Abstract
BACKGROUND
For patients who have a ventricular tachyarrhythmic event, implantable cardioverter–defibrillators (ICDs) are a mainstay of therapy to prevent sudden death. However, ICD shocks are painful, can result in clinical depression, and do not offer complete protection against death from arrhythmia. We designed this randomized trial to examine whether prophylactic radiofrequency catheter ablation of arrhythmogenic ventricular tissue would reduce the incidence of ICD therapy.
METHODS
Eligible patients with a history of a myocardial infarction underwent defibrillator implantation for spontaneous ventricular tachycardia or fibrillation. The patients did not receive antiarrhythmic drugs. Patients were randomly assigned to defibrillator implantation alone or defibrillator implantation with adjunctive catheter ablation (64 patients in each group). Ablation was performed with the use of a substrate-based approach in which the myocardial scar is mapped and ablated while the heart remains predominantly in sinus rhythm. The primary end point was survival free from any appropriate ICD therapy.
RESULTS
The mortality rate 30 days after ablation was zero, and there were no significant changes in ventricular function or functional class during the mean (±SD) follow-up period of 22.5±5.5 months. Twenty-one patients assigned to defibrillator implantation alone (33%) and eight patients assigned to defibrillator implantation plus ablation (12%) received appropriate ICD therapy (antitachycardia pacing or shocks) (hazard ratio in the ablation group, 0.35; 95% confidence interval, 0.15 to 0.78, P = 0.007). Among these patients, 20 in the control group (31%) and 6 in the ablation group (9%) received shocks (P = 0.003). Mortality was not increased in the group assigned to ablation as compared with the control group (9% vs. 17%, P = 0.29).
CONCLUSIONS
In this randomized trial, prophylactic substrate-based catheter ablation reduced the incidence of ICD therapy in patients with a history of myocardial infarction who received ICDs for the secondary prevention of sudden death.
(Current Controlled Trials number, ISRCTN62488166.)
Patients with a history of myocardial infarction who survive a spontaneous episode of ventricular arrhythmia are at high risk for subsequent sudden death from recurrent ventricular tachycardia or ventricular fibrillation. Implantable cardioverter–defibrillators (ICDs) decrease mortality and have therefore become the mainstay of treatment of these patients.1
However, ICDs are not a cure for ventricular arrhythmias. Defibrillator discharges (shocks) for treatment of recurrent arrhythmias are painful, and syncope may occur before delivery of therapy. Clinically significant anxiety and depression as a result of recurrent ICD shocks may occur in more than 50% of patients.2–4 Repeated ICD shocks within a short time interval, known as an ICD “storm,” occur in 10 to 25% of patients.5,6 Furthermore, ICDs do not provide absolute protection against death due to arrhythmia; the rate of sudden death among patients who do not have a response to ICDs is approximately 5%.7
Since no device is likely to be fully protective against ventricular tachycardia or fibrillation, it would be clinically valuable to adopt an approach that reduced the absolute incidence of ventricular tachycardia or fibrillation so that the ICD served solely as a backup device.8 One option is catheter ablation of the ventricular-tachycardia circuit as it traverses the scarred myocardial tissue. However, ablation in patients with ventricular tachycardia has been used infrequently in the past, except in patients with recurrent ventricular tachycardia that was resistant to drug therapy who received multiple ICD shocks. This is predominantly because more than 90% of ventricular-tachycardia episodes are associated with hemodynamic instability, rendering mapping of the ventricular-tachycardia circuit during the arrhythmia difficult or impossible.9
With the advent of advanced cardiac-mapping systems, substrate-based catheter ablation has emerged as a useful alternative to more conventional ablation methods.10–13 In the substrate-based approach, arrhythmogenic tissue is identified during sinus rhythm or pacing on the basis of its distinguishing characteristics on the electrogram, thus obviating the need for mapping during an episode of ventricular tachycardia.14,15 Indeed, initial studies revealed that surgical ablation with the use of the substrate-based approach successfully controlled both ventricular tachycardia and ventricular fibrillation.16,17 The Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia (SMASH-VT) study was initiated to examine the hypothesis that prophylactic catheter ablation (ablation after defibrillator implantation to prevent the occurrence of future shocks) can safely decrease the likelihood of subsequent ICD therapy in patients with myocardial infarction who receive an ICD after surviving a life-threatening ventricular arrhythmic event.
METHODS
STUDY DESIGN
The SMASH-VT study was a prospective, unblinded, randomized, controlled, multicenter trial (ISRCTN62488166). Institutional review board approval was obtained separately at each of the three participating centers. The trial began in August 2000, and the last patient was enrolled in June 2004; follow-up was completed in June 2006.
