Abstract
Objective
Human NK cell maturation involves the orderly acquisition of NK cell receptors. Our aim was to understand how stromal interactions and cytokines are important in this developmental process.
Methods
Human UCB CD34+/Lin−/CD38− cells were cultured on two murine stromal cell lines (AFT024 and EL08-1D2) in a switch culture to study NK cell development.
Results
When human progenitors were cultured on AFT024 with IL-3 and Flt3-L in the absence of IL-15, NK cell differentiation occurred, albeit at low frequency. These conditions favored the accumulation of CD56− NK cell precursors (CD34+CD7−, CD34+CD7+ and CD34−CD7+ cells), which are populations rare in adult blood but abundant in fresh UCB. In secondary culture, addition of IL-3 or IL-3+Flt3L to IL-15 increased the absolute number of CD56+ NK cells from precursors and the acquisition of CD94 and KIR. To further explore the microenvironment in early NK cell maturation, a cell line derived from murine embryonic liver (EL08-1D2) was studied. NK cell development and KIR acquisition was superior with EL08-1D2 which supported the differentiation of NK cell precursors, NK cell commitment, and proliferation.
Conclusion
Although the earliest events in NK cell maturation do not require exogenous human IL-15, it is required at a later stage of NK cell commitment. At a minimum, murine stroma, IL-3, and Flt3-L are required to recapitulate early NK cell development and differentiation into distinct NK cell precursors. EL08-1D2 induces KIR acquisition suggesting that extrinsic signals in NK cell development are conserved between mouse and man.
Introduction
Human NK cells represent a subpopulation of peripheral blood lymphocytes which express the CD56 antigen but no T-cell receptor antigens (CD3 negative). They mediate both MHC-independent and antibody-dependent killing of tumors and virally infected cells. In addition, they proliferate and secrete cytokines upon activation. Antibody-dependent cellular cytotoxicity (ADCC) by NK cells is mediated by binding of FcRγIII (CD16) to the Fc portion of antibodies. This initiates a sequence of cellular events culminating in the release of cytotoxic, granzyme-containing granules [1]. Different signaling pathways are engaged in the process by which NK cells lyse tumor or virally infected targets [2]. NK cell killing is non-MHC restricted, in terms of not requiring class I MHC for target recognition. However, recognition of class I by NK cells through killer immunoglobulin-like receptors (KIR), CD94/NKG2 heterodimers and other receptors influence whether target cell lysis occurs [3]. Whether or not a target is killed by NK cells is determined by a net balance of these positive and negative signals by several mechanisms [4] and factors inherent to different NK cell subsets [5]. We hypothesize that KIR and NKG2 receptor acquisition is determined at discrete developmental stages of NK cell maturation.
NK cells are derived from lineage negative CD34+/HLA-DR− (CD34+/Lin−/DR−) human progenitors and their differentiation is dependent on direct contact with stromal ligands and cytokines [6]. The development of NK cells from marrow progenitors has been corroborated in vitro [7,8] and in vivo after transplanting fetal sheep with CD34+/Lin−/DR− cells [9]. Marrow derived NK precursors eventually egress into the blood where CD56bright NK cells may be more primitive than CD56dim NK cells because they are less cytotoxic [10], lack FcRγIII (CD16) [10], constitutively express intermediate affinity IL-2 receptors [11], are highly proliferative and clonogenic [12,13], and express receptors for c-kit [14,15]. There is a continuum of differentiation from the most primitive hematopoietic stem cell to the terminally differentiated CD56dim KIR-expressing NK cell.
There has been significant interest in investigating the mechanisms by which the marrow microenvironment governs lymphoid differentiation through its supportive extracellular matrix [16], cell surface ligands, and production of soluble cytokines [17]. We have shown that cultured adult marrow CD34+/Lin−/DR− cells will differentiate into phenotypic and functional NK cells only if the progenitors are grown in direct contact with allogeneic stroma and IL-2 or IL-15. In mice, the ability of stroma to induce differentiation is, at least in part, regulated by the transcription factor interferon-regulatory factor-1 (IRF-1) [18]. IRF-1 knockout mice exhibit a severe NK deficiency, which is mediated by failure of transcriptional regulation of IL-15. In addition, knockout mice deficient in IL-15 itself or IL-15Rα have very few NK cells while IL-2 and IL-2Rα knockouts are unaffected [19,20]. In human studies, IL-15 is made by stroma and macrophages [21,22]. The importance of IL-15 in the homeostasis of NK [23,24] and T-cells provides further evidence of the physiologic role for this cytokine in the immune system [20]. These studies do not distinguish between the role of IL-15 in homeostatic expansion and survival versus differentiation. While IL-15 is undisputedly critical for NK cell commitment and for mature NK cell homeostasis, the focus of this study is to identify the signals operating prior to NK cell commitment which may be IL-15 independent.
Methods and Materials
Adult peripheral blood and umbilical cord blood progenitors
The use of all tissue was approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota according to the Declaration of Helsinki. Adult peripheral blood (PB) was obtained from normal adult donors and UCB was obtained from full-term mothers from local obstetrical units, the Memorial Blood Bank (Minneapolis, MN), Placental Blood Program of the New York Blood Center (New York, NY) or the St Louis Cord Blood Bank (St Louis, MO). Mononuclear cells were obtained by Ficoll-Hypaque (sp. grav. 1.077 Sigma Diagnostics, St. Louis, MO) density gradient centrifugation. Resulting UCB mononuclear cells were enriched for CD34+ cells by using the MACS magnetic micro bead selection system as recommended by the manufacturer (Miltenyi Biotech, Oberlin, CA). The positively selected cells were then stained with CD34 allophycocyanin antibody (APC, BD Biosciences, San Diego, CA), fluorescein isothiocyannate (FITC)-conjugated antibodies against CD2, CD3, CD4, CD5, CD7, CD8, CD10, and CD19 as the lineage (Lin) cocktail (all from BD Biosciences) and as a third color Phycoerythriln (PE)-conjugated CD38 (BD Biosciences) was used allowing for multicolor cell separation and sorting using the fluorescence activated cell sorter (FACS) DiVa and/or Aria (Becton Dickinson, San Jose, CA). CD34+/Lin−/38− stem cells were sorted for bulk culture experiments or directly into 96 well plates pre-established with irradiated AFT024 stroma as previously described [25] or a novel murine cell line designated EL08-1D2 [26,27].
