Abstract
The signal recognition particle (SRP) plays a pivotal role in transporting proteins to cell membranes. In higher eukaryotes, SRP consists of an RNA molecule and six proteins. The largest of the SRP proteins, SRP72, was found previously to bind to the SRP RNA. A fragment of human SRP72 (72c′) bound effectively to human SRP RNA but only weakly to the similar SRP RNA of the archaeon Methanococcus jannaschii. Chimeras between the human and M. jannaschii SRP RNAs were constructed and used as substrates for 72c′. SRP RNA helical section 5e contained the 72c′ binding site. Systematic alteration within 5e revealed that the A240G and A240C changes dramatically reduced the binding of 72c′. Human SRP RNA with a single A240G change was unable to form a complex with full-length human SRP72. Two small RNA fragments, one composed of helical section 5ef, the other of section 5e, competed equally well for the binding of 72c′, demonstrating that no other regions of the SRPR RNA were required. The biochemical data completely agreed with the nucleotide conservation pattern observed across the phylogenetic spectrum. Thus, most eukaryotic SRP RNAs are likely to require for function an adenosine within their 5e motifs. The human 5ef RNA was remarkably resistant to ribonucleolytic attack suggesting that the 240-AUC-242 “loop” and its surrounding nucleotides form a peculiar compact structure recognized only by SRP72.
Keywords: RNP, protein-RNA interactions, site-directed mutagenesis
INTRODUCTION
Signal recognition particle (SRP) is a ribonucleoprotein complex that captures cytosolic ribosomes with emerging signal peptides. SRP arrests the translation of secretory proteins and directs the stalled complexes to the membrane-associated SRP receptor. Translation resumes upon the dissociation of the SRP from the membrane-bound ribosomes, and the proteins are delivered into the translocation channel in order to cross the membrane (Nagai et al. 2003; Doudna and Batey 2004; Halic and Beckmann 2005; Shan and Walter 2005).
The dumbbell-shaped mammalian SRP is composed of six proteins (named SRP9 to SRP72 according to their molecular masses) and one ∼300-nucleotide (nt) RNA molecule arranged within the small (Alu) and large (S) domains of the SRP (Walter and Blobel 1983; Andrews et al. 1987; Siegel and Walter 1988b; Zwieb et al. 2005). The SRP9/14 heterodimer and the terminal regions of the SRP RNA (see Fig. 1A, sections 5a,5b, helices 2–4) are responsible for arresting the translation of nascent polypeptide chains (Siegel and Walter 1988a). The large domain of the SRP consists of helical sections 5e, 5f, helices 6–8, and proteins SRP19, SRP54, SRP68, and SRP72. SRP19 binds at the tetranucleotide loops (tetraloops) of helices 6 and 8 and maintains the parallel arrangement of both helices (Zwieb 1992; Hainzl et al. 2002; Diener and Wilson 2000; Oubridge et al. 2002). The multifunctional protein SRP54 (named Ffh in bacteria) is a constituent of every cell and binds to helix 8 of the SRP RNA (Samuelsson 1992; Batey et al. 2000). Modulated by highly conserved nucleotides and driven by GTP-hydrolysis, SRP54 plays a prominent role in signal peptide recognition and release (Spanggord et al. 2005; Siu et al. 2007).
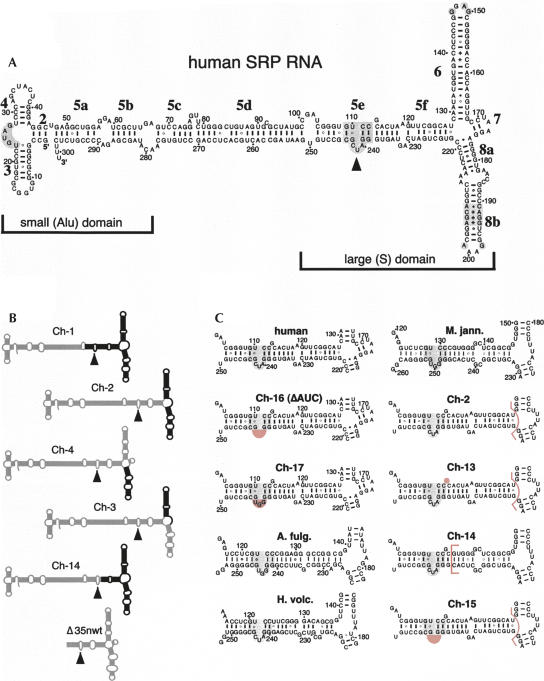
FIGURE 1.
Secondary structures of mutant SRP RNAs. (A) Secondary structure of human SRP RNA (hSR) with its small (Alu) and large (S) domains. Labeled are the 5′ and 3′ ends, the nucleotide residues in increments of 10, helices 2–8, and helical sections appended with letters a to f (Zwieb et al. 2005). Conserved regions are shaded gray and the 5e motif is marked by a solid arrowhead (Regalia et al. 2002). (B) Chimeric mutants Ch-1 to Ch-4 and Ch-14 are outlined with human SRP RNA sections in gray and Methanococcus jannaschii SRP RNA portions in black. (For details see Yin et al. 2001.) The Δ35nwt RNA corresponding to the large domain of the human SRP RNA is shown on the bottom. (C) Predicted secondary structures of the 5ef region in human SRP RNA (top, left column), Methanococcus jannaschii SRP RNA (M. jann., top, right column), the chimeric mutants Ch-2, Ch-13 to Ch-17, as well as the SRP RNAs of Archaeoglobus fulgidus (A. fulg.) and Haloferax volcanii (H. volc.). Ch-13 to Ch-15 are derivatives of Ch-2 as indicated by the pink bracket that separates the human from the M. jann. SRP RNA portions. Ch-16 (also named ΔAUC) and Ch-17 are alterations in the context of full-length human SRP RNA. The deletions of G113 in Ch-13, and of 240-AUC-242 (human SRP RNA numbering) in Ch-15 and Ch-16, as well as the AUC to GGU changes in Ch-17, are shaded pink. The 5e motif is shaded gray.