PATIENT RECRUITMENT
Men and women who were at least 18 years old were eligible for the study if they had a history of myocardial infarction more than 1 month before enrollment (as documented by an electrocardiogram or cardiac imaging) and if they had undergone a planned or a recent (within 6 months) implantation of a defibrillator for ventricular fibrillation, hemodynamically unstable ventricular tachycardia, or syncope with inducible ventricular tachycardia during invasive electrophysiological testing (in the latter group, syncope was assumed to be the qualifying spontaneous arrhythmic event). In an effort to foster enrollment, a further qualifying criterion was implemented after the study had commenced. This criterion allowed the enrollment of patients who had received an ICD for primary prophylaxis and subsequently had received appropriate ICD therapy for a single event. In this instance, the single ICD event was viewed as the first spontaneous arrhythmic event.
Patients were excluded if they were being treated with a class I or class III antiarrhythmic drug, if the substrate for the ventricular arrhythmia was thought not to be due to the myocardial infarction, if active and ongoing cardiac ischemia was thought to be the cause of the ventricular arrhythmia, or if they were having incessant or multiple episodes of ventricular tachycardia necessitating immediate treatment (with drugs or ablation). Additional exclusion criteria included the inability to give informed consent, stroke within 30 days before screening, contraindication to anticoagulation therapy, or any medical or non-medical condition likely to prevent completion of the trial.
STUDY PROCEDURES
Patients meeting the entry criteria who gave written informed consent were randomly assigned at a 1:1 ratio to either the control group or the ablation group with the use of sealed, prenumbered envelopes. Patients assigned to the control group received no further trial therapy. Patients assigned to the ablation group underwent catheter ablation of the arrhythmogenic substrate while the heart was in sinus rhythm (see the Supplementary Appendix, available with the full text of this article at www.nejm.org). The ablation procedure was performed either before or after ICD programming, which was standardized to ensure at least one ventricular-tachycardia zone in which antitachycardia pacing could occur. A transthoracic echocardiogram was obtained for all patients at baseline, and a second echocardiogram was obtained for those in the ablation group after the procedure had been completed. Nearly all patients received therapy with aspirin, beta-blockers, and angiotensin-converting–enzyme (ACE) inhibitors or angiotensin-receptor blockers.
FOLLOW-UP
Most patients were discharged home with a prescription for 4 to 6 weeks of oral anticoagulant therapy. Typically, this consisted of warfarin, but if a minimal number of ablation lesions had been placed (i.e., fewer than five), aspirin alone was given. Patients were followed in the ICD clinic at 3, 6, 9, 12, 18, and 24 months. Information was obtained from the ICD memory about the number of events, the number and type of treatments required, and cycle lengths during episodes of ventricular tachycardia. Additional clinical information obtained included New York Heart Association (NYHA) class, medication changes, changes in medical status, and transthoracic echocardiography at 3 and 12 months.
END POINTS
The primary end point of the study was survival free from any appropriate ICD therapy (shocks or antitachycardia pacing). The secondary end points included freedom from any appropriate ICD shock, death, and ICD storm. An ICD storm was defined as at least three shocks within a 24-hour period.
STATISTICAL ANALYSIS
On the basis of data from the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial, we projected that 50% of the patients in the control group would have a primary end-point event within 2 years after the time of enrollment (on the assumption of a patient population of whom 50% had ventricular fibrillation and 50% had hemodynamically unstable ventricular tachycardia).18 Considering the primary end point as a binary outcome rather than using a time-to-event calculation, we projected that there would be a 50% reduction in events in the ablation group. On the assumption of equal numbers of patients in each group, a sample size of 130 patients was required to achieve 80% power to detect a significant difference with a two-sided alpha error of 0.05.
An intention-to-treat analysis was performed that included all patients in their assigned trial groups, whether or not they actually underwent radiofrequency ablation. Continuous variables were compared by two-sample t-tests and categorical variables by Fisher’s exact test. Time-to-event curves describing the event-free survival of patients during the 24-month follow-up period were calculated by the Kaplan–Meier method and compared with the use of the log-rank test. Cox proportional-hazards regression models were used to estimate hazard ratios and 95% confidence intervals for the primary and secondary end points and to perform an analysis of the primary end point adjusted for baseline variables. All tests were two-tailed, and a P value less than 0.05 was considered to indicate statistical significance.