Isolation of Natural killer cell (NK) precursors
PB and UCB mononuclear cells were depleted of T cells, and monocytes using the MACS magnetic bead selection system as recommended by the manufacturer (Miltenyi Biotech) using micro beads coated with antibodies to CD3 and CD14 as a cocktail. The depleted cells were stained with CD56 and CD3 APC, CD34 FITC and CD7 PE (all from BD Biosciences). Four populations were identified gated off CD56−/CD3− cells and sorted as CD34+/CD7−, CD34+/CD7+, CD34−/CD7+ or CD34−/CD7− cells as a negative control population.
Culture of hematopoietic progenitors
CD34+/Lin−/CD38− cells (single cell or 50–300 cells/well) were plated in 96 well plates (Costar, Cambridge, MA) or at 1000 cells per well in 24 well plates (Costar) pre-established in contact with or suspended above a collagen coated 0.4 μm pore size transwell (Costar) with AFT024 or EL08-1D2 stroma. Media consisted of a 2:1 (vol:vol) mix of Dulbecco modified Eagle medium (DMEM high glucose without sodium pyruvate)/Ham F12-based medium (Gibco Laboratories, Grand Island, NY) and supplemented with 24 μM 2-mercaptoethanol, 50 μM ethanalomine, 20 mg/L ascorbic acid, 50 μg/L sodium selenite (Na2SeO3), 100 U/mL penicillin, 100 U/mL streptomycin (Gibco) and 20% heat inactivated human AB serum (Valley Biomedical, Inc., Winchester, VA). Cytokines were supplemented at a concentration of 10 ng/mL IL-21, 10 ng/mL IL-15, 10 ng/mL Flt-3 Ligand (Flt-3L), 20 ng/mL IL-7, 20 ng/mL c-kit ligand (KL) (all from R&D Systems, Minneapolis, MN). All cytokines were added fresh with weekly media changes with the exception of IL-3 (R&D Systems) where 5 ng/mL was added once at culture initiation. Cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C.
Antibodies and determination of absolute cell counts
FITC, PE, peridinin chlorophyll protein (PerCP), and APC coupled control immunoglobulins or specific antibodies directed at CD56 (NCAM 16.2; BD Pharmingen, San Diego, CA), CD34 (8G12; BD Biosciences), CD7 (M-T701, BD Pharmingen), CD3 (SK7; BD Biosciences) CD94 (HP-3D9; BD Pharmingen), NKG2A (Z199; Beckman Coulter), CD158a (EB6; Beckman Coulter; or HP-3E4, BD Pharmingen), CD158b (GL183; Beckman Coulter or CH-L, BD Pharmingen), CD158e1 (DX9; BD Bioscience or BD Pharmingen) and CD158i (FES172: Beckman Coulter) were used to evaluate fresh PB or UCB cells and the progeny NK cells from differentiation cultures. The CD7+ population was further evaluated for the expression of CD25, CD62L, CD117, CD127, CD45RA, CD161, NKp44, NKp46 and CD244 (all from BD Biosciences or BD Pharmingen) as indicated. Murine IL-15 was measured by ELISA (R&D) and IL-15Rα was analyzed using a polyclonal goat antibody with an anti-goat secondary reagent (R&D). MuIL-15 mRNA was detected using published primers [28]. All phenotype acquisition and analyses were performed with a FACSCalibur and CELLQuest PRO software (Becton Dickinson).
Absolute cell numbers were determined by addition of a known number of polystyrene micro spheres (Polysciences, Warrington, PA) to each aliquot of cultured progeny. After gating out debris, absolute cell numbers were calculated, using the method described [29]. The absolute number of cells per well was calculated as [(total number of beads added/well)/(number of beads collected) multiplied by the number of cells in the phenotype gate of interest) and again by the number of times the initial sample was divided)].
Statistics
Results from experimental points obtained from multiple experiments were reported as mean ± 1 SEM. Significance levels were determined by two-sided Student t test analysis and/or Chi Square.
Results
Direct contact with the stromal microenvironment induces the development of NK cells from bone marrow progenitors [30]. Although the importance of IL-15 in this process is undisputed [19,20,22–24], it is unclear whether IL-15 1) acts on early NK cell precursors, 2) is only needed at the terminal steps of NK cell development or 3) is important role in maintaining lymphocyte proliferation and homeostasis. To investigate this process, we cultured human umbilical cord blood (UCB) CD34+Lin−CD38− primitive progenitors on AFT024. We previously demonstrated that AFT024 can support NK cell development and the acquisition of killer immunoglobulin-like receptors (KIR) on CD56+ NK cells when exogenous human cytokines [IL-15, IL-7, IL-3, c-kit ligand (KL), and Flt3-ligand (FL)] are present. We hypothesized that the absence of IL-15 would lead to the accumulation of incompletely-differentiated, progenitor-derived NK precursors at various stages of differentiation. CD56 acquisition was used as a marker of NK cell commitment. While IL-3 or FL alone did not support the development of NK cells, the combination induced 117±32 CD56+ NK cells from 10 starting primitive cells after 4 weeks of culture. These NK cells did not express KIR but CD94 and NKG2A expression could be detected (data not shown). KL significantly increased NK cell development in the absence of exogenous IL-15 but IL-21 had no significant effect. Although AFT024, IL-3 and FL provide the minimally required signals for NK cell differentiation, as expected the number of NK cell progeny was 1–2 log lower in the absence of IL-15 than when IL-15 was included in culture (Fig. 1).
Figure 1. NK cell development on AFT024, IL-3 and FL can occur in the absence of IL-15.