Several high-resolution structures that include portions of the SRP RNA, SRP9/14, SRP19, and SRP54 have contributed significantly to our understanding of SRP structure and function (Batey et al. 2000; Weichenrieder et al. 2000; Kuglstatter et al. 2002; Rosendal et al. 2003). However, the two largest SRP proteins, SRP68 and SRP72, are difficult to isolate and have a propensity to aggregate. Therefore, no high-resolution data are currently available and the structure and function of SRP68 and SRP72 are relatively poorly understood. As both proteins play an important role in SRP-mediated protein targeting, a better understanding of their properties is highly desirable (Siegel and Walter 1988a). In the current study, we focus on human SRP72 and its recently discovered interaction with the SRP RNA (Iakhiaeva et al. 2005).
SRP72 was found to be present both in the cytoplasm and the nucleolus of transfected rat fibroblast cells, suggesting a function in SRP assembly (Politz et al. 2000). Exposing canine SRP to high ionic strength conditions released SRP72 from the RNA as a stable heterodimer with SRP68 (Scoulica et al. 1987). Early in vitro studies carried out in reticulocyte lysates suggested that SRP72 interacted with the RNA-bound SRP68 only through protein–protein interactions (Lütcke et al. 1993). More recently, however, human SRP72 was shown to bind to the SRP RNA independently of SRP68 or any other SRP proteins via a 56 amino acid residue domain (Iakhiaeva et al. 2005).
Enzymatic and chemical footprinting studies (Siegel and Walter 1988b; Menichelli et al. 2007) as well as cryoelectron microscopy of canine SRP bound to wheat germ ribosomes (Halic et al. 2004) revealed that SRP68/72 was part of the large domain of the SRP near the junction of helices 5, 6, and 8 (Zwieb et al. 2005). In agreement with this assertion, systematic site-directed mutagenesis of human SRP RNA showed that several nucleotide residues distributed throughout the large domain were required for the binding of SRP68/72. In contrast, the binding of SRP72 appeared more narrowly affected by alterations in SRP RNA helix 5 (Yin et al. 2007).
Our goal was to identify systematically and more precisely the residues within the large domain of human SRP RNA that were required for the binding of SRP72. We exploited the fortuitous observation that recombinant human SRP72 formed stable complexes with human SRP RNA, but associated only poorly with the similarly structured SRP RNA of the archaeon Methanococcus jannaschii. Chimeras between the human and the M. jannaschii SRP RNAs were used as substrates to demonstrate that the RNA-binding fragment of SRP72 bound preferentially to helical section 5e. Systematic site-directed mutagenesis within the 5e region revealed that SRP72 bound to a previously identified conserved motif of unknown function (Regalia et al. 2002). Here, we found that a single residue (A240 in human SRP RNA) situated within the 5e motif played a crucial role in the binding to SRP72. The conservation of the adenosine in the eukaryotic, but not the archaeal SRP RNAs, suggests that the 5e motif was recruited in the eukaryotes to bind to SRP72 in order to function in SRP transport, SRP assembly, and/or in modulating the shape of the SRP as the particle passes through the free, ribosome-bound, and membrane-bound stages.
RESULTS
A binding site for 72c′ within the large domain of human SRP RNA
For assessing the binding of SRP72 to human SRP RNA, we purified a recombinant polypeptide 72c′ that corresponded to amino acid residues 447–617 of human SRP72 and was shown previously to effectively form complexes with the SRP RNA (Fig. 2A; Iakhiaeva et al. 2005). In anticipation of solubility problems with the 72c′ polypeptide, we developed a method that used Ni-NTA paramagnetic agarose beads. This approach was a more flexible and speedier alternative to sucrose gradient centrifugation (Iakhiaeva et al. 2005). It also provided higher specificity and better reproducibility than the previously-employed double-filter binding assays (Yin et al. 2007). The three-component magnetic bead assay contained in vitro transcribed SRP RNA, N-terminally his-tagged human protein SRP19, and purified 72c′ polypeptide (Fig. 2B). Wild-type and mutant RNAs were synthesized by run-off transcription with T7 RNA polymerase, and the polypeptides were expressed and purified as described in Materials and Methods. The high affinity of his-tagged SRP19 (19-his) for the SRP RNA allowed us to prime the beads with any of the SRP RNA fragments that were capable of forming a complex with 19-his (Zwieb 1994; Henry et al. 1997). Binding of 72c′ to SRP RNA was observed by its association with the primed beads after separation in the magnetic field. The bound material was removed by incubating the beads with a buffer containing 250 mM imidazol followed by SDS-PAGE of the dissociated proteins and RNAs (see Materials and Methods).
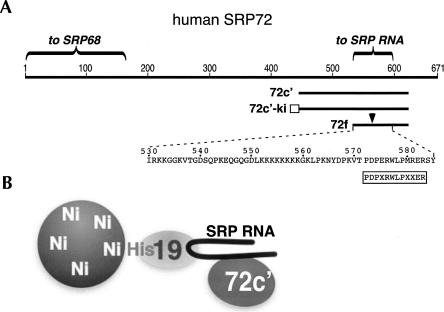
FIGURE 2.
Binding of human SRP72 fragments to SRP RNA. (A) Linear representation of human SRP72. Amino acid residues are numbered. Shown are the N-terminal region involved in the binding to SRP68 and the recently identified RNA binding domain near the C terminus. The SRP72 fragments 72c′, 72c′-ki, and 72f, described by Iakhiaeva et al. (2006) and in this publication, are labeled. The arrow indicates a region that is hypersensitive toward digestion by trypsin. The rectangle indicates a region in 72c′-ki used for radioactive labeling. The boxed sequence shows the Pfam motif (Andersen et al. 2006); X is for any amino acid residue. (B) Schematic representation of the three-component Ni-NTA paramagnetic bead assay showing his-tagged protein SRP19 bound to beads and RNA. Only RNA-bound 72c′ associates with the beads.