RESULTS
STUDY POPULATION
Over the course of 46 months, 128 patients were enrolled and randomly assigned to the ablation group or the control group (64 in each group) (Table 1). The mean age of the patients was 67 years, and 87% were male. The qualifying index arrhythmia was ventricular fibrillation in 18% of patients, ventricular tachycardia in 49%, syncope with inducible ventricular tachycardia in 21%, and recent ventricular fibrillation or tachycardia treated by a previously implanted ICD in 12%. Systematic information about patients who were screened but not enrolled was not collected.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Ablation Group (N = 64) | Control Group (N = 64) | P Value |
|---|---|---|---|
| Age — yr | 67±9 | 66±10 | 0.65† |
| Male sex — no. (%) | 59 (92) | 52 (81) | 0.12‡ |
| Interval between myocardial infarction and enrollment — yr§ | 8.8±8.5 | 7.9±7.8 | 0.66¶ |
| Index arrhythmia — no. (%) | 0.38‡ | ||
| Ventricular fibrillation | 13 (20) | 10 (16) | |
| Ventricular tachycardia | 30 (47) | 33 (52) | |
| Syncope with inducible ventricular tachycardia | 11 (17) | 16 (25) | |
| Recent ventricular fibrillation or tachycardia treated by a previously implanted ICD | 10 (16) | 5 (8) | |
| Left ventricular ejection fraction — % | 30.7±9.5 | 32.9±8.5 | 0.16† |
| Left ventricular ejection fraction ≤30% — no. (%) | 37 (58) | 30 (47) | 0.29‡ |
| Left ventricular ejection fraction ≤20% — no. (%) | 16 (25) | 7 (11) | 0.06‡ |
| New York Heart Association functional class — no. (%) | 0.37‡ | ||
| I or II | 54 (84) | 49 (77) | |
| III or IV | 10 (16) | 15 (23) | |
| Hypertension — no. (%) | 47 (73) | 43 (67) | 0.35‡ |
| Diabetes — no. (%) | 24 (38) | 32 (50) | 0.21‡ |
| Previous revascularization (PTCA or CABG) — no. (%) | 46 (72) | 40 (62) | 0.35‡ |
| Previous stroke — no. (%) | 3 (5) | 8 (12) | 0.21‡ |
| Medication — no. (%) | |||
| Class I or class III drugs | 0 | 0 | — |
| Beta-blockers | 60 (94) | 63 (98) | 0.37‡ |
| ACE inhibitors or angiotensin-receptor blockers | 59 (92) | 59 (92) | 1.0‡ |
| Statins | 37 (58) | 38 (59) | 1.0‡ |
| Aspirin | 52 (81) | 39 (61) | 0.02‡ |
| Type of ICD — no. (%) | 0.21‡ | ||
| Single-chamber | 23 (36) | 31 (48) | |
| Dual-chamber | 41 (64) | 33 (52) | |
Plus–minus values are means ±SD. ICD denotes implantable cardioverter–defibrillator, PTCA percutaneous transluminal coronary angioplasty, CABG coronary-artery bypass grafting, and ACE angiotensin-converting enzyme.
The P value was calculated by the t-test.
The P value was calculated by Fisher’s exact test.
The data are from 54 patients in the control group and 56 in the ablation group.
The P value was calculated by the Wilcoxon rank-sum test.
The two treatment groups were well balanced with respect to baseline demographic and clinical characteristics (Table 1). The only significant differences between the groups were in the fraction of patients with severely depressed ventricular function (which would be expected to result in higher mortality in the ablation group) and in the rate of aspirin use. No patients were being treated with membrane-active antiarrhythmic drugs, and most patients received both beta-blockers and ACE inhibitors or angiotensin-receptor blockers.
CATHETER ABLATION
Three patients assigned to the ablation group did not undergo the procedure. One died of congestive heart failure before the procedure could be performed, and two were lost to follow-up and did not undergo ablation (one of these presented at the clinic after having received an ICD shock 5 months after randomization).
The majority of patients (87%) underwent implantation of the defibrillator before ablation, but in 13% of patients the ablation procedure was performed before defibrillator implantation. Ventricular mapping was performed by means of either a retrograde aortic approach alone (26%) or a combined retrograde aortic and transseptal approach (74%). Ablation was performed with a standard ablation catheter in 10 patients (16%) and an irrigated ablation catheter in 48 patients (79%). In three patients (5%), no appreciable en-docardial scar was visualized and therefore no ablation lesions were placed.
Substantial ablation-related complications occurred in three patients: a pericardial effusion without tamponade, which was managed conservatively; an exacerbation of congestive heart failure requiring prolonged hospitalization; and a deep venous thrombosis requiring prolonged anticoagulation therapy. No ICD leads became dislodged, and no devices became infected. The 30-day mortality rate was zero.