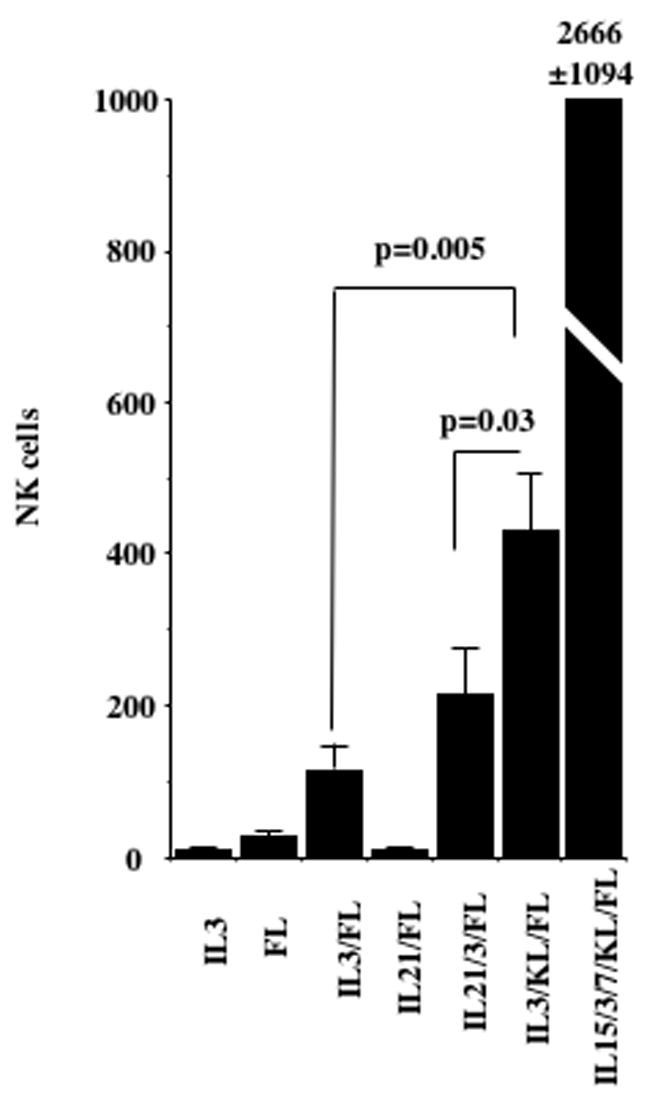
UCB CD34+/Lin−/CD38− cells (n=10) were cultured at 50 cells/well in direct contact with irradiated AFT024 and the indicated cytokines. On day 28, cells were harvested and analyzed by flow cytometry for the number of CD56+ NK cells. Results are normalized to 10 starting cells.
In the absence of IL-15, a number of CD56 negative NK cell precursors were defined by the expression of CD34 and CD7 (Fig. 2A). These same CD56 negative NK cell precursors were not present in IL-15 containing cultures, presumably because they are short lived under potent NK cell differentiation stimuli of IL-15. The differentiation of CD56 negative precursors (CD34+/CD7−, CD34+/CD7+ and CD34−/CD7+) from primitive CD34+Lin−CD38− progenitors was studied after culture with combinations of IL-3, FL, IL-21, and KL (Fig. 2B, C, D). After 28 days in culture on AFT024, IL-3 and FL consistently induced outgrowth of all three phenotypic NK cell precursors. Although IL-21 had no independent effect on NK cell commitment in the absence of IL-15 (Fig. 1), the addition of IL-21 to AFT024, IL-3 and FL only increased the CD34+/CD7+ cells (76±13 vs. 130±20, n=15; p <.01; Fig 2C). In contrast, addition of KL resulted in a net decrease of all three CD56 negative NK cell precursors.
Figure 2. CD56 negative NK cell precursors accumulate in the absence of IL-15.
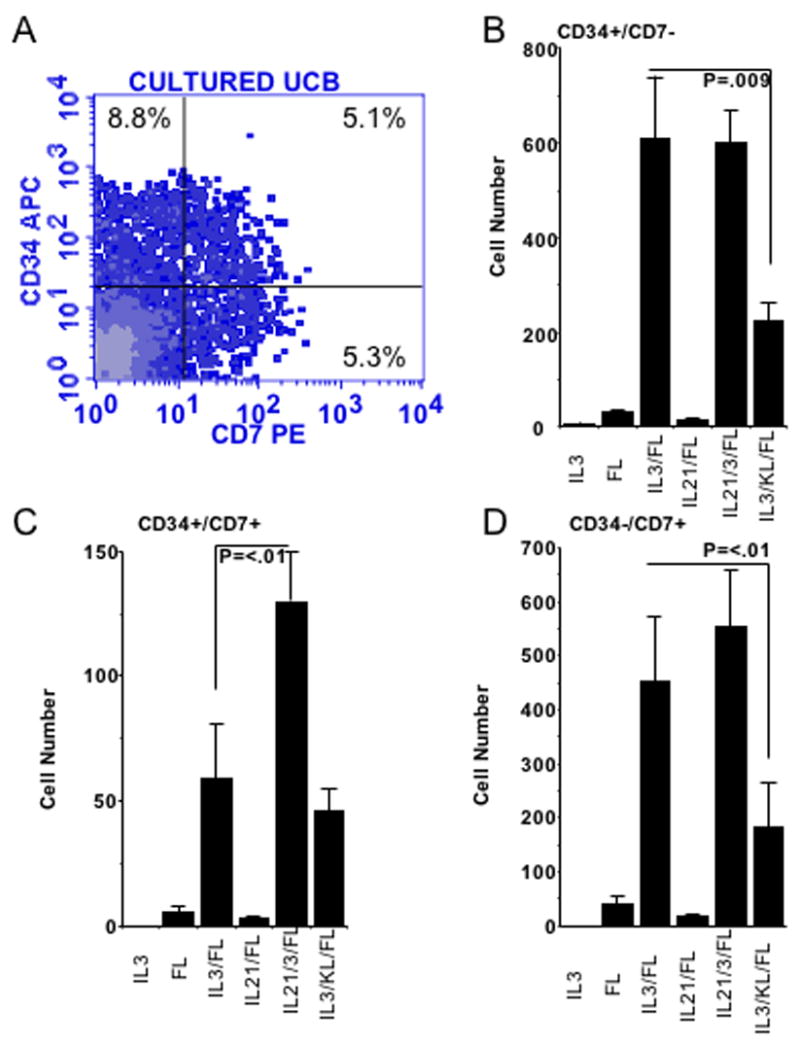
UCB CD34+/Lin−/CD38− cells (n=10) were cultured for 28 days on AFT024 and cytokines. A) Shown is a representative example of a culture supplemented with IL-3, IL-21 and Flt3L. Cultured progeny were gated on CD56 negative lymphocytes. The accumulation of (B) CD34+/CD7−, (C) CD34+/CD7+, and (D) CD34−/CD7+ precursors are shown for the indicated cytokines. Results are normalized to 10 starting cells.