We observed that 19-his bound to the beads as expected (Fig. 3A). 72c′ bound only when human SRP RNA (hR) was also present but not when hR was replaced with tRNA (Fig. 3A, lanes 2,3). Visual inspection of the Coomassie blue- and ethidium bromide-stained gel (Materials and Methods) confirmed that hR bound to the beads through its association with 19-his. Only 19-his and very small amounts of bound RNA were observed in reactions containing the control tRNA, most likely because tRNA did not bind to 19-his and/or was removed during the washing of the beads (Fig. 3A, lanes 2 and 8).
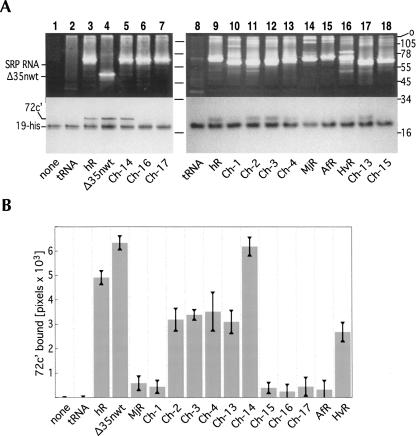
FIGURE 3.
Binding of fragment 72c′ (19.2 kDa, amino acid residues at positions 447–617 of human SRP72) to different RNAs in the presence of his-tagged human SRP19 (19-his). (A) 72c′-RNA complexes were incubated with RNA, 19-his, and Ni-NTA magnetic agarose beads. The bead-bound polypeptides and RNAs were analyzed by SDS-PAGE on 12.5% gels in Tris-tricine. Molecular mass markers in kDa are shown on the right. The top portion of the gels are shown stained with ethidium bromide indicating the positions of the full-length SRP RNAs and the Δ35nwt RNA; the bottom portions of the same gels show the Coomassie blue stained polypeptides 72c′ and 19-his. Due to the basic character of 72c′ (pKi 10.88) the polypeptide migrates considerably slower than what might be expected from the molecular weight. The various RNAs are indicated on the bottom of each panel including human SRP RNA (hSR), chimeras Ch-1 to Ch-4, Ch13 to Ch-17, as well as the SRP RNAs of Methanococcus jannaschii [MjR (31)], Archaeoglobus fulgidus (AfR), and Haloferax volcanii (HvR). (B) Quantitative analysis of 72c′ bound to the various RNAs. The standard errors of the mean are indicated by the vertical bars.
In preliminary experiments, 72c′ bound effectively not only to full-length hR, but also to the Δ35nwt RNA that corresponded to the large SRP domain (Fig. 3A, lane 4). (An outline of Δ35nwt secondary structure is shown in Fig. 1B.) This result agreed with previous data showing that a fragment of SRP72 containing the amino acid residues 530–586 of SRP72 bound to the RNA from the large SRP domain, but not to an RNA that lacked the large domain (Iakhiaeva et al. 2005).
Binding of 72c′ to chimeric SRP RNAs
Poor binding was observed with the in vitro synthesized SRP RNAs of Methanococcus jannaschii (MjR) and Archaeoglobus fulgidus (AfR) (Fig. 3B). This was surprising as the secondary structures of the archaeal and mammalian SRP RNAs were found to be very similar (Larsen and Zwieb 1991; Zwieb et al. 2005) and likely to be functionally interchangeable. Lack of binding could not be attributed to disrupted SRP19-RNA interactions as MjR and AfR were known to bind to human SRP19 with high affinity (Bhuiyan et al. 2000). Indeed, inspection of the ethidium bromide-stained SDS polyacrylamide gels showed that the beads had been properly primed with the two archaeal SRP RNAs (Fig. 3A, lanes 14,15). We concluded that subtle differences between the human, MjR, and AfR SRP RNAs were responsible for the dissimilar 72c′ binding activities.
The poor binding of MjR to 72c′ provided an opportunity to identify the site(s) in human SRP RNA responsible for the binding to SRP72. Our aim was to construct and analyze chimeric SRP RNAs with various regions exchanged between the human and M. jannaschii sequences (Fig. 1B). A similar approach was taken in a preceding study to identify the residues in helix 8 of human SRP RNA that were required for the SRP19-dependent binding of SRP54 (Yin et al. 2004). The availability of the chimeric RNAs Ch-1 to Ch-4 allowed us to quickly determine which of the helices in human SRP RNA contributed significantly to the binding of 72c′. As demonstrated by their association with the magnetic beads, Ch-1 to Ch-4 bound to 19-his, indicating that functional molecules had been synthesized (Fig. 3A, lanes 10–13). Binding of Ch-2, Ch-3, and Ch-4 to 72c′ was reduced only slightly when compared to hR. The Ch-1 RNA bound to 72c′ at the low level observed with the inactive MjR (Fig. 3B). We also observed pronounced binding of 72c′ to the chimeric mutant RNA Ch-14 containing an MjR-like helical section 5f in the context of Ch-2 (see Figs. 1, 3). These data suggested that the region corresponding to helical section 5e was responsible for the ability of the SRP RNA to bind to 72c′.
The looped-out guanosine (G113 in human SRP RNA), situated close to the 11-nt 5e motif, appeared to be a significant distinction between hR and MjR. This residue was absent in the inactive MjR, AfR, and Ch-1 RNAs (Fig. 1C). Thus, we speculated that G113 might play a role in the recognition of 72c′. However, Ch-13, a mutant RNA that lacked the guanosine, effectively formed complexes with 72c′(Figs. 1, 3).
Next, we considered the 240-AUC-242 loop located within the 5e motif. Deleting the loop from Ch-2 in the chimeric mutant RNA Ch15 or from hR in Ch16 completely abolished binding of the 72c′ fragment. The Ch-17 mutant RNA, in which 240-AUC-242 was replaced with the 249-GGU-251 triplet of MjR, was also inactive, suggesting a sequence-specific recognition of the trinucleotide by 72c′. Furthermore, the SRP RNA of Haloferax volcanii (HvR) that contained an AUC loop bound significant amounts of 72c′ (Fig. 3B). We concluded that 240-AUC-242, or residues within this trinucleotide, were responsible for the formation of a complex with 72c′.
