Abstract
Objective:
Shared neuropathological characteristics of patients with schizophrenia and their siblings may represent intermediate phenotypes that could be used to investigate genetic susceptibility to the illness. We sought to discover previously unidentified gray matter volume differences in patients with schizophrenia and their siblings using optimized Voxel-Based Morphometry (VBM).
Methods:
We studied 169 patients with schizophrenia, 213 of their unaffected siblings, and 212 healthy volunteers from the CBDB/NIMH Genetic Study of Schizophrenia with magnetic resonance imaging (MRI).
Results:
Patients with schizophrenia had significant regional gray matter decreases in the frontal, temporal, and parietal cortices compared with healthy volunteers. Their unaffected siblings tended to share gray matter decreases in the medial frontal, superior temporal and insular cortices, but these decreases were not significant after correction for multiple comparisons, even when we looked at a subgroup of siblings with a past history of mood disorder. As an exploratory analysis, we estimated heritability using regions of interest from the VBM analysis, as well as from the hippocampus. Hippocampal volume was significantly correlated within sibling-pairs.
Conclusions:
Our findings confirm and extend previous VBM analyses in ill subjects with schizophrenia. Furthermore, these data argue that while siblings may share some regional gray matter decreases with their affected siblings, the pattern of regional differences may be a weak intermediate phenotype for schizophrenia.
INTRODUCTION
A number of risk factors have been implicated in schizophrenia, including family history, lower social class, perinatal and other early developmental insults, and substance abuse (1). Amongst these, family history remains one of the strongest, with an estimated heritability of almost 80% (2). Deviations in brain morphology potentially reflecting genetic risk have been ubiquitous in the literature. Quantitative measures of brain structure using various neuroimaging techniques have a long history as putative intermediate phenotypes (3). Past twin studies have explored the heritability of this phenotype; a process that entails measuring the proportion of variation in this trait (i.e. brain structure) attributable to genetic variation.
The first neuroimaging study to investigate a familial intermediate phenotype of schizophrenia found an increased ventricle to brain ratio (VBR) in patients and their siblings (4). Since then, many twin studies have explored the putative heritability of brain structure using intraclass correlation (ICC), reporting high heritability estimates for total brain volume (94%) (5; 6) and lateral ventricular volume (82-85%) (7). Studies of twins discordant for schizophrenia have also found liability-related gray matter volume decreases in whole brain gray matter and frontal lobes (8), polar frontal and dorsolateral prefrontal (DLPFC) (9; 10), medial temporal (i.e., the hippocampus) (8; 11; 12), left medial frontal, and left sensory motor cortices (13), as well as liability-related white matter increases in the orbitofrontal cortex (13). The twin literature in schizophrenia points primarily to a variety of gray matter structural changes that may be heritable.
Investigating non-twin, healthy first-degree relatives represents a complementary approach to formal measures of heritability obtained in twin studies. Siblings who do not have schizophrenia or schizophrenia-spectrum disorder are considered ‘unaffected.’ These studies do not have the power to discern genetic from environmental risk factors as do twin studies, though advantageously lack potential confounders related to the intrauterine disadvantages of MZ twins (14). Region of interest studies have shown that first degree relatives may share regional brain volume abnormalities with their sick siblings in the amygdala-hippocampal complex, thalamus, and the temporal cortices (15-17).
Upon a background of complex and often contradictory structural findings within the volumetric literature, voxel-based morphometry (VBM) offers promise of clarifying the structural deficits in schizophrenia. Voxel-based morphometry performs voxel-wise comparisons of gray and white matter probabilities between groups of subjects. More recent studies have implemented ‘optimized VBM;’ a method described by Good and colleagues (18). A recent review of VBM findings in schizophrenia identified gray matter decreases in the superior temporal, medial temporal, inferior frontal, and medial frontal cortices plus the thalamus as regions most commonly reported to be abnormal, amidst a complex literature (19). The literature is still relatively small, though, and replications are needed to clarify these decreases.
VBM studies of unaffected siblings of patients with schizophrenia, or those at particularly high risk based on genetic background, have had with mixed findings of gray matter deficits (20-23). Borgwardt et. al. found that individuals at high risk who later developed psychosis had reduced gray matter in the right insula, inferior frontal, and superior temporal gyrus compared to those that remained healthy (22). Job et al (2003) report bilateral reductions in the anterior cingulate cortex in first episode patients compared with high risk individuals (20). Job et al (2005) reported density differences in the left temporal cortex and cerebellum over time, but no predictive effects at baseline (21). McIntosh and colleagues (23) found that liability for schizophrenia was related to volume decreases in the VLPFC and DLPFC. Summarizing across these ‘sibling’ VBM studies, the prefrontal and temporal cortices (primarily the superior temporal gyrus), hippocampus, insula, and cerebellum are regions that may be represent candidate targets for structural intermediate phenotypes in schizophrenia.
To date there has not been an optimized VBM study on a large group of patients with schizophrenia and their first degree/ unaffected siblings. In the present study, we investigated the hypothesis that genetic risk would be reflected in gray matter volume decreases in unaffected siblings. We used optimized VBM to evaluate structural MRI data from the CBDB/NIMH Genetic Study of Schizophrenia, an ongoing family study aimed at identifying biologic intermediate phenotypes and ultimately risk genes for the illness (24). Based on findings in twins discordant for schizophrenia, we hypothesized that gray matter changes in patients and corresponding but less significant changes in siblings would be identified primarily in the frontal and temporal cortex and medial temporal regions (6; 13). We also asked whether there were significant gray matter differences between a subgroup unaffected siblings with a history of mood disorder, namely depression (which made up 27.7% of our sample), and those that had no history of personality or mood disorder at all. In addition, we sought to further clarify the nature of these volume changes in patients and the whole group of siblings in relation to genetic risk by calculating heritability for sibling pairs (one unaffected and one affected), using volumes from the regions of interest identified by the VBM analysis, as well as the hippocampus, based on previous studies implicating this region in liability for schizophrenia (25).