FOLLOW-UP AND CLINICAL OUTCOME
All surviving patients completed the 2-year follow-up except the two who did not undergo ablation and were lost to follow-up. The mean (±SD) duration of follow-up was 22.5±5.5 months (range, 0 to 26). During follow-up, no patient received an antiarrhythmic agent (other than beta-blockers) before the primary end point was reached. Once a patient had had an ICD event, the physician was free to treat the patient as was clinically appropriate with membrane-active antiarrhythmic drugs (typically amiodarone or sotalol), catheter ablation (including repeat catheter ablation in patients who had been enrolled in the ablation group), or both.
As shown in Table 2, after 2 years of follow-up, 8 patients in the ablation group (12%) and 21 in the control group (33%) received at least one episode of appropriate ICD therapy, defined as either shocks or antitachycardia pacing (hazard ratio, 0.35; 95% confidence interval [CI], 0.15 to 0.78; P = 0.007). Kaplan–Meier analysis of this primary end point reveals progressive divergence of the curves for survival free from ICD therapy (Fig. 1). Multivariate analysis adjusted for patient characteristics and medication use at baseline did not reduce the magnitude of the effect observed (hazard ratio, 0.31; 95% CI, 0.13 to 0.76; P=0.01).
Table 2.
End Points.*
| Variable | Ablation Group (N = 64) | Control Group (N = 64) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| no. of patients (%) | ||||
| ICD events* | 8 (12) | 21 (33) | 0.35 (0.15–0.78) | 0.007† |
| ICD shocks | 6 (9) | 20 (31) | 0.27 (0.11–0.67) | 0.003† |
| ICD storms | 4 (6) | 12 (19) | 0.30 (0.09–1.00) | 0.06‡ |
| Death | 6 (9) | 11 (17) | 0.59 (0.22–1.59) | 0.29† |
| Congestive heart failure | 3 (5) | 6 (9) | ||
| Ventricular tachycardia storm | 0 | 1 (2) | ||
| Cancer | 1 (2) | 0 | ||
| Pulmonary embolism | 1 (2) | 0 | ||
| Unknown | 1 (2) | 4 (6) | ||
Implantable cardioverter–defibrillator (ICD) events include ICD shocks and antitachycardia pacing.
The P value was calculated by the log-rank test.
The P value was calculated by Fisher’s exact test.
Figure 1. Kaplan–Meier Estimate of the Primary End Point of Survival Free from ICD Therapy.
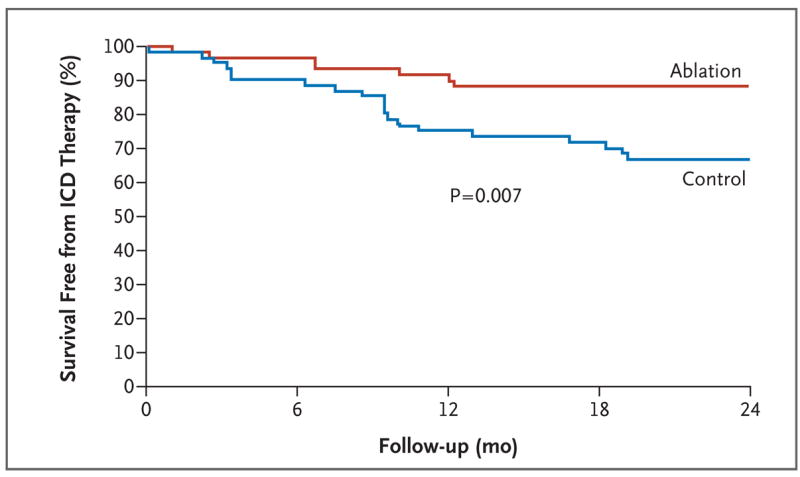
ICD denotes implantable cardioverter–defibrillator.