Systematic alterations of 240-AUC-242
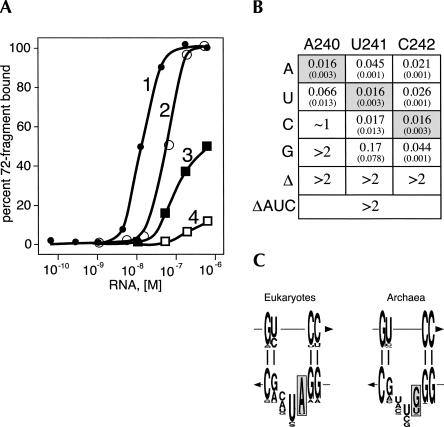
In further dissecting the function of the 240-AUC-242 trinucleotide loop, individual nucleotides were changed by PCR site-directed mutagenesis and the mutant RNAs were synthesized by run-off transcription of BamHI-digested plasmids using T7 RNA polymerase. To determine quantitatively the binding affinities of the mutant RNAs, we modified the 72c′ expression plasmid to encode a polypeptide with an N-terminal peptide tag containing the sequence MALRRASAVEL. When incubated with protein kinase and γ-32P-labeled ATP, the 72c′-ki fusion protein was radioactively labeled at the serine of the tag. Paramagnetic Ni-NTA agarose beads were primed with 19-his and incubated with increasing concentrations of hR while maintaining a fixed amount of radioactive peptide until the binding was saturated. The phosphorylated peptide remained stable throughout the binding procedure as monitored by SDS-PAGE of aliquots on a 20% polyacrylamide gel (not shown). The amount of radioactivity, both free and bead-bound, was measured in a scintillation counter, and the apparent association constant K′a was calculated by determining the concentration of unbound RNA required to bind 50% of the labeled 72c′-ki. Assuming the binding of one molecule of RNA to one molecule of 72c′-ki the value of K′a for the interaction between the peptide and wild-type human SRP RNA was determined to be ∼1.6 × 108 M−1. With the exception of the U241C change, significantly weaker binding was observed with the altered RNAs (Fig. 4B). Deleting A240, U241, or C242 reduced binding to concentrations (>2 mM) that were outside the range of the assay. Similar effects were caused by the A240G transition and, to a lesser extent, by the A240C transversion. Overall, changes at positions 241 and 242 were less detrimental than alterations of A240. These data highlighted a prominent role for A240 in the binding to 72c′-ki.
FIGURE 4.
Effects of the mutations within the 5e motif on the binding of 32P-labeled 72c′-ki. (A) Titration of radioactive 72c′-ki (0.5 nM) with human SRP RNA using purified his-tagged SRP19 and Ni-NTA magnetic beads: (1) wild-type human SRP RNA; (2) A240U mutant RNA; (3) A240C mutant RNA; (4) A240G mutant RNA. (B) Summary of the effects of the nucleotide substitutions on the apparent association constant (in 106 M−1). Numbers in parentheses denote measurement errors. (C) Structure logos of the 5e motif in eukaryotes and archaea. The height of each letter is proportional to the residue frequency (47). The arrows indicate the 5′ to 3′-directions of the RNA chains. The conserved adenosine (A240 in human SRP RNA) and its corresponding nucleotides in the archaeal SRP RNAs are highlighted in gray.
Comparative analysis of the 5e motif
Originally, the 11-nt 5e motif had been identified by comparative sequence analysis as one of the conserved features of the eukaryotic and archaeal SRP RNAs (Regalia et al. 2002). We recalculated the nucleotide frequencies in both phylogenetic groups by generating updated representative alignments from the SRP RNA sequences available at the RNP database (http://rnp.uthct.edu). The comparison of the eukaryotic (141 sequences) and archaeal SRP RNA (28 sequences) alignments revealed a conservation of A240 in the eukaryotic SRP RNAs. In a small number of sequences, this adenosine was changed to uracil but not to other nucleotides (Fig. 4C). Interestingly, the A240U alteration had the least dramatic effect on the binding of 72c′-ki (Fig. 4B). In the archaeal sequences, only HvR possessed an adenosine at the first position of an AUC trinucleotide loop. In agreement with the biochemical binding data, the HvR sequence provided fortuitous evidence for the importance of the AUC triplet (Fig. 3). Moreover, the nucleotide frequency observed by comparative sequence analysis correlated with the experimentally observed effects of the 5e loop mutations on the binding of 72c′ and 72c′-ki.
Effect of A240G on the binding of full-length human SRP72
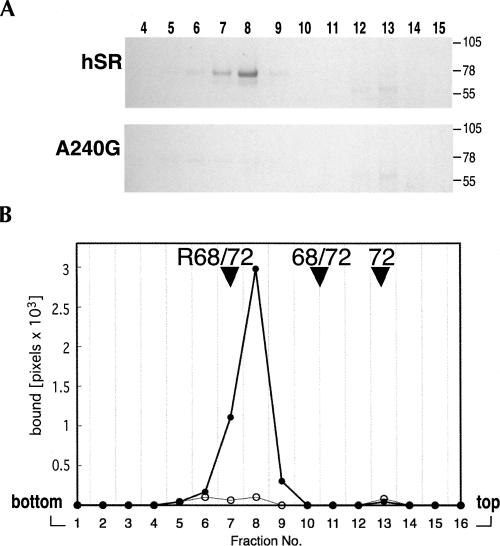
We investigated the possibility that the A240 mutation affected the binding of the 72c′ fragment but not of full-length SRP72. An N-terminally his-tagged version of full-length human SRP72 (72-his) was isolated and incubated with wild-type hR or the A240G mutant RNA. Complexes were loaded on top of 10%–40% sucrose gradients and subjected to centrifugation. Aliquots of the gradient fraction were analyzed by SDS-PAGE and the polypeptides were stained with Coomassie blue. A distinct complex was observed in fractions with wild-type hR (Fig. 5, fractions 7,8). In contrast, only minuscule amounts of complex were recovered with the A240G mutant RNA. Comparing the mobility of the hR/72-his complex with complexes of known stoichiometry suggested that one molecule of hR was bound to one 72-his molecule (Fig. 5B).
FIGURE 5.