METHODS
Subjects
All subjects were recruited as part of the CBDB/NIMH Genetic Study of Schizophrenia [NIH Study ID NCT00001486] described in detail elsewhere (26). In brief, subjects included 169 patients with schizophrenia-spectrum disorders (SCZ), 213 of their unaffected siblings (SIB), and 212 healthy volunteers (i.e. normal controls, or NC) (Table 1). All patients were on antipsychotic medications at the timing of scan acquisition. Exclusionary criteria for the Sibling Study is described in detail elsewhere (26). Patients were included if they had a schizophrenia spectrum disorder, which included patients with schizophrenia, schizoaffective disorder, Psychosis NOS, or schizoid personality disorder (see supplementary material, Table 1). Siblings were included if they had a past history of mood (∼ 37 %) or personality (5 %) disorder, leaving a majority with no history (∼ 58%). Subjects were excluded if they had a past history of drug or alcohol abuse. Medication and family representation are detailed in supplemental material. All scans from subjects with available, usable structural imaging data free of visible artifact, distortion, incomplete acquisition, or evidence of neurological abnormality, were processed for the whole-brain VBM analysis. Differences in demographic means between each diagnostic group were tested using an ANOVA in SPSS version 13.0 (27) (Table 1).
Table 1.
Group demographics for VBM Analysis
| Demographics (Means +/− SD) |
||||||
|---|---|---|---|---|---|---|
|
Variable |
SCZ (n=169) |
SIB (n=213) |
NC (n=212) |
NC vs. SCZ |
SCZ vs. SIB |
NC vs. SIB |
| Age | (36.39, 9.46) | (36.5, 9.75) | (33.31, 9.86) | 0.00* | 0.88 | 0.00* |
| Gender (% male) | 78.2% | 41.8% | 48.6% | 0.00* | 0.00* | 0.18 |
| Education (years) | (14.41, 2.23) | (16, 2.48) | (16.89, 2.82) | 0.00* | 0.00* | 0.00* |
| Mean WAIS-full scale IQ | (94.89, 11.18) | (106.4, 12.73) | (107.69, 11.72) | 0.00* | 0.00* | 0.28 |
| Handedness | (71.09, 57) | (73.36, 54.65) | (78.25, 49.58) | 0.09 | 0.45 | 0.33 |
| Relative Gray matter volume (mm3) |
(0.73, .06) | (0.73, .06) | (0.74, 0.06) | 0.08 | 0.9 | 0.09 |
| Positive Syndrome Score | (12.79, 5.69) | (7.06, .32) | (7.06, .35) | 0.00* | 0.00* | 0.89 |
| Negative Syndrome Score | (19.52, 9.40) | (8.45, 3.46) | (8.14, 2.8) | 0.00* | 0.00* | 0.33 |
| No Past or Present Diagnosis | 0% | 57.80% | 85.40% |
Significant at p<.05 in ANOVA comparison of means, SPSS
Structural image processing and analyses
Image acquisition and data processing software have been described in previous studies from this group (28). Customized template creation was based on a sample of 100 healthy volunteers, 100 unaffected siblings, and 100 patients with schizophrenia or schizoaffective disorder taken from our study sample. They were matched for age (NC mean = 35.6 (± standard deviation 9.97), SIB = 36.27(±9.17), SCZ = 36.18 (±9.54); p>.05).
We used optimized VBM performed in SPM2, including the non-uniformity correction option (18; 29). Modulated images were smoothed using a 6mm FWHM isotropic Gaussian kernel for the small volume extractions for ICC calculation, and 10mm for the whole brain VBM analysis. We used a smaller smoothing kernel for the ICC calculation so that the extracted regions would better represent the gray matter volume present in that region.
The images were analyzed using the General Linear Model as implemented in SPM2. For the across-group analysis, we used an analysis of covariance model with diagnostic group as factor, age, gender, and IQ as covariates of interest, and total gray matter volume and second-order polynomial age expansions as covariates of no interest. Voxel-by-voxel t-tests were computed across the whole brain to determine global differences in gray matter volumes based on diagnosis, contrasting SCZ vs. NC, SCZ vs. SIB, and SIB vs. NC. In addition, we examined sibling subgroups broken down by past history of a major depressive episode since this represented the largest uniform diagnostic subgroup (SIBpast, n = 44) and those with no past history (SIBnopast, n = 128). Both SIBpast and SIBnopast were contrasted with SCZ and NC. Significant voxels (thresholded at .05 FWE corrected, cluster (k) > 5 contiguous voxels) are reported in Talairach spaceas in prior reports (30).
We masked the gray matter decreases of SIB vs. NC with SCZ vs. NC to identify overlap in voxel-wise variation in gray matter. This method identifies voxels that are significantly different in both contrasts (NC>SIB, NC>SCZ) (p< 0.001 uncorrected), i.e. a conjunction analysis. Since the findings from the NC>SIB analysis were seen only at the exploratory threshold of p<.001 uncorrected, we used that threshold for the conjunction analysis. We then extracted regional volume measures from this masked analysis using a 2mm sphere around the peak voxels and plotted the regional gray matter volumes across diagnosis to visualize the distribution across the three groups.
Finally, gray matter volumes from these regions as well as the hippocampus were extracted from the 6mm smoothed gray matter images in patients and their unaffected sibling pair using automatic ROI's from the Wakeforest University Pickatlas (WFU) (www.fmri.wfubmc.edu) (31). In addition we extracted volumes from Brodmann's area 17 to serve as a negative control for an intermediate phenotype, as gray matter volume in patients and unaffected siblings has not been identified as abnormal in this region by previous VBM studies (19). For ICC calculations, data from 116 sibling pairs were imported into Statistica 6 for Windows (http://www.statsoft.com/) and analyzed using the Variance Components and Mixed Model ANOVA module, with a significance level of p<.05 (for more detail, see supplemental material).
To test for confounds of drug ingestion in patients, we did a post-hoc analysis to look at medication effects on brain structure. We ran a multiple regression with a constant in SPM2 in the patients that had chlorpromazine equivalents (n = 79), regressing out effects of age, gender, years of education, mean gray matter volume, and IQ and testing for a linear relationship between medication and regional gray matter volume.
RESULTS
We first contrasted gray matter in SCZ vs. NC using an ANCOVA. A widespread distribution of significant gray matter decreases was found in patients compared with healthy volunteers (FWE p<.05, cluster size > 5) (Table 2, Figure 1). The most robust differences in regional gray matter were observed in the superior and inferior frontal, the medial prefrontal, the insular, left superior temporal, and the inferior parietal cortices, the medial thalamus and the posterior cerebellum. There were also increases in gray matter in SCZ compared with NC in the medial cerebellum, globus pallidus, left inferior temporal cortex, right middle temporal gyrus, left precentral gyrus, left anterior cingulate, and orbitofrontal cortex (Table 3).