CD56 negative CD34+/CD7−, CD34+/CD7+ and CD34−/CD7+ NK cell precursors were sorted to purity and plated in secondary cultures with fresh AFT024 feeders under NK cell differentiation conditions (IL-15, IL-7, IL-3, KL, and FL). A simultaneously collected control population with the CD34−/CD7− phenotype did not generate NK cells. In contrast, all other precursors gave rise to CD56+ NK cell progeny after secondary culture bearing NK cell receptors (CD94 or KIR) verifying their NK cell lineage (Fig 3). IL-15 alone was able to generate NK cells from CD34−/CD7+ cells while the more primitive CD34+ NK cell precursors required other cytokines (data not shown). This supports the premise that the CD34−/CD7+ precursor requires IL-15 for final NK cell commitment (defined by the acquisition of CD56).
Figure 3. CD56 negative NK precursors give rise to CD56+ NK cells in secondary culture.
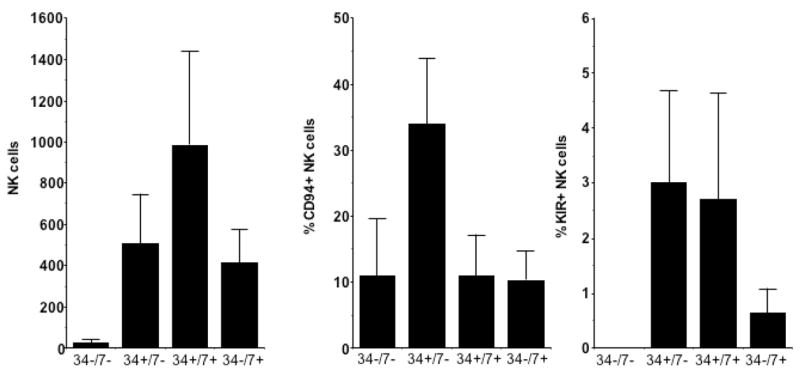
UCB CD34+/Lin−/CD38− cells (n=5) were cultured for 28 days on AFT024 supplemented with IL-3 and FL and then sorted into CD34+/CD7−, CD34+/CD7+, and CD34−/CD7+ NK cell precursors and plated into a secondary culture with cytokines as indicated for an additional 28 days. The absolute cell number of CD56+ NK cells and their ability to express KIR and CD94 is shown.
Having shown that NK cell precursors can differentiate from human hematopoietic stem cells, we wanted to exclude the possibility that these precursors were artifacts of in vitro culture. For these studies, we evaluated fresh UCB units that provide a source of developing hematopoietic cells. The developmental intermediates were highly enriched in UCB compared to adult peripheral blood from normal volunteers (all P values < 0.0018) for the CD34+/CD7− (31±4.8% vs. 1.9±0.7%), CD34+/CD7+ (3.7±0.8 vs. 0±0%) and CD34−/CD7+ (28±3.9% vs. 0.6±0.4%) population (Fig. 4). These fresh UCB NK cell precursors were studied for their capacity to give rise to CD56+ NK cells (Table 1). Fresh UCB CD56 negative NK cell precursors generated more NK cell progeny than did cultured precursors of the same phenotype. IL-15 alone was sufficient to induce further maturation of CD56 negative CD34−/CD7+ precursors to express CD56, CD94 and KIR.
Figure 4. CD56 negative NK cell precursors are markedly enriched in UCB.
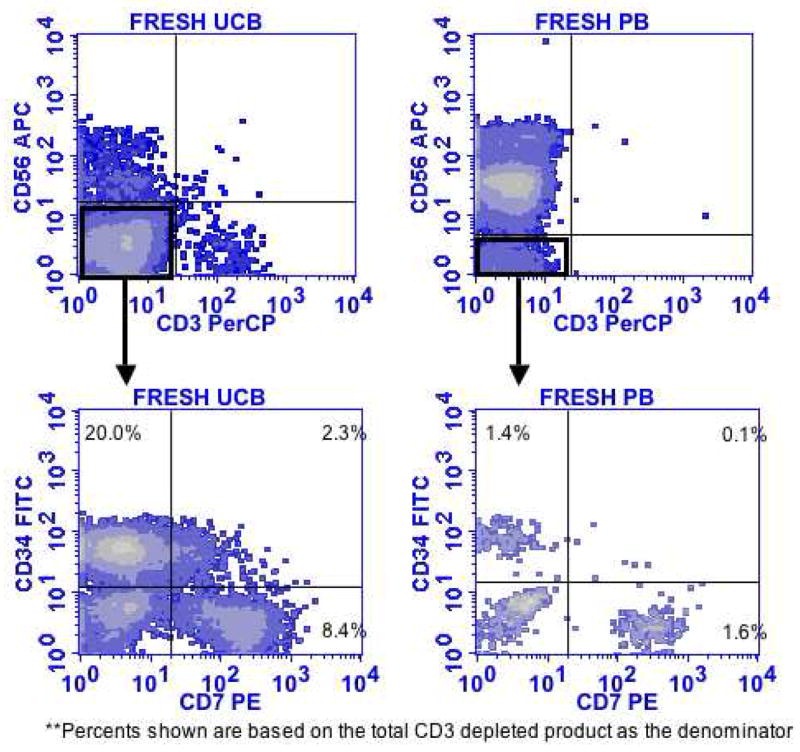
Fresh adult blood (n=10) and UCB (n=20) were depleted of CD3+ T cells and stained for CD56, CD3, CD34 and CD7. This representative phenotypic shows a 10-fold enrichment of CD56 negative NK precursors in UCB.
Table 1.
Fresh UCB NK precursors have different requirements for NK cell differentiation (multiple replicates from 3–6 separates experiments).