Separation of SRP72 complexes by sucrose gradient centrifugation. (A) Equal amounts of full-length human SRP72 containing an N-terminal his-tag (72-his) were incubated with wild-type or the A240G mutant RNA and loaded on top of 10%–40% sucrose gradients. Polypeptides in the fractions (numbered on top) were separated by SDS-PAGE on 8% gels in Tris-tricine and stained with Coomassie blue. Molecular mass markers in kDa are indicated on the right side of each panel. (B) Graphical representation of the data derived from the scanned gel. Filled circles: human SRP RNA; open circles: A240G mutant SRP RNA. The arrowheads point to the tops of the peaks obtained with the hSR-SRP68/72 complex (R68/72), the free SRP68/72 heterodimer (68/72), and free SRP72 (72). In the absence of an active RNA only small amounts of 72-his were recovered in distinct fractions due to the tendency of the protein to aggregate (Iakhiaeva et al. 2006).
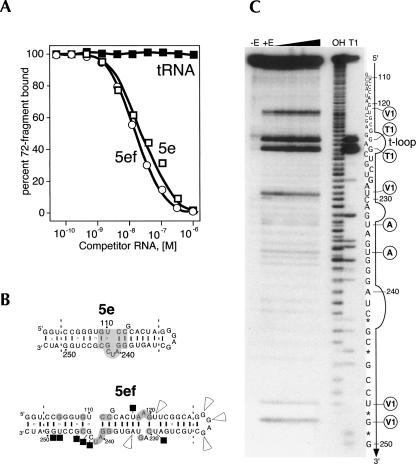
Binding activity of helical sections 5e and 5ef
The specificity of the interaction between 72c′-ki and the SRP RNA was assessed further in competition experiments with two small RNA hairpins expected to fold into structures representing helical sections 5ef and 5e with a GGAG tetraloop (Fig. 6B). Using radioactive 72c′-ki and unlabeled Δ35nwt RNA as the active components, the 5ef and 5e RNAs competed successfully and specifically at molar concentrations similar to those observed with Δ35nwt RNA or hR (Fig. 6A). No significant differences between 5ef and 5e were observed with respect to their effectiveness as competitors, suggesting that the 5e region, containing at its center an 11-nt motif, was sufficient to bind 72c′-ki with high affinity. No competition was observed with tRNA or 5S rRNA at the ∼200-fold higher concentrations needed to reduce the hR binding by 50%.
FIGURE 6.
Competition experiments and structure probing. (A) Binding of radioactive 72c′-ki (0.5 nM) to Δ35nwt RNA (50 nM) in the presence of unlabeled 5ef RNA (open circles), 5e RNA (open squares), or E. coli tRNA (black solid squares) as competitors. (B) Assumed secondary structures of the 5e and 5ef RNAs. The 5e motif is shown on a gray background. The dashed lines subsume nucleotides that are specific to the human SRP RNA and are numbered accordingly. Conserved residues in 5ef are shown on a gray background. The black squares mark RNase protection by 72f. Arrowheads indicate enhanced ribonucleolytic cleavages. (C) RNA structure probing. 5ef RNA was transcribed in vitro, radioactively labeled at the 3′-end by incubation with 32P-pCp and T4 RNA ligase and subjected to mild digestions with RNases A, T1, and V1. 5ef RNA was held constant at 1 μM, and the 72f:RNA ratio in the analyzed complexes was increased from 0.1 to 4 as indicated by the wedge. The fragments were separated on a 15% polyacrylamide gel. Lanes are labeled for no enzyme (-E), mixture of RNases added (+E), ladder obtained by alkaline digestion (OH), and partial digestion with RNase T1 (T1). The sequence is numbered in reference to the human SRP RNA. Stars mark bands generated by fragments containing cyclic phosphates. The RNase T1-accessible tetranucleotide loop (t-loop) is indicated. Sites of prominent ribonucleolytic attack are labeled with circled letters A, T1, or V1 for the RNase that cleaved this residue.
Structure probing of the 5ef RNA hairpin
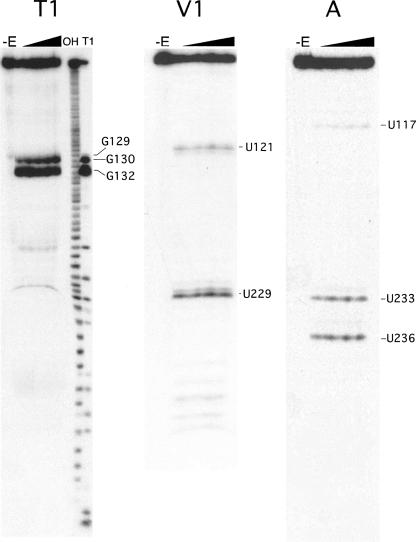
To determine the surface-accessible nucleotide residues in the 5ef hairpin, the 3′-end of the in vitro-transcribed 5ef RNA was labeled with 32P. The radioactive RNA was used as a substrate for partial digestions with a battery of single-strand and double-strand specific ribonucleases followed by the separation of the RNA fragments on denaturing polyacrylamide gels as described in Materials and Methods. Enzymatic probing revealed that the 5ef RNA was remarkably resistant toward digestions by RNase T1, A, T2, S1, and even the double-strand specific RNase V1 (examples are shown in Fig. 7). In particular, the 231-GA-232 and 240-AUC-242 “loops” were cleaved at unexpectedly low levels. Only the residues within the GGAG-tetraloop of the hairpin were prominently digested by RNases T1, T2, and S1. No significant changes in the nucleotide exposure of the 5ef RNA internal loops were observed at urea concentrations of up to 4 M in conditions that disrupted tertiary interaction in tRNA (not shown). Consistent with the predicted secondary structure, residues U121, C229, U248, and G249 were weak substrates for RNase V1, whereas residues U233 and U236 were subject to mild attacks by RNase A (Fig. 6B,C). The weakly susceptible residues tended to form two clusters of conserved nucleotides (Fig. 6B, shaded gray). The double-strand specific RNase V1 cleaved only near the apex of the hairpin (Fig. 6B, residues U121,U229) and close to the paired termini (Fig. 6B, residues U248,G249), but not within the central region of the 5ef RNA. These results suggested that the central portion of 5ef formed an unknown structure that was neither double-stranded nor single-stranded but nevertheless accessible to SRP72.