Table 2.
Summary of the results obtained by voxel-wise wtatistical analysis of the GM Maps of decreases in SCZ and SIB compared with NC. Only the peak voxels are listed. Left is defined as “L” and Right is defined as “R”.
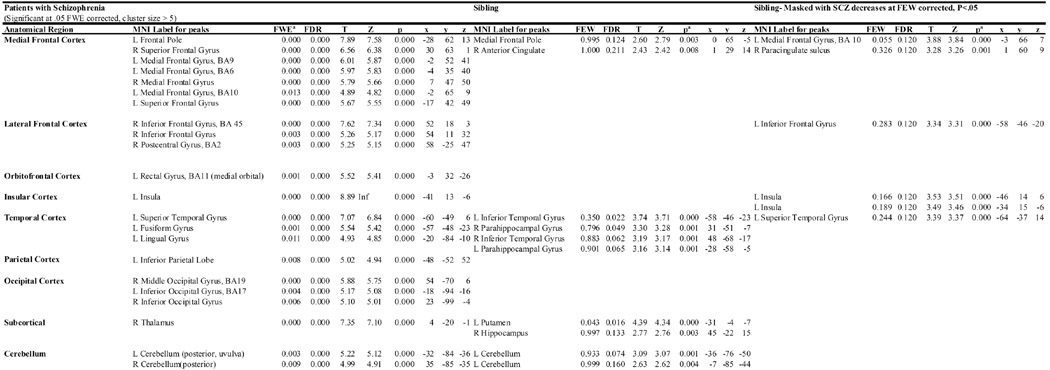 |
Statistical threshold used for inclusion. FWE-corrected for patient decreases and p- uncorrected for sibling and conjunction analysis.
Figure 1.
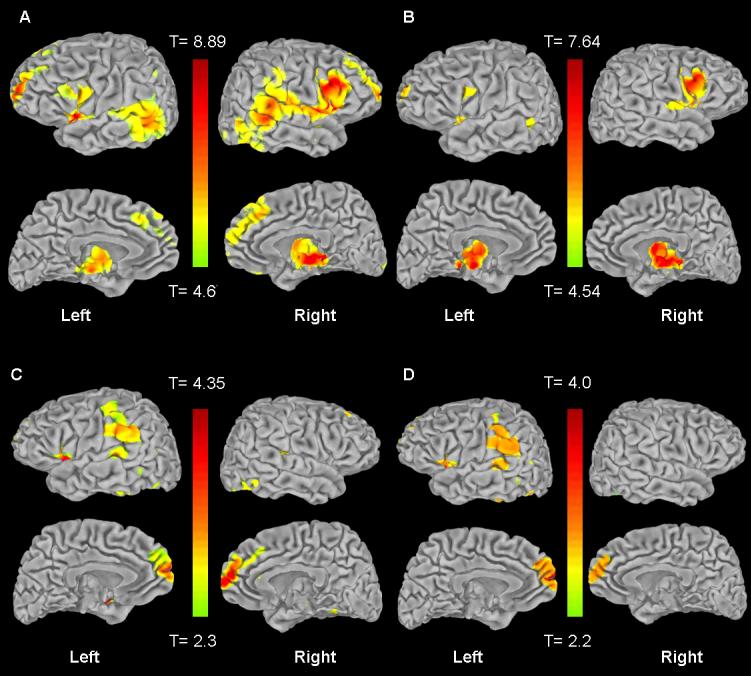
Statistical maps of the entire brain showing gray matter volume reductions in: A. SCZ compared with NC (at a threshold of p<.05 FWE corrected, cluster size > 5), B. SCZ compared to SIB (at a threshold of p<.05 FWE corrected, cluster size > 5), C. SIB compared with NC (at a threshold of p<.001 uncorrected, cluster size > 5),. D. SIB compared with NC, only results that overlap with SCZ are shown (at a threshold of p<.001 uncorrected, cluster size >5, small volume corrected with patient decreases). Thresholded images have been transformed from MNI space into Talairach space and converted to T-scores, which were projected onto the pial surface of a representative standard surface ; the range is shown in the corresponding color bars. The lateral surface is in the top row of each section, and the medial surface is shown below the lateral.
Table 3.
Summary of the results obtained by voxel-wise statistical analysis of the gray matter maps of increases in SCZ compared with NC listed rostal to caudal. Below that are results of the analysis of gray matter increases in SIB compared with NC. Patients results are significant (p<.05 FWE corrected, k > 5), and sibling results are not significant, trends shown at p<.001 uncorrected, k > 5 for comparison with their affected siblings.