| Cytokine Condition | CD34+/CD7− Precursor | CD34+/CD7+ Precursor | CD34−/CD7+ Precursor |
|---|---|---|---|
| NK cell number from 10 starting cells of indicated phenotype | |||
| IL-15 | No cells | QNS | 560±281 |
| IL-15/FL | 97±28 | QNS | 1780±477 |
| IL-15/IL-3/FL | 2495±868 | QNS | 1336±342 |
| Compare IL15/FL±IL3 | P=0.04 | QNS | P=NS |
| IL-15/IL-3/IL-7/KL/FL | 4138±1396 | 2787±693 | 2879±1256 |
| Percent NK cells expressing KIR | |||
| IL-15 | No cells | QNS | 14±12 |
| IL-15/FL | 4.2±2.4 | QNS | 27±6.8 |
| IL-15/IL-3/FL | 14±4.3 | QNS | 19±5.4 |
| Compare IL15/FL±IL3 | P=0.08 | QNS | P=NS |
| IL-15/IL-3/IL-7/KL/FL | 7.8±2.5 | 1.8±0.8 | 13±3.9 |
| Percent NK cells expressing CD94 | |||
| IL-15 | No cells | QNS | 16±9.0 |
| IL-15/FL | 30±13 | QNS | 43±9.6 |
| IL-15/IL-3/FL | 71±5.3 | QNS | 51±5.9 |
| Compare IL15/FL±IL3 | P=0.02 | QNS | P=NS |
| IL-15/IL-3/IL-7/KL/FL | 64±6.0 | 32±6.3 | 43±7.5 |
IL, interleukin, FL, Flt-3 ligand, KL, c-kit ligand, NS, not significant QNS, quantity not insufficient for analysis
CD56− NK cell precursors from fresh UCB were further evaluated for receptors found on mature NK cells. CD16, CD161, CD94, CD159 (NKG2A), CD244 (2B4), NKp44, NKp46 and KIR were not expressed on CD34+ positive cells (data not shown). In contrast, CD34−/CD56−/CD7+ cells variable expressed these NK cell receptors except NKp44 and NKp46, which were not expressed. We found that CD16 (FcRγIII) was expressed on 30±5% of CD34−/CD56−/CD7+ cells and was a good marker to divide the CD34−/CD56−/CD7+ population into cells with distinct phenotypic characteristics (Table 2). CD34−/CD56−/CD7+/CD16− cells expressed significantly more CD25, CD62L, CD117 and CD127, markers of immaturity. CD34−/CD56−/CD7+/CD16+ cells were more mature based on their higher relative expression of CD45RA, CD161, CD94, CD159 (NKG2A), CD244 and KIR. These results suggest that the CD34−/CD56−/CD7+ population is heterogeneous and CD16+/CD56− cells may represent a unique stage of NK cell development where NK cell receptor acquisition has already been initiated.
Table 2.
CD7+/CD56− NK cell precursors can be further divided by co-expression of FcRγIII (CD16) (n=8).
| CD7+/CD34−/CD56−/CD3− Cells | |||
|---|---|---|---|
| Surface Expression | Gated on CD16− | Gated on CD16+ | P Value |
| CD25 | 15±3.9 | 0.5±0.5 | <0.01 |
| CD62L | 9.3±1.8 | 2.5±1.0 | <0.01 |
| CD117 | 21±4.7 | 8.4±2.7 | 0.049 |
| CD127 | 25±4.7 | 3.6±1.8 | <0.01 |
| CD45RA | 77±6.4 | 92±4.0 | <0.01 |
| CD161 | 15±2.7 | 24±6.7 | <0.01 |
| CD94 | 25±4.8 | 48±4.7 | .01 |
| NKG2A | 22±3.2 | 38±3.0 | <0.01 |
| CD244 | 9.9±2.1 | 38±6.0 | <0.01 |
| KIR* | 13±2.3 | 43±6.5 | <0.01 |
Cocktail including CD158a, CD158b, CD158i
Our group has previously shown IL-15 (or IL-2) signals are required for KIR acquisition. However, we had not tested the potential role of other factors. On fresh UCB CD56− NK cell precursors we tested the role of FL and IL-3 on NK cell receptor expression during development. IL-3 had a significant additive effect on the CD34+/CD7− but not CD34−/CD7+ cells to increase acquisition of KIR and NKG2A (Table 1) suggesting that the effects of IL-3 are on early development. This identifies a novel role for IL-3 and FL as early acting factors that can influence NK cell receptor expression.
Having identified a system to recapitulate early stages of NK cell development, we evaluated a novel murine cell line, designated EL08-1D2, for its capacity to support NK cell development and KIR acquisition. The use of KIR expression in the definition of NK cell maturation has distinguished our stromal-based cultures from those described by others where KIR expression is low or absent under stroma-free conditions [31]. Under NK cell differentiation conditions (IL-15, IL-7, IL-3, KL, and FL), EL08-1D2 supported NK cell development earlier and more effectively than did AFT024 (Fig. 5). The supportive capacity of EL08-1D2, like AFT024, was most efficient when progenitors were in direct contact with the murine feeder compared to separation by a microporous membrane.
Figure 5. EL08-1D2 is superior to AFT024 to support NK cell differentiation.
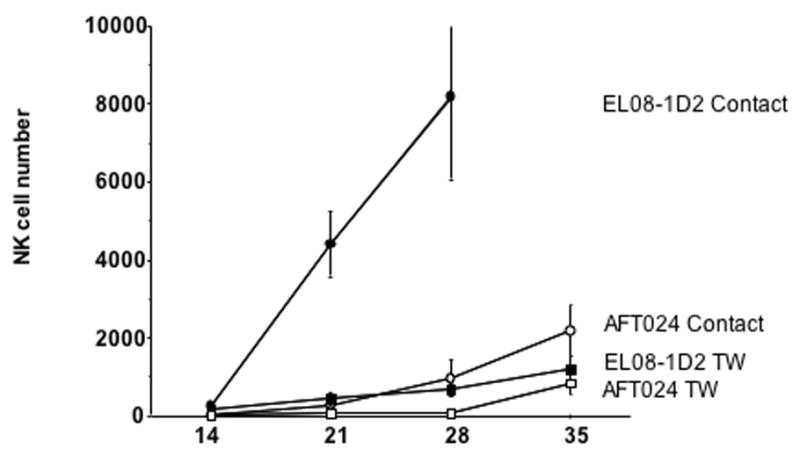
CD34+/Lin−/CD38− cells were cultured on AFT024 (n=4) or EL08-1D2 (n=4) separated from stroma by a Transwell (TW) membrane prohibiting cell-cell contact. NK cell differentiation (measured by the CD56 expressing cells) from 10 starting cells was followed over time after culture with IL-15, IL-3, IL-7, KL, FL. For CD34+/Lin−/CD38− cells in contact with EL08-1D2, counts are not shown beyond day 28 as cell numbers decreased. EL08-1D2 supported NK cell differentiation better than all other conditions tested (P=<0.03 for all points at days 21 and 28).
To definitively understand the differential roles played by the two murine feeders, single CD34+Lin−CD38− cells were cultured on EL08-1D2 and compared to cells cultured on AFT024. The absolute number of NK cells derived from a single primitive progenitor was significantly greater on EL08-1D2 compared to AFT024 after 28 days of culture (123,852±14006 vs. 23143±8117, p=<.0001). Of the 66 EL08-1D2 and 46 AFT024 clones analyzed, 86% of EL08-1D2 cultured cells expressed KIR in a polyclonal pattern compared to 45% on AFT024 (p=<.0001 using Chi Square). The absolute number of KIR+ NK cell progeny from a single primitive progenitor on EL08-1D2 feeders was 3689±801 versus 799±491 when on AFT024 (p=.0027).