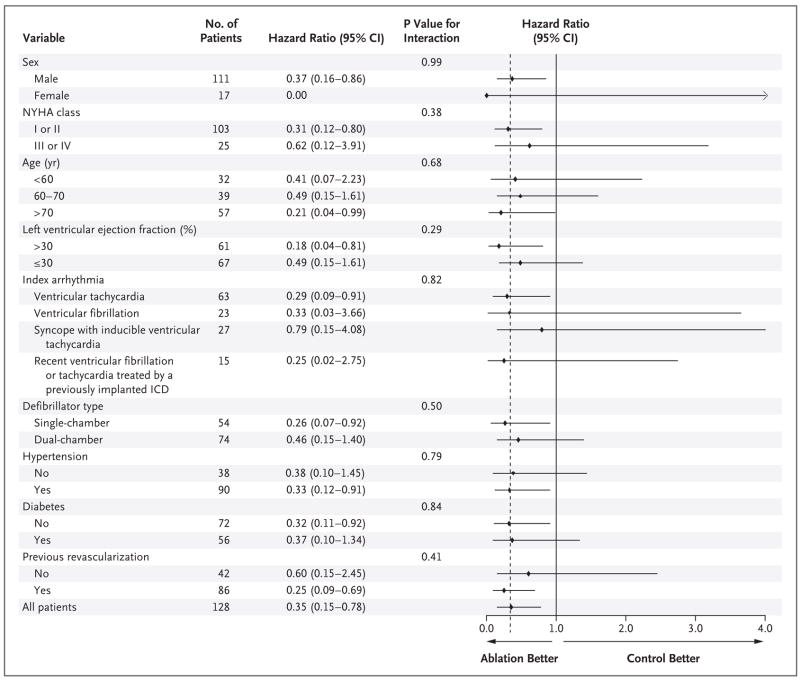
As shown in Figure 2, there were no significant differences in the effect of ablation on the primary end point of any subsequent ICD therapy in any of the subgroup analyses performed. These included stratification according to sex, functional class, age, ventricular function (including those patients with the most severely depressed function), index arrhythmia, type of ICD, and the presence or absence of hypertension, of diabetes, and of previous revascularization.
Figure 2. Hazard Ratios and 95% Confidence Intervals for Subsequent ICD Therapy According to Subgroup.
The chart shows hazard ratios (black diamonds) with 95% confidence intervals (horizontal lines) and P values for the interaction between the treatment effect and each subgroup variable. Because of the limited number of patients in certain subgroups, wide confidence intervals are noted. The dashed vertical line indicates the hazard ratio for the study entire population. NYHA denotes New York Heart Association, and ICD implantable cardioverter–defibrillator.
Six of the patients assigned to ablation (9%) and 20 of the control patients (31%) received at least one appropriate ICD shock (excluding antitachycardia pacing) (P = 0.003) (Fig. 3A). There was a trend toward decreased mortality in the ablation group as compared with the control group (9% vs. 17%, P = 0.29) (Table 2). ICD storms occurred in 4 patients assigned to ablation (6%) and 12 control patients (19%) (P = 0.06).
Figure 3. Kaplan–Meier Estimates of Secondary End Points.
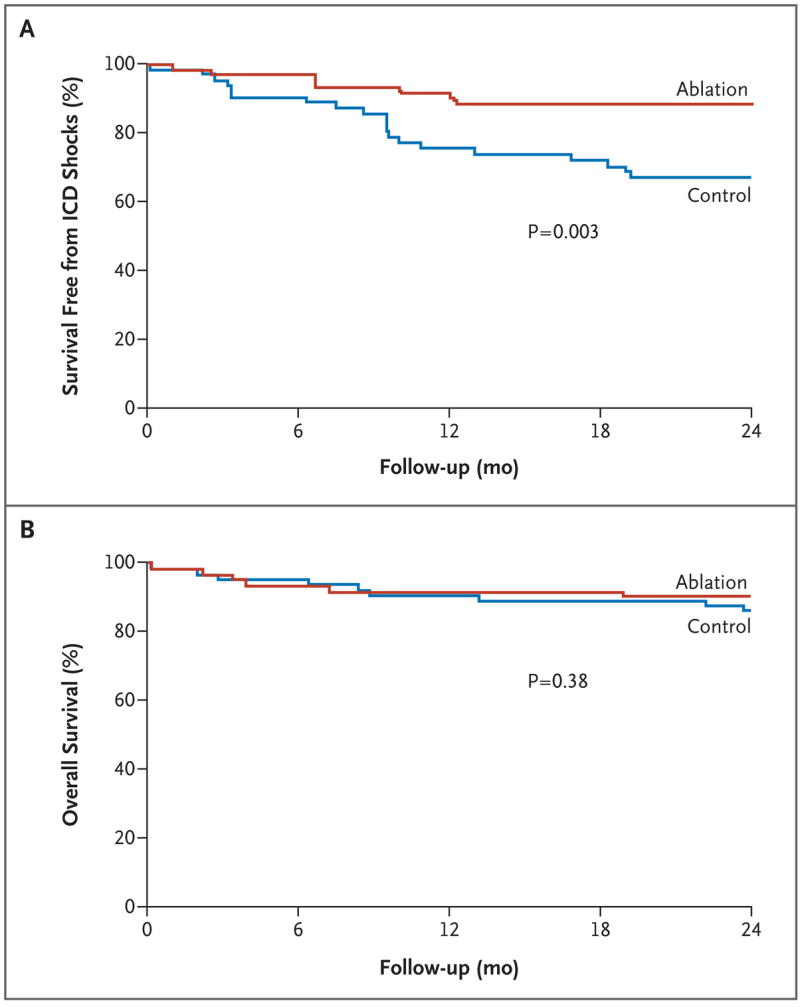
ICD denotes implantable cardioverter–defibrillator.