| Group | MNI Label | FWE | FDR | T | Z | p | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| SCZ | R Orbitofrontal Cortex | 0.002 | 0.000 | 5.37 | 5.27 | 0.000 | 28 | 50 | −7 |
| L Orbitofrontal Cortex | 0.000 | 0.000 | 5.87 | 5.74 | 0.000 | −16 | 40 | −13 | |
| R Orbitofrontal Cortex | 0.001 | 0.000 | 5.56 | 5.44 | 0.000 | 18 | 38 | −15 | |
| Anterior Cingulate | 0.000 | 0.000 | 5.85 | 5.72 | 0.000 | −6 | 27 | −5 | |
| L Globus Pallidus | 0.000 | 0.000 | 7.65 | 7.37 | 0.000 | −13 | 4 | −2 | |
| R Globus Pallidus | 0.000 | 0.000 | 6.46 | 6.28 | 0.000 | 12 | 4 | −4 | |
| L Precentral Gyrus, BA 6 |
0.028 | 0.000 | 4.71 | 4.64 | 0.000 | −41 | −4 | 41 | |
| L Inferior Temporal Gyrus |
0.002 | 0.000 | 5.35 | 5.24 | 0.000 | −49 | −14 | −27 | |
| R Middle Temporal Gyrus |
0.000 | 0.000 | 5.66 | 5.54 | 0.000 | 50 | −32 | −19 | |
| R Medial Cerebellum | 0.000 | 0.000 | 10.0 | Inf | 0.000 | 20 | −64 | −24 | |
| SIB | R Inferior Frontal Gyrus, BA 45 |
0.937 | 0.505 | 3.39 | 3.37 | 0.000 | 49 | 15 | 18 |
| R Inferior Frontal Gyrus, BA 9 |
0.739 | 0.505 | 3.64 | 3.61 | 0.000 | 61 | 8 | 24 | |
| R Parahippocampal Gyrus |
0.742 | 0.505 | 3.64 | 3.61 | 0.000 | 26 | −22 | −25 | |
| R Inferior Temporal Gyrus |
0.101 | 0.467 | 4.38 | 4.33 | 0.000 | 51 | −29 | −20 | |
| R Precuneus | 0.906 | 0.505 | 3.45 | 3.42 | 0.000 | 12 | −55 | 36 |
The ANCOVA comparing SCZ vs. SIB revealed significant gray matter decreases in the patients when compared to their healthy siblings (FWE p<.05) (Table 4, Figure 1). These decreases in patients were in the fronto-polar cortex (superior and middle frontal gyri, BA 10), inferior frontal gyrus, right pre- and post-central gyrus, left middle temporal gyrus, right medial parietal cortex, left prestriate cortex, right ventrolateral nucleus of the thalamus and the right ventral cerebellum. In addition, the ANCOVA comparing SCZ vs. SIBnopast revealed similar significant gray matter decreases in the patients, namely in the left and right superior and middle frontal gyri (BA 9 and 10), left inferior frontal gyrus (BA 45), and the right ventrolateral nucleus of the thalamus. Peaks unique to this subgroup with no past psychiatric history were in the left insula, right superior temporal gyrus (BA 22) and right parietal cortex (BA 40) (Table 4). The ANCOVA comparing SCZ vs. SIBpast revealed similar frontal cortical regions as the combined group and the no past depression group, with the addition of left and right BA 46, the left middle frontal gyrus (BA 6), the right precentral gyrus (BA 6), the right thalamus, and the right superior temporal gyrus (BA 22) (Table 4). In all three analyses the patients had two large clusters of increased gray matter in the cerebellum (bilateral culmen, p<.001 FWE, k=40,690, 28, −53, −38), and the left lateral globus pallidus (lentiform nucleus, p<.000 FWE, k=9,661, −18, −3 −3) compared to the unaffected siblings.
Table 4.
Summary of the results obtained by voxel-wise statistical analysis of the gray matter maps of decreases in SCZ compared with SIB, SIBpast, and SIBnopast. Only peak findings that reached our threshold for significance are listed (FWE p<.05, cluster size > 5). Findings are listed rostral to caudal.
| Sibling Subgroup |
MNI Label | FWE a |
FDR | T | Z | p | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| All | L Superior Frontal Gyrus, BA 10 | 0.000 | 0.000 | 6 | 5.86 | 0.000 | −27 | 62 | 14 |
| (SIB) | R Middle Frontal Gyrus, BA 10 | 0.021 | 0.000 | 4.68 | 4.61 | 0.000 | 33 | 62 | 3 |
| R Inferior Frontal Gyrus | 0.000 | 0.000 | 6.58 | 6.39 | 0.000 | 56 | 16 | 22 | |
| L Inferior Frontal Gyrus | 0.000 | 0.000 | 5.7 | 5.58 | 0.000 | −53 | 14 | 24 | |
| L Inferior Frontal Gyrus, BA 47 | 0.001 | 0.000 | 5.49 | 5.38 | 0.000 | −43 | 13 | −4 | |
| R Precentral gyrus | 0.028 | 0.000 | 4.62 | 4.55 | 0.000 | 60 | 3 | 28 | |
| R Thalamus (ventrolateral nucleus) |
0.000 | 0.000 | 7.64 | 7.36 | 0.000 | 12 | −10 | 6 | |
| R Postcentral gyrus | 0.008 | 0.000 | 4.92 | 4.84 | 0.000 | 65 | −29 | 37 | |
| L Middle Temporal Gyrus, BA 21 |
0.015 | 0.000 | 4.77 | 4.7 | 0.000 | −61 | −48 | 4 | |
| L Prestriate | 0.019 | 0.000 | 4.72 | 4.65 | 0.000 | −16 | −94 | −15 | |
| R Cuneus | 0.011 | 0.000 | 4.85 | 4.78 | 0.000 | 21 | −99 | −6 | |
|
Past Depression |
L Superior Frontal Gyrus, BA 10 | 0.003 | 0.000 | 5.48 | 5.28 | 0.000 | −29 | 65 | 15 |
| (SIBpast) | R Superior Frontal Gyrus, BA 10 |
0.033 | 0.000 | 4.90 | 4.76 | 0.000 | 37 | 60 | −1 |
| R Middle Frontal Gyrus, BA 46 | 0.006 | 0.000 | 5.34 | 5.15 | 0.000 | 49 | 50 | 12 | |
| L Middle Frontal Gyrus, BA 46 | 0.017 | 0.000 | 5.07 | 4.91 | 0.000 | −54 | 33 | 18 | |
| L Middle Frontal Gyrus, BA 9 | 0.008 | 0.000 | 5.24 | 5.07 | 0.000 | −44 | 33 | 38 | |
| R Inferior Frontal Gyrus, BA 9 | 0.000 | 0.000 | 6.71 | 6.37 | 0.000 | 60 | 16 | 24 | |
| L Inferior Frontal Gyrus | 0.000 | 0.000 | 5.89 | 5.65 | 0.000 | −55 | 13 | 25 | |
| L Middle Frontal Gyrus, BA 6 | 0.003 | 0.000 | 5.36 | 5.17 | 0.000 | −52 | 7 | 47 | |
| R Superior Temporal Gyrus, BA 22 |
0.000 | 0.000 | 6.54 | 6.22 | 0.000 | 63 | 1 | 2 | |
| R Precentral Gyrus, BA 6 | 0.021 | 0.000 | 5.02 | 4.87 | 0.000 | 64 | −8 | 39 | |
| R Thalamus | 0.013 | 0.000 | 5.13 | 4.97 | 0.000 | 8 | −16 | 0 | |
| R Thalamus | 0.005 | 0.000 | 5.35 | 5.17 | 0.000 | 11 | −33 | −1 | |
| No Past | L Middle Frontal Gyrus, BA 10 | 0.000 | 0.000 | 6.30 | 6.09 | 0.000 | −29 | 66 | 14 |