The increased NK cell differentiation seen with EL08-1D2 can be explained by its increased ability to support progenitor differentiation, to support proliferation or by a combination of both. This question was addressed by evaluating the NK cell precursors that accumulate in the absence of IL-15. All CD56 negative NK cell precursors are significantly increased in EL08-1D2 supported cultures compared to AFT024 (Fig. 6) suggesting that EL08-1D2 exerts its effect on all stages of NK cell development.
Figure 6. EL08-1D2 better supports NK cell precursor differentiation.
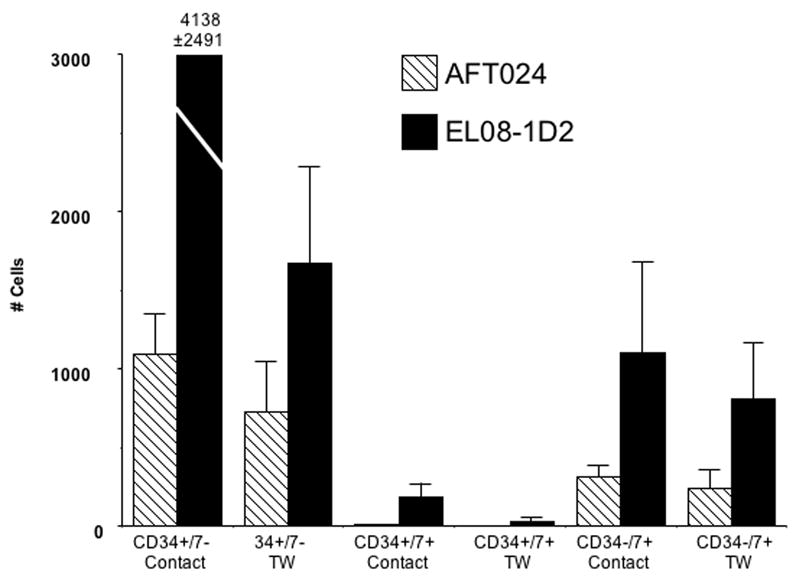
CD34+/Lin−/CD38− cells were cultured on AFT024 (n=7) or EL08-1D2 (n=7) for 28 days. The importance of cell-cell contact was determined by culture in direct contact or separated from stroma by a Transwell (TW) membrane prohibiting cell-cell contact. CD56 negative NK cell precursor differentiation from 10 starting cells is reported after culture with IL-3 and Flt3L.
Even though exogenous huIL-15 was not added to cultures, we questioned whether small amounts of muIL-15 could be produced by mouse stroma, could cross-reactive with human cells and whether the signal could be amplified by trans-presentation with IL-15Rα on the stromal cell surface. Although low level IL-15 transcripts were found on both feeders (data not shown), neither EL08-1D2 nor AFT024 made enough muIL-15 to be detected by ELISA in supernatants collected after 2, 7, or 14 days (data not shown). Flow cytometry showed the absence of muIL-15Rα on AFT024 and a low level of expression on EL08-1D2 (data not shown). To understand the functional significance of these results, we performed additional experiments using a bioassay readout. Purified NK cells, exquisitely sensitive to IL-15, were plated on AFT024 or EL08-1D2. After 14 days of culture in the absence of exogenous huIL-15, no NK cell recovery was seen. Therefore, even if assays for muIL-15 and muIL-15Rα missed some low level of detection, the aggregate functional IL-15 signaling is not enough to support mature human NK survival and likely does not play a significant role in the developmental assays used here.
Discussion
Using a two-step culture, we have been able to separate early and late events in NK cell development. Early acting cytokines IL-3 and FL and a mouse stromal feeder induced progenitor differentiation into CD34+/CD7−, CD34+/CD7+, and CD34−/CD7+ NK cell precursors in the absence of IL-15. These intermediates are not merely a result of culture artifact since similar cells were found in fresh UCB, a site of developing hematopoiesis. This study agrees with our earlier work [30] and work published by others suggesting that heterogeneous CD34+ populations can be divided into a number of lymphoid precursors based on the expression of CD45RA, CD161, CD117 (c-kit) and CD127 (the IL7 receptor) [32–34]. Testing these markers with the expression of NK cell receptors was especially informative to further characterize the CD34−/CD56−/CD7+ population. The presence or absence of CD16 resulted in unique populations of NK cells at different developmental stages. The CD16− population was more immature with characteristics of lymphoid precursors (higher CD117, CD127, CD62L and CD25). The CD16+ population was different and expressed significantly more NK cell receptors including KIR and NKG2A but no expression of natural cytotoxicity receptors NKp44 and NKp46.
Finding a KIR expressing CD56−/CD16+ population is unique and may define an important developmental checkpoint in the path by which NK cells become educated. It also suggests that CD16 may precede CD56 expression in some circumstances. These conclusions are supported by the finding of hypofunctional NK cells in UCB that become CD56+/CD16+ after stimulation with IL-15 [35]. In human disease, HIV infection also seems to uniquely expand a population of dysfunctional CD56−/CD16+ NK cells in vivo [36]. The receptor repertoire of NK cells found in HIV infected viremic individuals is similar to what we describe here. Specifically, CD56−/CD16+ NK cells expressed KIR, NKG2A and CD244 but no NKp46. It is possible that the CD56−/CD16+ cells are at a developmental stage ready to be “licensed”, the recently described process where lytic function is acquired on NK cells expressing “self” inhibitory receptors [37,38].
We show that IL-15 is required for NK cell commitment but not early stages of NK cell development. Early CD34+ progenitors are dependent on stroma, IL-3 and FL for the development of NK cell precursors but are unable to efficiently move forward without huIL-15. In contrast, after loss of the CD34 progenitor marker, IL-15 alone is capably of pushing CD34−/CD7+ cells into CD56+ NK cells in the absence of IL-3 and FL. FL in combination IL-15 can induce NK cell commitment from CD34+/CD7− precursors but IL-3 plays an important role in KIR and NKG2A acquisition suggesting that transcription factors induced by this combination are important in determining the transcriptional regulation of the developing KIR repertoire. Recent studies in the mouse support that these fate decisions are made at early developmental stages [39,40].