FIGURE 7.
Enzymatic probing of 5ef RNA bound to the 72f polypeptide. Examples of sequencing gels displaying partial digests of 32P-labeled 5ef RNA-72f polypeptide complexes with increasing concentrations of RNases A, T1, and V1 as indicated by the wedges. The peptide:RNA ratio was 2:1. Ten-microliter reaction mixtures contained 0.01, 0.02, 0.04 and 0.05 units of RNase T1, 0.05, 0.1, 0.15, and 0.2 units of RNase V1, or 0.00001, 0.0001, 0.0005, and 0.001 units of RNase A.
Despite the relatively poor sensitivity of the 5ef hairpin stem toward numerous ribonucleolytic probes, we identified several nucleotides that were protected or became more accessible upon binding of protein. In order to limit any potential interference by extraneous regions of SRP72, we chose a short fragment of human SRP72 (see Fig. 2A, human SRP72, 72f, positions 539–617) that still contained the RNA binding region (Iakhiaeva et al. 2005). The RNase protection experiments were carried out with complexes formed by mixing 32P-labeled 5ef RNA with increasing amounts of purified 72f. Samples were incubated at different temperatures (4°C or 22°C) and ionic conditions (100 mM or 300 mM sodium acetate). The intensities of the radioactive bands on the footprinting polyacrylamide gels were recorded as described in Materials and Methods. Similar changes in the band intensities were observed under all conditions but were most pronounced at 22°C in a buffer containing 100 mM potassium acetate. Residues U117, U229, C242, G243, G245, U248, and C249 located at or near the two internal loops of the 5ef hairpin became less accessible upon the addition of 72f, suggesting that 72f bound prominently in the 242–249 region (Fig. 6B). Enhanced digestion by RNase V1 was observed at residues U121 and A234. Also, the three guanosine residues within the GGAG tetraloop were cleaved more prominently in the presence of 72f. These enhancements indicated that conformational changes had occurred in the 5f region upon the binding of 72f.
DISCUSSION
A more versatile magnetic bead assay for studying protein–RNA interactions overcame the numerous problems associated with determining the SRP72 binding site in the human SRP RNA. First, we used chimeric RNAs to identify helical section 5e as the major determinant. Next, the deletion of 240-AUC-242 was found to completely abolish binding, suggesting a role of the 11-nt 5e motif (Regalia et al. 2002). Finally, A240 was shown to be crucial for the formation of stable complexes with SRP72.
The comparison of eukaryotic and archaeal SRP RNAs highlighted the conservation of A240 in eukaryotes (Fig. 4C). The importance of this adenosine for binding to SRP72 was not immediately apparent because the archaeon Haloferax volcanii contained an AUC triplet within its 5e motif despite the lack of an H. volcanii SRP72 gene (Zwieb and Eichler 2002). A renewed extensive search of the completed H. volcanii genome did not reveal an SRP72 homolog (Schneider et al. 2006). Interestingly, HvR bound to the 72c′ fragment of human SRP72 at levels observed with some of the active chimeric SRP RNAs (Fig. 3B), and, when analyzed by sucrose gradient centrifugation, HvR formed stable complexes with human SRP72 (data not shown). Typically, however, adenosine was underrepresented in the archaeal 5e motif, and, presumably because the archaea lacked SRP72, the first residue of the triplet was free to be a guanosine or a uracil residue.
We do not know why a cytosine at the first position of the trinucleotide loop was not observed either in the eukaryotic or the archaeal sequences. We suggest that such a change would disrupt the structure of 5e motif and effectively render the site nonfunctional. Because of the overall conservation of the 5e motif in archaea, this region might take part in yet another function that is different from binding to SRP72.
RNA fragments 5ef and 5e competed about equally well with hR or the Δ35nwt RNA, demonstrating that the 34-nt residues corresponding to the 5e region were sufficient to bind to 72c′-ki with high affinity. These results indicated that the 5e region bound independently of the neighboring regions of the SRP RNA. Although we did not attempt to reduce the size of the SRP72 binding site even further, is seems reasonable that an RNA smaller than 5e might still be capable to compete. We also refrained from introducing changes that disrupted the four base pairs of the 11-nt 5e motif because these pairings were maintained in both the eukaryotic and archaeal SRP RNAs.
The footprinting results were difficult to interpret because of the unusually high resistance of the 5ef hairpin stem toward ribonucleolytic probes (Fig. 7). Initially, the data seemed to indicate that 72f bound throughout the entire 5ef region. However, because the 5ef and the shorter 5e hairpin competed equally well with the Δ35nwt RNA (Fig. 6A), the protections observed at residues 117 and 229 were more likely the result of steric hindrance despite the use of the relatively small 72f polypeptide. Furthermore, enhancements were seen only in 5f, suggesting that 72f bound at or near 5e and increased the accessibility of the neighboring 5f region.
The 5e motif was initially discovered in an alignment of eukaryotic SRP RNAs (Regalia et al. 2002) as one of four conserved SRP RNA elements (Fig. 1A, shown in gray). Our results suggest that, like other conserved SRP RNA sites, the 5e motif plays a role in the binding to the SRP proteins, most likely during SRP assembly. The protections and enhancements observed in helical section 5ef were consistent with the possibility that SRP72 bound at or near the 5e region and exposed sites at the neighboring 5f region to promote the binding of another protein to the SRP RNA, perhaps SRP68 as supported by the work of Menichelli et al. (2007). As another possibility, SRP72 might approach the RNA as a heterodimer already bound to SRP68 and then contribute to the overall affinity of SRP68/72 by making additional contacts with 5e. In both cases, SRP19 is likely to initiate eukaryotic SRP assembly by preparing the SRP68/72 binding sites because only unspecific protections by SRP68/72 were observed in the absence of SRP19 (Menichelli et al. 2007). SRP72, using section 5e of the SRP RNA as a “foothold,” might also stabilize the 90° angle observed by cryo-electron microscopy (Halic et al. 2004) at a hinge region involving nucleotides at positions 100 and 250 (Fig. 1A, indicated as “h”). This theoretical possibility would explain the conservation of the 5e motif in the archaea SRPs where SRP68 and SRP72 are absent.