ADVERSE EFFECTS
As shown in Figure 4, there was no evidence of an adverse effect of catheter ablation on ventricular function. In both groups, there were no significant changes in the distribution of patients according to NYHA functional class from before enrollment to 3 months after enrollment. In the group assigned to ablation, 84% were in NYHA class I or II and 16% were in class III or IV at baseline; the corresponding percentages at 3 months were 88% and 12%. In the control group, 77% were in NYHA class I or II, and 23% were in class III or IV at baseline; the corresponding percentages at 3 months were 85% and 15%. Three patients (two in the ablation group and one in the control group) received inappropriate defibrillator shocks as a result of supraventricular tachycardias (two with atrial fibrillation and one with atrial flutter).
Figure 4. Effect of Substrate Ablation on Ventricular Function.
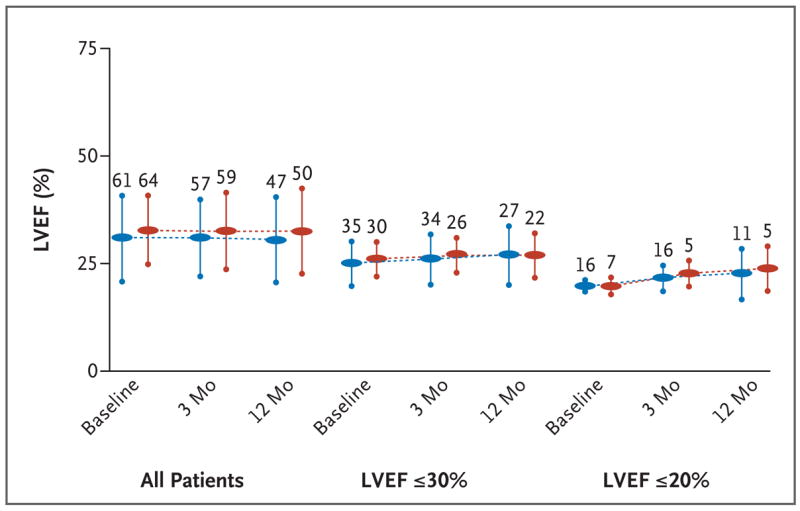
The left ventricular ejection fractions (LVEF) at baseline and at 3-month and 12-month follow-up visits are shown for the ablation (blue) and control (red) groups. To assess a preferential negative effect on function in the most severely dysfunctional ventricles, results are also shown for the subgroups with an LVEF of 30% or less and an LVEF of 20% or less. The numbers of patients analyzed (those for whom echocardiographic data were available) are shown above the vertical bars, which indicate the standard deviation.
DISCUSSION
Our trial was initiated to objectively examine the use of substrate ablation in patients with a previous myocardial infarction who were receiving an ICD for the secondary prevention of sudden death. As compared with the control population, patients in the ablation group had a 65% reduction in the risk of receiving ICD therapy during the subsequent 2 years. When antitachycardia-pacing therapy was excluded from this analysis, there remained a 73% reduction in the risk of receiving subsequent ICD shocks. This effect may be clinically important, not only because of the reduction in the unpleasant experience of device discharge, but also in terms of subsequent adverse cardiovascular events. The Defibrillator in Acute Myocardial Infarction Trial (DINAMIT) and the Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II) both found an increase in congestive heart failure and mortality in patients receiving ICD shocks (although the shocks may not have been causally related to heart failure or mortality).19,20 These end points consisted not of a decrease in the number of ICD events but, rather, of a decrease in the total number of patients who received any ICD therapy. In addition, all the patients were followed for 2 years or until death. The two groups were well balanced with respect to baseline characteristics, and a high percentage received beta-blockers and antagonists of the renin–angiotensin–aldosterone system. The groups were also well balanced with respect to the use of statin drugs, which some studies suggest may decrease the risk of ICD therapy, although not all patients used these drugs.21
Our study is unusual in including not only patients with monomorphic ventricular tachycardia but also those whose index arrhythmic episode was ventricular fibrillation. However, patients with ventricular fibrillation constituted a smaller percentage of the study cohort than did patients with other qualifying arrhythmias, and it is premature to draw specific conclusions about the salutary effects of catheter ablation in this group.