The finding of NK cell commitment in the absence of exogenous IL-15 suggests that IL-15 may exert its dominant effects after NK cell commitment (e.g. maturation and homeostatic expansion). The IL-15 independent effects need to be interpreted carefully. It is now well established that IL-15 signaling may be amplified by interaction with membrane bound or soluble IL-15Rα resulting in a “hyper IL-15” signal [41–45]. We questioned whether murine IL-15 was produced by the stroma itself, which could interact with IL-15Rα to account for our findings. This possibility is unlikely because of the absence of muIL-15 protein produced by AFT024 and EL08-1D2, the absence of IL-15Rα on the AFT024 feeder and inability of either feeder to permit survival and expansion of mature NK cells. In future studies, we have engineered EL08-1D2 with human IL-15Rα to better understand the role of IL-15 trans-presentation on different stages of human NK cell development.
How and where NK cells differentiate has been of great interest especially given the recent excitement that they play a role in allogeneic transplant outcomes. Although NK cells derive from marrow derived CD34+ cells [6,30], recent data support a role for differentiation in peripheral lymph nodes [46,47]. Although we and others have used the mouse feeder AFT024 to induce NK cell differentiation from CD34+ progenitors [25,48], here we show that EL08-1D2, another mouse microenvironment, is significantly better at recapitulating NK cell development. Differences between these feeders may provide important information on factors important in NK cells development.
The difference between the two mouse feeders may be related to the developmental stage from which they were derived. During embryonic development, definitive hematopoiesis is first established in the aorta-gonads-mesonephros (AGM) region at around day 10.5 after gestation [49]. Slightly later, at around E11 the embryonic liver is colonized with hematopoietic cells and the liver develops into the major hematopoietic tissue during embryogenesis. The stromal line EL08-1D2 was cloned from a culture of livers from murine embryos at E11 [26,27], whereas the AFT024 line is derived from fetal livers of a later timepoint of gestation [50]. EL08-1D2 shows characteristics in common with vascular smooth muscle cells [51] as well as osteoblastic cells [52], which are suspected to be the main stromal cell type involved in HSC regulation in the bone marrow [53]. The EL08-1D2 cell line was selected to support maintenance of hematopoietic stem cells (HSC) [26]. In particular, EL08-1D2 maintained HSC without the requirement of direct stroma-stem cell contact in TW cultures [52]. Interestingly, in a study of over twenty different embryo-derived cell lines, EL08-1D2 uniquely supported the prolonged production of human hematopoietic progenitors from CD34+ UCB cells without the addition of any cytokines [54]. This finding and the present study indicate that EL08-1D2 produces factors involved in hematopoiesis and NK cell differentiation which act across species barriers. Further study is required to precisely identify these factors.
In summary, the finding of CD34+/CD7−, CD34+/CD7+, and CD34−/CD7+ (± CD16) NK cell precursors in UCB is intriguing and may be important for our clinical studies using allogeneic NK cells. We have recently shown that haploidentical adult donor NK cells are capable of expanding in vivo [55]. The finding that only those patients with circulating NK cells 14–28 days after cell infusions achieved a clinical complete remission supports the importance of the NK cell infusion. However, 80% of patients did not expand NK cells or respond clinically. We hypothesize that in vivo expansion of appropriately alloreactive NK cells will correlate with clinical responses. Since CD34+ progenitors can differentiate into significantly more NK cells per single cell than either adult CD56bright or CD56dim NK cells, infusion of UCB containing a spectrum of NK precursors (from stem cell to committed NK cells) at a much greater frequency than adult blood may provide a cell source capable of supporting better in vivo expansion. These clinical trials are underway.
Acknowledgments
This work was supported in part by National Institutes of Health Grant P01-CA-65493 (JSM, JEM), R01-HL-55417 (JSM) and R01-AI-50656 (JSM, CTL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leibson PJ. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6:655–661. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 2.Leibson P. Viewpoint: signal transduction during natural killer cell activation. Nat Immun. 1995;14:117–122. [PubMed] [Google Scholar]
- 3.Lanier LL, Corliss B, Phillips JH. Arousal and inhibition of human NK cells. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 4.Raulet DH, Held W. Natural killer cell receptors: the offs and ons of NK cell recognition. Cell. 1995;82:697–700. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 5.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–2229. [PubMed] [Google Scholar]
- 6.Miller JS, Verfaillie C, McGlave P. The generation of human natural killer cells from CD34+/DR- primitive progenitors in long-term bone marrow culture. Blood. 1992;80:2182–2187. [PubMed] [Google Scholar]
- 7.Silva MR, Hoffman R, Srour EF, Ascensao JL. Generation of human natural killer cells from immature progenitors does not require marrow stromal cells. Blood. 1994;84:841–846. [PubMed] [Google Scholar]
- 8.Lotzova E, Savary CA, Champlin RE. Genesis of human oncolytic natural killer cells from primitive CD34+CD33− bone marrow progenitors. J Immunol. 1993;150:5263–5269. [PubMed] [Google Scholar]
- 9.Srour EF, Zanjani ED, Cornetta K, Traycoff CM, Flake AW, Hedrick M, Brandt JE, Leemhuis T, Hoffman R. Persistence of human multilineage, self-renewing lymphohematopoietic stem cells in chimeric sheep. Blood. 1993;82:3333–3342. [PubMed] [Google Scholar]
- 10.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–3191. [PubMed] [Google Scholar]
- 11.Nagler A, Lanier LL, Phillips JH. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990;171:1527–1533. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierson BA, Gupta K, Hu WS, Miller JS. Human natural killer cell expansion is regulated by thrombospondin-mediated activation of transforming growth factor-beta 1 and independent accessory cell-derived contact and soluble factors. Blood. 1996;87:180–189. [PubMed] [Google Scholar]