| (SIBnopast) | R Superior Frontal Gyrus, BA 10 |
0.006 | 0.000 | 5.24 | 5.12 | 0.000 | 35 | 65 | 1 |
| R Middle Frontal Gyrus, BA 10 | 0.014 | 0.000 | 5.04 | 4.92 | 0.000 | 37 | 63 | 11 | |
| R Middle Frontal Gyrus, BA 9 | 0.002 | 0.000 | 5.53 | 5.38 | 0.000 | 44 | 39 | 40 | |
| R Middle Frontal Gyrus, BA 9 | 0.000 | 0.000 | 6.32 | 6.10 | 0.000 | 58 | 14 | 33 | |
| L Inferior Frontal Gyrus, BA 45 | 0.002 | 0.000 | 5.48 | 5.34 | 0.000 | −55 | 14 | 23 | |
| L Insula | 0.002 | 0.000 | 5.48 | 5.34 | 0.000 | −42 | 12 | −5 | |
| R Superior Temporal Gyrus, BA 22 |
0.003 | 0.000 | 5.44 | 5.30 | 0.000 | 62 | −2 | 3 | |
| R Thalamus (ventrolateral nucleus) |
0.000 | 0.000 | 7.24 | 6.93 | 0.000 | 13 | −8 | 6 | |
| R Inferior Frontal Gyrus, BA 40 | 0.021 | 0.000 | 4.95 | 4.85 | 0.000 | 48 | −36 | 57 |
Significant at .05 FWE corrected, cluster size > 5
The ANCOVA comparing SIB vs. NC revealed several focal gray matter decreases in the SIB when compared to NC, but these did not survive correction across the whole brain. At an exploratory threshold (p<.001, uncorrected) (Table 2), these decreases were in the medial frontal cortex, the right anterior cingulate, the inferior temporal gyrus, the parahippocampal gyrus, right hippocampus, left putamen, and left cerebellum. The ANCOVA comparing SIBpast vs. NC revealed no suprathreshold voxels for increases or decreases, even at an uncorrected threshold at p<.001. The ANCOVA comparing SIBnopast vs. NC resulted in the same clusters listed from the SIB vs. NC analysis above, such that there was no significant contribution of mood disorder history on the gray matter decreases compared with healthy controls. SIBs had increased gray matter compared to NC at an exploratory threshold (p<.001, uncorrected) in the right inferior frontal cortex (BA 45 and BA 9), right parahippocampal gyrus, right inferior frontal gyrus, and right precuneus (Table 3).
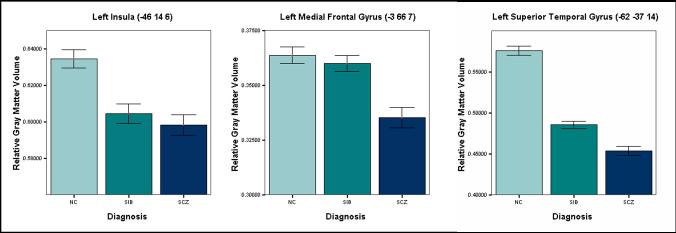
The masked analysis revealed regions that were commonly decreased in the SCZ and SIB when compared with NC (Table 2, Figure 1). These were the left medial frontal polar cortex, paracingulate cortex, left inferior frontal gyrus, left insula, and left superior temporal gyrus. The most significant differences lay in the left medial frontal gyrus, left insula, and left superior temporal gyrus. These volumes were extracted as described and plotted in Figure 2.
Figure 2.
Mean differences in regional gray matter volume in regions identified by the masked analysis. The left insula, left medial temporal gyrus, and left superior temporal gyrus (sphere of 2mm around listed coordinate) were decreased in SCZ compared with NC, were decreased in SIB compared to NC, and were decreased in SCZ relative to SIB (p<.001, all p values at voxel level, uncorrected, 1 standard error shown with the error bars). Measures for regional volume have been extracted from the significant cluster and transformed into percentage volume relative to class total gray matter, while controlling for confounding variables such as age and gender.
The ICC calculations within gray matter regions of interest, as well as calculated p-values are listed in Table 5. The left hippocampus, right hippocampus, and bilateral hippocampus had significant intra-class correlation coefficients within sib-pairs. The three regions implicated in the masked analysis were not significantly correlated within sib-pairs. In addition, the ICC analysis was not significant within Brodmann's area 17.
Table 5.
Summary of ICC results from ROI analysis of mean gray matter volumes between 116 sibling pairs.
| Components of Variance (ICC) |
|||||
|---|---|---|---|---|---|
| ROI | MSF | MSE | F | ICC (η2) | p |
| L Hippocampus | 0.000593 | 0.004971 | 14.961 | 0.106 | **0.0001 |
| R Hippocampus | 0.000519 | 0.004036 | 16.032 | 0.114 | **0.0000 |
| Bilateral Hippocampus | 0.002236 | 0.016196 | 17.152 | 0.121 | **0.0000 |
| L BA 17 | −0.000045 | 0.007798 | 0.3315 | 0.000 | 0.56531 |
| R BA 17 | −0.000073 | 0.008935 | 0.0389 | 0.000 | 0.84364 |
| L Superior Temporal Gyrus* | −0.000062 | 0.007201 | 0.0005 | 0.000 | 0.98145 |
| L Insula* | 0.000006 | 0.005472 | 1.1314 | 0.001 | 0.28856 |
| L Medial Frontal Gyrus* | −0.000030 | 0.003597 | 0.0366 | 0.000 | 0.84831 |
2 mm region of interest used from masked analysis, see coordinates in Figure 2
significant at p<.05
DISCUSSION
We report the results of the largest optimized VBM study to our knowledge addressing gray matter changes in patients with schizophrenia (and related psychoses) and their siblings. Our findings confirm previous VBM analyses of gray matter in patients with schizophrenia. The analysis of siblings compared with healthy controls revealed trends for decreases and increases in the siblings. The masked analysis showed some shared gray matter decreases between patients and their unaffected siblings in the left medial frontal, left superior temporal, and left insular cortices. In our analysis of heritability, we found these three regions were not significantly correlated within the 116 sibling pairs. Interestingly, when we calculated ICCs for the hippocampal region in an exploratory analysis, we found significant correlations within sibling pairs. In general, brain structure is highly heritable and siblings may share regional gray matter variation (4; 5; 9), however it is difficult to determine how much of this is genetically mediated by risk genes for schizophrenia.