In order for substrate ablation to be a clinically viable option, the safety profile of this interventional procedure is of paramount importance. There was no significant change in ventricular function or functional status during follow-up, perhaps because the ablation lesions were placed exclusively within infarcted tissue, which contributes little to contractile function. This remained true even for those patients with the most depressed cardiac function. Overall mortality was not greater in the patients assigned to ablation; indeed, there was even a trend toward decreased mortality in the ablation group, although it was not statistically significant.
In this study, the use of prophylactic class III antiarrhythmic drugs was not evaluated. However, the efficacy of the available class III drugs (other than amiodarone) in preventing shocks is unclear, and the unfavorable safety profile of amiodarone makes it an unattractive prophylactic drug option.22–25 There are few data directly comparing catheter ablation with antiarrhythmic medications for the management of ventricular tachycardia. The only randomized study of which we are aware involved 105 patients who had already received ICD shocks. In that study, the primary end point, ventricular tachycardia, recurred in 49% and 75% of the ablation and drug-treatment groups, respectively.26
It is unknown whether the results of our study can be applied to patients with ventricular arrhythmia due to other causes, such as patients with dilated cardiomyopathy. Such other disease substrates often involve ventricular tachycardia circuits that are partly or predominantly epicardial, thereby necessitating pericardial mapping and ablation — an approach not evaluated in this study.
This study was limited by the small number of participants. Another limitation was the absence of quality-of-life information — a measurement well known to be substantially affected by ICD shocks. In addition, a formal economic analysis would be needed to adequately understand the relationship between the immediate costs of the ablation procedure and the long-term costs of care for patients with ICDs. During enrollment, screening data were not collected; this may limit our understanding of how to select the most appropriate patients for ablation and how to generalize the data to other patients. Finally, this study did not compare ablation with antiarrhythmic drug therapy, and therefore we cannot comment directly on the relative efficacy of these two therapeutic approaches in this setting.
In summary, the SMASH-VT trial evaluated the role of radiofrequency substrate ablation for the control of ventricular arrhythmias in patients with ischemic heart disease who received an ICD for a ventricular tachyarrhythmic event. At a mean follow-up of 2 years, the frequency of subsequent episodes of any ICD therapy, and of ICD shocks, was significantly lower among patients undergoing ablation than among those not undergoing this intervention.
Supplementary Material
Acknowledgments
Supported in part by a National Institutes of Health K23 award (HL68064) to Dr. Reddy.
Dr. Reddy reports receiving consulting fees, lecture fees, and grant support from Biosense Webster and St. Jude Medical, lecture fees and grant support from Boston Scientific, and lecture fees from Medtronic. Dr. Reynolds reports receiving consulting fees from Biosense Webster. Dr. Jongnarangsin reports having equity ownership in Medtronic. Dr. Ruskin reports receiving consulting fees from Medtronic and Biosense Webster and lecture fees from St. Jude Medical and Boston Scientific and having equity ownership in Cameron Health and InnerPulse. Dr. Josephson reports receiving consulting fees from Medtronic and Biosense Webster. No other potential conflict of interest relevant to this article was reported.
Footnotes
Presented in part as an abstract at the 2006 Annual Scientific Sessions of the Heart Rhythm Society, Boston, May 17–20, 2006.