- 13.Pierson BA, Miller JS. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood. 1996;88:2279–2287. [PubMed] [Google Scholar]
- 14.Matos ME, Schnier GS, Beecher MS, Ashman LK, William DE, Caligiuri MA. Expression of a functional c-kit receptor on a subset of natural killer cells. J Exp Med. 1993;178:1079–1084. doi: 10.1084/jem.178.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson WE, Haldar S, Baiocchi RA, Croce CM, Caligiuri MA. The c-kit ligand suppresses apoptosis of human natural killer cells through the upregulation of bcl-2. Proc Natl Acad Sci U S A. 1994;91:7553–7557. doi: 10.1073/pnas.91.16.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurley RW, McCarthy JB, Verfaillie CM. Direct adhesion to bone marrow stroma via fibronectin receptors inhibits hematopoietic progenitor proliferation. J Clin Invest. 1995;96:511–519. doi: 10.1172/JCI118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verfaillie CM. Direct contact between human primitive hematopoietic progenitors and bone marrow stroma is not required for long-term in vitro hematopoiesis. Blood. 1992;79:2821–2826. [PubMed] [Google Scholar]
- 18.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, Waldmann TA, Taniguchi T, Taki S. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 21.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- 22.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, Croce CM, Baumann H, Caligiuri MA. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 24.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JS, McCullar V, Punzel M, Lemischka IR, Moore KA. Single adult human CD34(+)/Lin−/CD38(−) progenitors give rise to natural killer cells, B-lineage cells, dendritic cells, and myeloid cells. Blood. 1999;93:96–106. [PubMed] [Google Scholar]
- 26.Oostendorp RA, Harvey KN, Kusadasi N, de Bruijn MF, Saris C, Ploemacher RE, Medvinsky AL, Dzierzak EA. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood. 2002;99:1183–1189. doi: 10.1182/blood.v99.4.1183. [DOI] [PubMed] [Google Scholar]
- 27.Oostendorp RA, Medvinsky AJ, Kusadasi N, Nakayama N, Harvey K, Orelio C, Ottersbach K, Covey T, Ploemacher RE, Saris C, Dzierzak E. Embryonal subregion-derived stromal cell lines from novel temperature-sensitive SV40 T antigen transgenic mice support hematopoiesis. J Cell Sci. 2002;115:2099–2108. doi: 10.1242/jcs.115.10.2099. [DOI] [PubMed] [Google Scholar]
- 28.Gravisaco MJ, Mongini C, Alvarez E, Ruybal P, Escalada A, Sanchez-Lockhart M, Hajos S, Waldner C. IL-2, IL-10, IL-15 and TNF are key regulators of murine T-cell lymphoma growth. Int J Mol Med. 2003;12:627–632. [PubMed] [Google Scholar]
- 29.Pribyl JR, Dittel BN, LeBien TW. In vitro studies of human B lymphopoiesis. Ann N Y Acad Sci. 1995;764:9–18. doi: 10.1111/j.1749-6632.1995.tb55799.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83:2594–2601. [PubMed] [Google Scholar]
- 31.Yu H, Fehniger TA, Fuchshuber P, Thiel KS, Vivier E, Carson WE, Caligiuri MA. Flt3 ligand promotes the generation of a distinct CD34(+) human natural killer cell progenitor that responds to interleukin-15. Blood. 1998;92:3647–3657. [PubMed] [Google Scholar]
- 32.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 33.Kouro T, Medina KL, Oritani K, Kincade PW. Characteristics of early murine B-lymphocyte precursors and their direct sensitivity to negative regulators. Blood. 2001;97:2708–2715. doi: 10.1182/blood.v97.9.2708. [DOI] [PubMed] [Google Scholar]
- 34.Loza MJ, Perussia B. Final steps of natural killer cell maturation: a model for type 1-type 2 differentiation? Nat Immunol. 2001;2:917–924. doi: 10.1038/ni1001-917. [DOI] [PubMed] [Google Scholar]
- 35.Gaddy J, Broxmeyer HE. Cord blood CD16+56- cells with low lytic activity are possible precursors of mature natural killer cells. Cell Immunol. 1997;180:132–142. doi: 10.1006/cimm.1997.1175. [DOI] [PubMed] [Google Scholar]
- 36.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, O’Shea MA, Kinter A, Kovacs C, Moretta A, Fauci AS. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 39.Saleh A, Davies GE, Pascal V, Wright PW, Hodge DL, Cho EH, Lockett SJ, Abshari M, Anderson SK. Identification of probabilistic transcriptional switches in the Ly49 gene cluster: a eukaryotic mechanism for selective gene activation. Immunity. 2004;21:55–66. doi: 10.1016/j.immuni.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Anderson SK. Transcriptional regulation of NK cell receptors. Curr Top Microbiol Immunol. 2006;298:59–75. doi: 10.1007/3-540-27743-9_3. [DOI] [PubMed] [Google Scholar]
- 41.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 42.Giron-Michel J, Giuliani M, Fogli M, Brouty-Boye D, Ferrini S, Baychelier F, Eid P, Lebousse-Kerdiles C, Durali D, Biassoni R, Charpentier B, Vasquez A, Chouaib S, Caignard A, Moretta L, Azzarone B. Membrane-bound and soluble IL-15/IL-15Ralpha complexes display differential signaling and functions on human hematopoietic progenitors. Blood. 2005;106:2302–2310. doi: 10.1182/blood-2005-01-0064. [DOI] [PubMed] [Google Scholar]
- 43.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, Hughes TL, Marburger TB, Sung J, Baiocchi RA, Guimond M, Caligiuri MA. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JS, McCullar V. Human natural killer cells with polyclonal lectin and immunoglobulinlike receptors develop from single hematopoietic stem cells with preferential expression of NKG2A and KIR2DL2/L3/S2. Blood. 2001;98:705–713. doi: 10.1182/blood.v98.3.705. [DOI] [PubMed] [Google Scholar]
- 49.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 50.Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–4347. [PubMed] [Google Scholar]
- 51.Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20:205–214. doi: 10.1634/stemcells.20-3-205. [DOI] [PubMed] [Google Scholar]
- 52.Oostendorp RA, Robin C, Steinhoff C, Marz S, Brauer R, Nuber UA, Dzierzak EA, Peschel C. Long-term maintenance of hematopoietic stem cells does not require contact with embryo-derived stromal cells in cocultures. Stem Cells. 2005;23:842–851. doi: 10.1634/stemcells.2004-0120. [DOI] [PubMed] [Google Scholar]
- 53.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 54.Kusadasi N, Oostendorp RA, Koevoet WJ, Dzierzak EA, Ploemacher RE. Stromal cells from murine embryonic aorta-gonad-mesonephros region, liver and gut mesentery expand human umbilical cord blood-derived CAFC(week6) in extended long-term cultures. Leukemia. 2002;16:1782–1790. doi: 10.1038/sj.leu.2402615. [DOI] [PubMed] [Google Scholar]
- 55.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]