MATERIALS AND METHODS
In vitro synthesis of human and archaeal SRP RNAs
Human SRP RNA (hR) was synthesized by run-off transcription of DraI-restricted phR with T7 RNA polymerase as described (Zwieb 1991). The Δ35nwt RNA corresponded to the large domain of human SRP RNA (nucleotide residues at positions 100–253) and produced a 154-nt RNA by run-off transcription of the BamHI-restricted plasmid (Yin et al. 2007). The synthesis of the SRP RNAs of Methanococcus jannaschii (MjR) and Archaeoglobus fulgidus (AfR) has been described (Bhuiyan et al. 2000). SRP RNA of Haloferax volcanii (HvR) was synthesized as previously reported (Tozik et al. 2002).
Preparation of chimeric SRP RNAs
The design of chimeras between the human and M. jannashii SRP RNAs Ch-1 to Ch-4 has been described (Yin et al. 2004). For each of the chimeric constructs Ch-13 to Ch-17, two PCR reactions were carried out with the appropriate mutagenic primers and flanking primers CCATGATTACGAATTCTAATACGACTC and GTGCTGCAAGGCGATTAAGTTGGGTAA in a rapid cycler (Idaho Technology) for 35 cycles at an annealing temperature of 40°C. The PCR products were mixed to amplify the full-length gene using only the flanking primers. The amplified DNAs were digested with EcoRI and BamHI, ligated to purified Δ35 vector (Zwieb 1991) that had been restricted with EcoRI and BamHI, followed by screening of the transformants, restriction mapping, and sequence verification. Identical amplification conditions were employed for the construction of Ch-13 to Ch-17 using appropriate primers and pCh-2 or phR as templates.
Changes at positions 240–242 of human SRP RNA
Alterations in the phR plasmid were made by two-step PCR site-directed mutagenesis as described for the construction of the chimeric mutants Ch-13 to Ch-17 above.
Synthesis 5ef and 5e RNAs
The appropriate complementary oligonucleotides were annealed and ligated to agarose gel-purified Δ35 vector that had been restricted by run-off transcriptions with T7 RNA polymerase as described above for the full-length hR.
Preparation of his-tagged human SRP19 and SRP72
For the purification of human SRP19 with an N-terminal addition of six histidine residues, E. coli Rosetta pLysS cells were transformed with pET-His6-SRP19 (Henry et al. 1997) and grown overnight on LB plates containing ampicillin and chloramphenicol. Several colonies were used to inoculate 50 mL of LB medium containing antibiotics followed by shaking at 37°C. At an A600 of 0.2, IPTG was added to a final concentration of 1 mM and the incubation was continued overnight at room temperature. Cells were harvested by centrifugation and the pellet was resuspended in 12 mL of ice-cold lysis buffer (50 mM sodium phosphate pH 7.5, 500 mM sodium chloride, 10 mM imidazol, 1 mM 2-mercaptoethanol) and frozen at –70°C. One milliliter of the frozen cell suspension was thawed on ice, mixed with 1 mL of lysis buffer and sonicated six times for 15 sec using a model 300 dismembrator (Fisher Scientific) at a setting of 35% with 1 min pauses while the sample was placed on ice. The lysate was subjected to centrifugation in a microfuge for 20 min and the supernatant was added to 200 μL of Ni-NTA Superflow beads (Quiagen) that had been pre-equilibrated in lysis buffer. The sample was mixed by slow rotation for 1 h at 4°C and transferred to 0.5 mL mini-columns (Evergreen Scientific). After the columns had settled by gravity flow, the beads were washed with 1 mL of lysis buffer. His-tagged SRP19 was eluted with 200 μL of 50 mM sodium phosphate pH 7.5, 500 mM sodium chloride, 1 mM 2-mercaptoethanol containing 150 mM imidazole. The protein was stored at 4°C and used within 2 wk.
A bacterial expression plasmid encoding an N-terminally his-tagged version of full-length human SRP72 was assembled and the induction and purification of 72-his were carried out as described for SRP72 (Iakhiaeva et al. 2005).
Purification and radioactive labeling of tagged SRP72c′ (72c′-ki)
The construction of the bacterial expression plasmid for the synthesis and purification of an RNA binding fragment of SRP72 (72c′, amino acid residues 447–617) has been described (Iakhiaeva et al. 2005). To attach an N-terminal tag suitable for the radioactive labeling using protein kinase, the DNA of p72c′ was digested with NcoI and ligated to annealed oligonucleotides CATGGCCCTCCGGCGGGCCTCTGCAGTCGAGCT and CATGAGCTCGACTGCAGAGGCCCGCCGGAGGGC. Samples were processed as described above, and the sequence of p72c′-ki was confirmed using a commercial provider. The 72c′-ki polypeptide was purified essentially as 72c′, described above.
A 30-μL protein labeling reaction was carried out in 20 mM HEPES at pH 7.9, 5mM DTT, 10 mM MgCl2 containing 0.5 μg of purified 72c′-ki polypeptide, 40 units of the catalytic subunit of protein kinase A from bovine heart (Sigma), and γ-32P-ATP (3000 Ci/mmol, PE Biosystems). The sample was incubated at room temperature for 30 min and stored at –70°C. More than 50% of the radioactivity was incorporated as determined by SDS-PAGE followed by autoradiography and Cerenkov radiation counting in a Beckman Coulter LS6500 Multi-Purpose Scintillation Counter.
Purification of 72f
The plasmid for the expression of a peptide composed of amino acid residues 529–617 of human SRP72 (72f, 89 amino acids, 9.9 KDa) was constructed by ligating NcoI-BstXI restricted vector 72c′ to an NcoI-BstXI restriction fragment of plasmid 72d (Iakhiaeva et al. 2005). Samples were processed and the protein was purified essentially as described above.