References
- 1.Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA. 2006;295:809–18. doi: 10.1001/jama.295.7.809. [DOI] [PubMed] [Google Scholar]
- 2.Sears SE, Jr, Conti JB. Understanding implantable cardioverter defibrillator shocks and storms: medical and psychosocial considerations for research and clinical care. Clin Cardiol. 2003;26:107–11. doi: 10.1002/clc.4960260303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamphuis HCM, de Leeuw RJ, Derksen R, Hauer RN, Winnubst JA. Implantable cardioverter defibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study. Europace. 2003;5:381–9. doi: 10.1016/s1099-5129(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 4.Bilge AK, Ozben B, Demircan S, Cinar M, Yilmaz E, Adalet K. Depression and anxiety status of patients with implantable cardioverter defibrillator and precipitating factors. Pacing Clin Electrophysiol. 2006;29:619–26. doi: 10.1111/j.1540-8159.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 5.Villacastín J, Almendral J, Arenal A, et al. Incidence and clinical significance of multiple consecutive, appropriate, high-energy discharges in patients with implanted cardioverter-defibrillators. Circulation. 1996;93:753–62. doi: 10.1161/01.cir.93.4.753. [DOI] [PubMed] [Google Scholar]
- 6.Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol. 1998;32:1909–15. doi: 10.1016/s0735-1097(98)00495-1. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KP. Sudden cardiac death unresponsive to implantable defibrillator therapy: an urgent target for clinicians, industry and government. J Interv Card Electrophysiol. 2005;14:71–8. doi: 10.1007/s10840-005-4547-9. [DOI] [PubMed] [Google Scholar]
- 8.Pacifico A, Johnson JW, Stanton MS, et al. Comparison of results in two implantable defibrillators. Am J Cardiol. 1998;82:875–80. doi: 10.1016/s0002-9149(98)00495-0. [DOI] [PubMed] [Google Scholar]
- 9.Josephson ME. Electrophysiology of ventricular tachycardia: an historical perspective. J Cardiovasc Electrophysiol. 2003;14:1134–48. doi: 10.1046/j.1540-8167.2003.03322.x. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson WG. Catheter ablation of monomorphic ventricular tachycardia. Curr Opin Cardiol. 2005;20:42–7. [PubMed] [Google Scholar]
- 11.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–96. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 12.Reddy VY, Neuzil P, Taborsky M, Ruskin JN. Short-term results of substrate mapping and radiofrequency ablation of ischemic ventricular tachycardia using a saline-irrigated catheter. J Am Coll Cardiol. 2003;41:2228–36. doi: 10.1016/s0735-1097(03)00492-3. [DOI] [PubMed] [Google Scholar]
- 13.Arenal A, Glez-Torrecilla E, Ortiz M, et al. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41:81–92. doi: 10.1016/s0735-1097(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy DM, Vassallo JA, Buxton AR, Doherty JU, Marchlinski FE, Josephson ME. The value of catheter mapping during sinus rhythm to localize site of origin of ventricular tachycardia. Circulation. 1984;69:1103–10. doi: 10.1161/01.cir.69.6.1103. [DOI] [PubMed] [Google Scholar]
- 15.Kienzle MG, Miller J, Falcone RA, Harken A, Josephson ME. Intraoperative endocardial mapping during sinus rhythm: relationship to site of origin of ventricular tachycardia. Circulation. 1984;70:957–65. doi: 10.1161/01.cir.70.6.957. [DOI] [PubMed] [Google Scholar]
- 16.Guiraudon G, Fontaine G, Frank R, Escande G, Etievent P, Cabrol C. Encircling endocardial ventriculotomy: a new surgical treatment for life-threatening ventricular tachycardias resistant to medical treatment following myocardial infarction. Ann Thorac Surg. 1978;26:438–44. doi: 10.1016/s0003-4975(10)62923-2. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan SC, Josephson ME. Surgery for postinfarction ventricular tachycardia: is it obsolete? Pacing Clin Electrophysiol. 2000;23:1295–301. doi: 10.1111/j.1540-8159.2000.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 18.The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 19.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter–defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 20.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 21.Goldberger JJ, Subacius H, Schaechter A, et al. Effects of statin therapy on arrhythmic events and survival in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1228–33. doi: 10.1016/j.jacc.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 22.Pacifico A, Hohnloser SH, Williams JH, et al. Prevention of implantable defibrillator shock by treatment with sotalol. N Engl J Med. 1999;340:1855–62. doi: 10.1056/NEJM199906173402402. [DOI] [PubMed] [Google Scholar]
- 23.Seidl K, Hauer B, Schwick NG, Zahn R, Senges J. Comparison of metoprolol and sotalol in preventing ventricular tachyarrhythmias after the implantation of a cardioverter/defibrillator. Am J Cardiol. 1998;82:744–8. doi: 10.1016/s0002-9149(98)00478-0. [DOI] [PubMed] [Google Scholar]
- 24.Mazur A, Anderson ME, Bonney S, Roden DM. Pause-dependent polymorphic ventricular tachycardia during long-term treatment with dofetilide: a placebo-controlled, implantable cardioverter-defibrillator-based evaluation. J Am Coll Cardiol. 2001;37:1100–5. doi: 10.1016/s0735-1097(01)01106-8. [DOI] [PubMed] [Google Scholar]
- 25.Connolly SJ, Dorian P, Roberts RS, et al. Comparison of β-blockers, amiodarone plus β-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC study: a randomized trial. JAMA. 2006;295:165–71. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 26.Epstein AJ, Wilber DJ, Calkins H, et al. Randomized controlled trial of ventricular tachycardia treatment by cooled-tip catheter ablation vs drug therapy. J Am Coll Cardiol. 1998;31:118A. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.