemical studies on homoterpene biosynthesis in higher plants: Mechanistic, phylogenetic, and ecological aspects. Helv. Chim. Acta 74 1773–1789. [Google Scholar]
- Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., Ward, E., Kessmann, H., and Ryals, J. (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 262 754–756. [DOI] [PubMed] [Google Scholar]
- Gibeaut, D.M., Hulett, J., Cramer, G.R., and Seemann, J.R. (1997). Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 115 317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J., Chen, W., Estes, B., Chang, H.S., Nawrath, C., Metraux, J.P., Zhu, T., and Katagiri, F. (2003). Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J. 34 217–228. [DOI] [PubMed] [Google Scholar]
- Guzman, P., and Ecker, J.R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner, J., Ketchum, R.E., and Croteau, R. (1998). Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase from Taxus canadensis and assessment of the role of this prenyltransferase in cells induced for taxol production. Arch. Biochem. Biophys. 360 62–74. [DOI] [PubMed] [Google Scholar]
- Heinekamp, T., Kuhlmann, M., Lenk, A., Strathmann, A., and Droge-Laser, W. (2002). The tobacco bZIP transcription factor BZI-1 binds to G-box elements in the promoters of phenylpropanoid pathway genes in vitro, but it is not involved in their regulation in vivo. Mol. Genet. Genomics 267 16–26. [DOI] [PubMed] [Google Scholar]
- Hopke, J., Donath, J., Blechert, S., and Boland, W. (1994). Herbivore-induced volatiles: The emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a beta-glucosidase and jasmonic acid. FEBS Lett. 352 146–150. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck, J., and Ronquist, F. (2001). MrBayes: Bayesian inference of phylogeny. Bioinformatics 17 754–755. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant, M.R., Ament, K., Sabelis, M.W., Haring, M.A., and Schuurink, R.C. (2004). Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 135 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers, I.F., Aharoni, A., van Herpen, T.W., Luckerhoff, L.L., Dicke, M., and Bouwmeester, H.J. (2005). Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309 2070–2072. [DOI] [PubMed] [Google Scholar]
- Karimi, M., De Meyer, B., and Hilson, P. (2005). Modular cloning in plant cells. Trends Plant Sci. 10 103–105. [DOI] [PubMed] [Google Scholar]
- Kessler, A., and Baldwin, I.T. (2001). Defensive function of herbivore-induced plant volatile emissions in nature. Science 291 2141–2144. [DOI] [PubMed] [Google Scholar]
- Koch, T., Krumm, T., Jung, V., Engelberth, J., and Boland, W. (1999). Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol. 121 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton, K., Weymann, K., Friedrich, L., Vernooij, B., Uknes, S., and Ryals, J. (1995). Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol. Plant Microbe Interact. 8 863–870. [DOI] [PubMed] [Google Scholar]
- Martin, D.M., Fäldt, J., and Bohlmann, J. (2004). Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol. 135 1908–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath, C., and Metraux, J.P. (1999). Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, K., Saito, T., Nakagawa, T., Kawamukai, M., and Kamiya, Y. (2000). Five geranylgeranyl diphosphate synthases expressed in different organs are localized into three subcellular compartments in Arabidopsis. Plant Physiol. 122 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, R.D.M. (1996). TREEVIEW: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12 357–358. [DOI] [PubMed] [Google Scholar]
- Park, J.H., Halitschke, R., Kim, H.B., Baldwin, I.T., Feldmann, K.A., and Feyereisen, R. (2002). A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 31 1–12. [DOI] [PubMed] [Google Scholar]
- Penninckx, I.A., Thomma, B.P., Buchala, A., Metraux, J.P., and Broekaert, W.F. (1998). Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10 2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky, E., Lewinsohn, E., and Croteau, R. (1995). Purification and characterization of S-linalool synthase, an enzyme involved in the production of floral scent in Clarkia breweri. Arch. Biochem. Biophys. 316 803–807. [DOI] [PubMed] [Google Scholar]