Gray Matter Changes Specific to Schizophrenia
Our ANCOVA comparing patients with schizophrenia to healthy volunteers was largely consistent with previous VBM (19) and ROI studies (32). Patients with schizophrenia had highly significant decreases in the frontal cortex, primarily the bilateral medial frontal cortex and inferior frontal gyri, consistent with other VBM studies (19). Frontal cortex ROI analyses have shown similar evidence for gray matter volume changes in the prefrontal and medial frontal cortices (32). There were significant insular decreases in patients with schizophrenia, consistent with studies showing volume changes in the insula (33-36). These data may support a re-evaluation of the role the insula may play in schizophrenia, though this region may be subject to artifact in VBM previously mentioned.
Patients with schizophrenia also had significant decreases in the medial thalamus and pulvinar compared to the healthy volunteers, consistent with some (37; 38), but not all previous reports (39-41). This discrepancy may be caused by issues specific to imaging (39). for instance lack of sensitivity in this region and tissue classification issues (40). Smaller changes have been reported in the post-mortem brain (42), though not replicated in others (43), making it a difficult finding to interpret in schizophrenia.
We found significantly decreased gray matter volume in the superior and middle temporal gyri, but not in the medial temporal gyri. Decreased volume in the superior temporal gyrus is one of the most consistently found morphometric abnormalities in patients with schizophrenia (44-46) and has been found both in ROI (32) and a majority, but not all VBM studies (19; 34). Our study did not reveal grossly abnormal volume in the hippocampus of patients with schizophrenia. Findings of abnormality in the medial temporal cortex extend from VBM studies (reviewed by (19)) to manually drawn ROI analyses on MRI (reviewed by (32)), post-mortem studies (reviewed by (47)), and genetic studies (reviewed by (48)). While found more often in MRI studies (49) than in postmortem studies (47), hippocampal volume decrease in patients is not the only region were such discrepancies exist (50), and a lack of decreased gross volume does not exclude abnormalities at the subcellular level in patients. To clarify whether diagnostic subtype affected this finding (51), we performed an analysis looking at gray matter variation in a subgroup of patients with only schizophrenia compared with those that had broader spectrum psychosis, and found no significant differences in the results, or a finding of hippocampal decrease in these subgroups (data available upon request). We also found no relationship between gray matter volume and chlorpromazine equivalent dosage (see supplemental material). It is likely that our lack of finding of hippocampal decrease is due to methodological limitations; especially considering that another overlapping analysis, using subcortical segmentation, did find hippocampal volume decreases (Goldman et al., in submission).
The most significant region in which patients had increased gray matter relative to the controls was the bilateral globus pallidus. Other regions of increased gray matter are discussed more in detail in supplemental material. Enlargement of basal ganglia volumes in patients with schizophrenia was originally described in MRI studies by DeLisi et al and Jernigan et al in 1991 (52), and may vary with antipsychotic drug treatment. This is inconclusive in post-mortem studies (53; 54). However, most MRI studies have found increased caudate volumes in patients with schizophrenia (reviewed in (32)). Some have concluded that such changes reflect an iatrogenic effect of antipsychotic treatment (55). Data from our group would suggest the same (see Goldman et al. in submission).
Gray Matter Changes Specific to Siblings
Overall, the gray matter decreases in the unaffected siblings when compared with healthy controls were small compared to differences found in their affected siblings. No regions were significantly increased or decreased (FWE). Some studies looking at genetic liability for schizophrenia have failed to find any differences in healthy relatives of patients, though their samples have been smaller (56; 57). Overall, the regions in which we see tendencies for volume decreases in unaffected siblings are qualitatively consistent with the liability literature (9; 58). For instance, findings of decreased gray matter in the superior temporal gyrus and medial frontal cortex have been found in both studies of high risk subjects that later transitioned to schizophrenia (22), unaffected siblings (59), and siblings from multiply-affected families (58). The anterior cingulate and medial frontal cortex have also been reported to be decreased in the Edinburg high risk group (20). Temporal lobe decreases have been reported from density measurements in those at risk for schizophrenia (21), as well as volume decreases in siblings with fetal hypoxia (17), the latter demonstrating possible gene-environment interactions affecting the temporal cortices. Our sibling group had medial temporal lobe decreases in the parahippocampal and hippocampal cortices. The hippocampus and hippocampalamygdala complex have been reported to be a vulnerability indicator for schizophrenia (15; 16). In addition, the insula has been reported to be decreased in subjects at high risk (22). Our unaffected siblings also had trends for decreases in the cerebellum compared to controls, similar to Job et al., 2005 (21). In general, our sibling decreases are supported by the liability literature, but should be interpreted with caution in light of the lack of significance.
There were unique gray matter volume increases that reached a trend for significance (p < .001 uncorrected, k > 5) in the right inferior frontal cortex, with clusters in BA 9 and BA 45, as well as a small region in the right parahippocampal gyrus, the right inferior temporal gyrus, and the right precuneus. In support of our findings, increases of bilateral parahippocampal gyrus volume (60) and temporal lobe volumes (though middle temporal) have been reported in unaffected relatives of schizophrenia (59). The largest increases compared with healthy controls were in BA 9 and BA 46 in the right inferior frontal cortex. These regions were also significantly increased (p < .05 FWE corrected) when compared with patients with schizophrenia, in the combined group, as well as the past history and no past history subgroups (Table 4). More regional decreases in the prefrontal cortex have been reported in siblings in one study (23), though overall frontal lobe decreases are more common (reviewed in (61)). Increased gray matter in siblings or high-risk subjects compared to healthy controls are measures not always reported in liability studies (9; 57), though Seidman et al. (1977) found increased cerebral volume in a small group of relatives (62). These increases could represent a protective or compensatory phenomenon in unaffected family members, though this is highly speculative.
Sibling and Patient overlap: ICC Analysis
Our masked analysis revealed that there were shared gray matter decreases in the left superior temporal gyrus, left medial frontal gyrus, and left insula between siblings and patients, but these were not significant. We extracted these regions and calculated ICC, but they were not significantly correlated between sibling pairs. Cortical gray matter variations in these regions, at least as measured by optimized VBM, may be only weakly related to genetic risk for schizophrenia. If the volumes of these regions are affected by genetic factors, other factors would seem to be obscuring these effects. For example, future studies could investigate other cortical regions of interest in unaffected non-twin siblings.