- Pichersky, E., Raguso, R.A., Lewinsohn, E., and Croteau, R. (1994). Floral scent production in Clarkia (Onagraceae) (I. Localization and developmental modulation of monoterpene emission and linalool synthase activity). Plant Physiol. 106 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond, P., Weber, H., Damond, M., and Farmer, E.E. (2000). Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro, D.K., Ehlting, J., Keeling, C.I., Lin, R., Mattheus, N., and Bohlmann, J. (2006). Microarray expression profiling and functional characterization of AtTPS genes: Duplicated Arabidopsis thaliana sesquiterpene synthase genes At4g13280 and At4g13300 encode root-specific and wound-inducible (Z)-gamma-bisabolene synthases. Arch. Biochem. Biophys. 448 104–116. [DOI] [PubMed] [Google Scholar]
- Ronquist, F., and Huelsenbeck, J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 1572–1574. [DOI] [PubMed] [Google Scholar]
- Roslan, H.A., Salter, M.G., Wood, C.D., White, M.R., Croft, K.P., Robson, F., Coupland, G., Doonan, J., Laufs, P., Tomsett, A.B., and Caddick, M.X. (2001). Characterization of the ethanol-inducible alc gene-expression system in Arabidopsis thaliana. Plant J. 28 225–235. [DOI] [PubMed] [Google Scholar]
- Sabelis, M.W., Janssen, A., and Kant, M.R. (2001). Ecology. The enemy of my enemy is my ally. Science 291 2104–2105. [DOI] [PubMed] [Google Scholar]
- Schilmiller, A.L., Koo, A.J., and Howe, G.A. (2007). Functional diversification of acyl-coenzyme A oxidases in jasmonic acid biosynthesis and action. Plant Physiol. 143 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee, C., Kollner, T.G., Gershenzon, J., and Degenhardt, J. (2002). The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-beta-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol. 130 2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions, A., et al. (2002). A high-throughput Arabidopsis reverse genetics system. Plant Cell 14 2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel, S.H., et al. (2003). NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi, A., Weber, H., Reymond, P., Browse, J., and Farmer, E.E. (2001). Plant defense in the absence of jasmonic acid: The role of cyclopentenones. Proc. Natl. Acad. Sci. USA 98 12837–12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines, B., Katsir, L., Melotto, M., Niu, Y., Mandaokar, A., Liu, G., Nomura, K., He, S.Y., Howe, G.A., and Browse, J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448 661–665. [DOI] [PubMed] [Google Scholar]
- Tholl, D. (2006). Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 9 297–304. [DOI] [PubMed] [Google Scholar]
- Tholl, D., Chen, F., Petri, J., Gershenzon, J., and Pichersky, E. (2005). Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J. 42 757–771. [DOI] [PubMed] [Google Scholar]
- Trapp, S.C., and Croteau, R.B. (2001). Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 158 811–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon, J.J.A., De Boer, J.G., and Dicke, M. (2000). Parasitoid-plant mutualism: Parasitoid attack of herbivore increases plant reproduction. Entomol. Exp. Appl. 97 219–227. [Google Scholar]
- Van Poecke, R.M., Posthumus, M.A., and Dicke, M. (2001). Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 27 1911–1928. [DOI] [PubMed] [Google Scholar]
- van Wees, S.C., Chang, H.S., Zhu, T., and Glazebrook, J. (2003). Characterization of the early response of Arabidopsis to Alternaria brassicicola infection using expression profiling. Plant Physiol. 132 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Malek, B., van der Graaff, E., Schneitz, K., and Keller, B. (2002). The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216 187–192. [DOI] [PubMed] [Google Scholar]
- Wildermuth, M.C., Dewdney, J., Wu, G., and Ausubel, F.M. (2001). Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414 562–565. [DOI] [PubMed] [Google Scholar]
- Williams, L., III, Rodriguez-Saona, C., Pare, P.W., and Crafts-Brandner, S.J. (2005). The piercing-sucking herbivores Lygus hesperus and Nezara viridula induce volatile emissions in plants. Arch. Insect Biochem. Physiol. 58 84–96. [DOI] [PubMed] [Google Scholar]
- Xie, D.X., Feys, B.F., James, S., Nieto-Rostro, M., and Turner, J.G. (1998). COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280 1091–1094. [DOI] [PubMed] [Google Scholar]
- Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L., and Gruissem, W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.