There is an apparent conundrum presented by the competing observations of increased gray matter volume in our three regions (SIB > SCZ) against non-significant ICC in the setting of smaller areas of shared gray matter reductions (NC > SIB, SCZ). We think this is an important issue to address, because an exploratory analysis using λs suggests significant familiality in these three regions (data upon request). Hopefully, future experiments using some combined methodological approach to heritability and a larger sample may solve this paradox.
Sibling and Patient overlap: ICC Analysis within the Hippocampus
The hippocampus had significant heritability values between sibling pairs, arguing for genetic effects on the volume of this region. Hippocampal volume changes measured with MRI are not specific to schizophrenia, as they are also seen in dementia (63; 64), depression (65; 66), and post-traumatic stress disorder (67; 68). However, several “risk” genes for schizophrenia have been shown to affect hippocampal structure (28; 30). Moreover, the hippocampus has been tagged as a key region under genetic control from various family studies (15; 25), though a recent imaging study of monozygotic twins did not find liability related density changes in the limbic cortex (13). Despite this, the significant ICC values for sibling pairs reported here may still reflect heritability of gray matter volume in the hippocampus, though we are not able to measure whether or not this heritability is related to schizophrenia and can only posit such relationship based on other studies showing similar results in twins (8; 12).
Limitations
There are limitations to VBM, including sensitivity to methodological choices in normalization, smoothing kernel and template (29; 69). In this study, we paid close attention to these issues, using a custom template which could for instance reduce ventricle-related artifact. However, the size of the smoothing kernel can affect which results reach significance, and while we attempted to choose a moderate kernel, it may have contributed to false-negatives, especially in the sibling group as the findings might be expected to be small relative those of patients verses controls. Further discussion of study limitations is included as supplemental material.
Conclusions
Our whole brain VBM study replicated previous work showing regional gray matter volume abnormalities in patients with schizophrenia compared with healthy volunteers. Furthermore, our evaluation of gray matter volume in sibling pairs demonstrated that while some areas appear heritable (e.g., the hippocampus), others do not share as much gray matter variation as one might expect (e.g., the superior temporal gyrus, medial frontal gyrus and the insula). While evidence for functional brain neuroimaging intermediate phenotypes have been found within the frontal and temporal cortices (25; 70), gray matter regional volume changes may not fully characterize the deficit to make it an intermediate phenotype for schizophrenia, or they may simply be in very specific regions. Possible explanations for this discrepancy include strong environmental factors impacting on these regional gray matter changes or the influence of compensatory mechanisms. After all, unaffected siblings, while not always free of major mental illness, remain free of schizophrenia. Their affected siblings, on the other hand, experience factors that could impact on measures of brain volume that are related to clinical state (i.e. antipsychotic medication, metabolic and nutritional factors, or smoking). Further investigation using healthy sibling pairs or twins may prove useful for the identification and clarification of structural intermediate phenotypes in schizophrenia.
Supplementary Material
Acknowledgments
We thank the staff of the Clinical Brain Disorders Branch Sibling Study of Schizophrenia and all participating families. We thank M. Weirich and staff for subject recruitment. In addition, we wish to thank Aaron Goldman and Brad Zoltick for contributions relating to the structural archive, and Joshua W. Buckholtz for helpful comments during manuscript preparation. This work was supported by grants from the NIMH intramural program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors report no competing interests.
REFERENCES
- 1.Bromet EJ, Fennig S. Epidemiology and natural history of schizophrenia. Biol Psychiatry. 1999;46:871–881. doi: 10.1016/s0006-3223(99)00153-5. [DOI] [PubMed] [Google Scholar]
- 2.Jones P, Cannon M. The new epidemiology of schizophrenia. Psychiatr Clin North Am. 1998;21:1–25. doi: 10.1016/s0193-953x(05)70358-0. [DOI] [PubMed] [Google Scholar]
- 3.Callicott JH, Weinberger DR. Neuropsychiatric dynamics: the study of mental illness using functional magnetic resonance imaging. Eur J Radiol. 1999;30:95–104. doi: 10.1016/s0720-048x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger DR, DeLisi LE, Neophytides AN, Wyatt RJ. Familial aspects of CT scan abnormalities in chronic schizophrenic patients. Psychiatry Res. 1981;4:65–71. doi: 10.1016/0165-1781(81)90009-3. [DOI] [PubMed] [Google Scholar]
- 5.Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120(Pt 2):257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- 6.Rijsdijk FV, van Haren NE, Picchioni MM, McDonald C, Toulopoulou T, Pol HE, et al. Brain MRI abnormalities in schizophrenia: same genes or same environment? Psychol Med. 2005;35:1399–1409. doi: 10.1017/S0033291705005167. [DOI] [PubMed] [Google Scholar]
- 7.Reveley AM, Reveley MA, Murray RM. Cerebral ventricular enlargement in non-genetic schizophrenia: a controlled twin study. Br J Psychiatry. 1984;144:89–93. doi: 10.1192/bjp.144.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Baare WF, van Oel CJ, Hulshoff Pol HE, Schnack HG, Durston S, Sitskoorn MM, et al. Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry. 2001;58:33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- 9.Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon TD, Thompson PM, van Erp TG, Huttunen M, Lonnqvist J, Kaprio J, et al. Mapping heritability and molecular genetic associations with cortical features using probabilistic brain atlases: methods and applications to schizophrenia. Neuroinformatics. 2006;4:5–19. doi: 10.1385/NI:4:1:5. [DOI] [PubMed] [Google Scholar]
- 11.Reveley AM, Reveley MA, Clifford CA, Murray RM. Cerebral ventricular size in twins discordant for schizophrenia. Lancet. 1982;1:540–541. doi: 10.1016/s0140-6736(82)92047-5. [DOI] [PubMed] [Google Scholar]
- 12.Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- 13.Hulshoff Pol HE, Schnack HG, Mandl RC, Brans RG, van Haren NE, Baare WF, et al. Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry. Neuroimage. 2006;31:482–488. doi: 10.1016/j.neuroimage.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 14.Dube J, Dodds L, Armson BA. Does chorionicity or zygosity predict adverse perinatal outcomes in twins? Am J Obstet Gynecol. 2002;186:579–583. doi: 10.1067/mob.2002.121721. [DOI] [PubMed] [Google Scholar]
- 15.Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, et al. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry. 2002;59:839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- 16.Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, et al. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49:811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- 17.Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59:35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 19.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 20.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64:1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 21.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, et al. Regional Gray Matter Volume Abnormalities in the At Risk Mental State. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, et al. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141:76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- 24.Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 25.Callicott JH, Egan MF, Bertolino A, Mattay VS, Langheim FJ, Frank JA, et al. Hippocampal N-acetyl aspartate in unaffected siblings of patients with schizophrenia: a possible intermediate neurobiological phenotype. Biol Psychiatry. 1998;44:941–950. doi: 10.1016/s0006-3223(98)00264-9. [DOI] [PubMed] [Google Scholar]
- 26.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 27.SPSS . SPSS Base 10.0 for Windows User's Guide. Chicago IL: 1999. SPSS Inc. [Google Scholar]
- 28.Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 30.Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 32.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasai K, Shenton ME, Salisbury DF, Onitsuka T, Toner SK, Yurgelun-Todd D, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60:1069–1077. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- 35.Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK. Mapping of grey matter changes in schizophrenia. Schizophr Res. 1999;35:1–14. doi: 10.1016/s0920-9964(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 36.Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83:155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Gaser C, Nenadic I, Buchsbaum BR, Hazlett EA, Buchsbaum MS. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am J Psychiatry. 2004;161:154–156. doi: 10.1176/appi.ajp.161.1.154. [DOI] [PubMed] [Google Scholar]
- 38.Andreasen NC, Arndt S, Swayze V, 2nd, Cizadlo T, Flaum M, O'Leary D, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 39.Bridle N, Pantelis C, Wood SJ, Coppola R, Velakoulis D, McStephen M, et al. Thalamic and caudate volumes in monozygotic twins discordantfor schizophrenia. Aust N Z J Psychiatry. 2002;36:347–354. doi: 10.1046/j.1440-1614.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 40.Deicken RF, Eliaz Y, Chosiad L, Feiwell R, Rogers L. Magnetic resonance imaging of the thalamus in male patients with schizophrenia. Schizophr Res. 2002;58:135–144. doi: 10.1016/s0920-9964(01)00330-9. [DOI] [PubMed] [Google Scholar]
- 41.Davatzikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, et al. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62:1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 42.Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, et al. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- 43.Danos P, Schmidt A, Baumann B, Bernstein HG, Northoff G, Stauch R, et al. Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: a replication study. Psychiatry Res. 2005;140:281–289. doi: 10.1016/j.pscychresns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Pearlson GD. Superior temporal gyrus and planum temporale in schizophrenia: a selective review. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1203–1229. doi: 10.1016/s0278-5846(97)00159-0. [DOI] [PubMed] [Google Scholar]
- 45.Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry. 2004;161:1121–1124. doi: 10.1176/appi.ajp.161.6.1121. [DOI] [PubMed] [Google Scholar]
- 46.Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, et al. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res. 1998;32:161–167. doi: 10.1016/s0022-3956(97)00038-1. [DOI] [PubMed] [Google Scholar]
- 47.Dwork AJ. Postmortem studies of the hippocampal formation in schizophrenia. Schizophr Bull. 1997;23:385–402. doi: 10.1093/schbul/23.3.385. [DOI] [PubMed] [Google Scholar]
- 48.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2004 doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 49.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 50.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 51.Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 52.Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:881–890. doi: 10.1001/archpsyc.1991.01810340013002. [DOI] [PubMed] [Google Scholar]
- 53.Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz U, et al. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr Res. 1990;3:295–301. doi: 10.1016/0920-9964(90)90013-w. [DOI] [PubMed] [Google Scholar]
- 54.Falke E, Han LY, Arnold SE. Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res. 2000;93:103–110. doi: 10.1016/s0165-1781(00)00104-9. [DOI] [PubMed] [Google Scholar]
- 55.Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- 56.Marcelis M, Suckling J, Woodruff P, Hofman P, Bullmore E, van Os J. Searching for a structural endophenotype in psychosis using computational morphometry. Psychiatry Res. 2003;122:153–167. doi: 10.1016/s0925-4927(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 57.McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56:544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 58.McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 59.Goghari VM, Rehm K, Carter CS, Macdonald AW., 3rd Regionally Specific Cortical Thinning and Gray Matter Abnormalities in the Healthy Relatives of Schizophrenia Patients. Cereb Cortex. 2006 doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- 60.Seidman LJ, Wencel HE. Genetically mediated brain abnormalities in schizophrenia. Curr Psychiatry Rep. 2003;5:135–144. doi: 10.1007/s11920-003-0030-4. [DOI] [PubMed] [Google Scholar]
- 61.McIntosh AM, Job D, Whalley HC, Johnstone EC, Lawrie SM. Genetic liability, brain structure and symptoms of schizophrenia. In: Gattaz WF, Hafner H, editors. Search for the causes of schizophrenia. Springer-Verlag; Berlin: 2004. [Google Scholar]
- 62.Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Matsuda G, et al. Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: a pilot magnetic resonance imaging study. Am J Med Genet. 1997;74:507–514. doi: 10.1002/(sici)1096-8628(19970919)74:5<507::aid-ajmg11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 63.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- 64.Ishii K, Sasaki H, Kono AK, Miyamoto N, Fukuda T, Mori E. Comparison of gray matter and metabolic reduction in mild Alzheimer's disease using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging. 2005;32:959–963. doi: 10.1007/s00259-004-1740-5. [DOI] [PubMed] [Google Scholar]
- 65.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 66.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 67.Hedges DW, Allen S, Tate DF, Thatcher GW, Miller MJ, Rice SA, et al. Reduced hippocampal volume in alcohol and substance naive Vietnam combat veterans with posttraumatic stress disorder. Cogn Behav Neurol. 2003;16:219–224. doi: 10.1097/00146965-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 69.Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- 70.Winterer G, Egan MF, Radler T, Hyde T, Coppola R, Weinberger DR. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr Res. 2001;49:129–143. doi: 10.1016/s0920-9964(00)00128-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.