Three-component magnetic bead assays
To measure binding of 72c′ to its target, the RNAs (2 μM) were mixed at room temperature with purified his-tagged human SRP19 (1.5 μM) in binding buffer (100 μL of 50 mM sodium phosphate at pH 7.5, 300 mM potassium acetate, 5 mM MgCl2, 1 mM 2-mercaptoethanol, 0.05% Tween 20, 10 mM imidazol, and 10% glycerol) and incubated for 5 min at room temperature. Purified 72c′ (2 μM) was added, the samples were mixed gently with the pipette tip and incubated at 37°C for 10 min. Fifteen microliters of a 5% suspension of Ni-NTA magnetic agarose beads (Quiagen) were added with a yellow tip that had been cut with a razor blade to enlarge the diameter of the opening. The samples were incubated at room temperature for 1 h with mixing by pipetting five times every 15 min. The beads were concentrated for ∼30 sec in a magnetic separator (Invitrogen) and the unbound material was removed. The beads were washed by adding 50 μL binding buffer and pipetting up and down four times followed by separation in the magnetic field. The wash solution was removed and the bead-bound material was eluted by the addition of 15 μL of 50 mM sodium phosphate at pH 8.0, 300 mM sodium chloride, 250 mM imidazol, 0.05% Tween 20, followed by magnetic separation. Fifteen microliters of the sample were removed from the beads, mixed with 6 μL twice-concentrated SDS loading buffer (100 mM Tris-HCl at pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% glycerol) and analyzed by electrophoresis on 12.5% polyacrylamide Tricine-SDS gels, followed by staining of the polypeptides with Coomassie blue. A picture was taken with a digital camera and the number of pixels in each band was measured using NIH Image (available at http://rsb.info.nih.gov/nih-image/). The gel was stained with 0.1 μg/mL ethidium bromide for visualization of the RNA. After ∼2 min the gel was placed onto a UV-transilluminator (Bio-Rad) and a picture was recorded.
To determine the binding affinities, 15 μL aliquots of the magnetic bead suspension were mixed at room temperature in 50 μL binding buffer (see above) with His-tagged SRP19 (1 μM) and increasing amounts of the wild-type or mutant RNAs. Samples were incubated on ice for 1 h with mixing by pipetting five times every 15 min. The unbound material was removed using a magnetic separator, the beads were washed with 50 μL binding buffer, and 2000–5000 cpm (Cerenkov) of 32P-labeled Δ72c′-ki polypeptide was added to the washed beads in 50 μL binding buffer, followed by 1 h incubation on ice with mixing by pipetting five times every 15 min. Unbound material was removed and bound molecules was eluted with 50 μL of 50 mM sodium phosphate at pH 8.0, 300 mM sodium chloride, 250 mM imidazol, and 0.05% Tween 20. The radioactivity levels in the samples were measured by Cerencov radiation counting in 1.5 mL reaction tubes inserted into a standard glass scintillation vial using a Beckman Coulter LS6500 scintillation counter.
For the competition experiments, the magnetic beads suspension was primed with 1 nM of His-tagged SRP19 and 0.05 μM of Δ35nwt RNA or full-length human SRP RNA followed by removal of the unbound material in the magnetic field. 32P-labeled Δ72c′-ki peptide was mixed with increasing amounts of the various competitor RNAs, incubated with the beads for 1 h on ice and processed as described above.
Isolation of SRP72-RNA complexes by sucrose gradient centrifugation
Human SRP72 with an N-terminal 6xHis tag was expressed and purified as described (Iakhiaeva et al. 2006). The protein was mixed with RNA (1 μM) in 200 μL of 50 mM Tris-HCl at pH 7.9, 300 mM potassium acetate, 5 mM MgCl2, 1 mM DTT, incubated for 10 min at 37°C, and loaded on top of a 10%–40% sucrose gradient prepared in 50 mM sodium phosphate at pH 7.5, 150 mM sodium chloride, 5 mM MgCl2, and 1 mM DTT at 4°C. After centrifugation (Beckman NVT65 at 55,000 rpm for 4.5 h at 4°C) 16 gradient fractions were collected. The main portion of each fraction was precipitated by trichloroacetic acid and analyzed by SDS-PAGE on 8% gels in Tris-tricine. To determine the distribution of the RNA, 10 μL aliquots of the sucrose gradient fractions were analyzed by electrophoresis in 2% agarose gels followed by staining with ethidium bromide.
Enzymatic footprinting of the 5ef RNA and its complex with the 72f polypeptide
Conditions for partial nuclease digestions of 5ef RNA in the absence or presence of 72f polypeptide were adopted from Christiansen et al. (1990). The ribonucleoprotein complexes were formed in 20 mM Tris-HCl at pH 7.5, 5 mM MgCl2, 1 mM DTT buffer containing either 100 mM or 300 mM potassium acetate. Footprinting experiments were conducted with 1 μM of the 5e RNA at protein:RNA ratios varying from 0.1, 1, 2, and 4. These concentrations ensured formation of the complex, since the Kd of 72f polypeptide for 5ef RNA was determined to be ∼0.016 μM. Digestions were carried out at room temperature in 10 μL reaction mixtures containing one or more of the following enzymes: 0.2 unit RNase V1 (USB Corporation), 0.05 unit RNase T1 (Sankyo), 0.001 unit RNase A (Ambion), 0.5 unit RNase T2 (GIBCO BRL). For footprinting with RNase S1 (USB Corporation), 2.7 unit of enzyme and a buffer containing 50 mM HEPES at pH 7.5, 20 mM magnesium acetate, and 1 mM zinc acetate buffer were used. The digestion products were resolved by electrophoresis in gels containing 15% polyacrylamide in 100 mM Tris-100 mM H3BO4 at pH 8.3, 2.5 mM EDTA, 8 M urea, and visualized by autoradiography. The amount of radioactivity in each band was measured using a Typhoon 9410 phosphoimager and ImageQuant software (Amersham Biosciences).
ACKNOWLEDGMENTS
This work was supported by NIH grant GM-49034 to C.Z.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.979508.
REFERENCES
- Andersen E.S., Rosenblad M.A., Larsen N., Westergaard J.C., Burks J., Wower I.K., Wower J., Gorodkin J., Samuelsson T., Zwieb C. The tmRDB and SRPDB resources. Nucleic Acids Res. 2006;34:D163–D168. doi: 10.1093/nar/gkj142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D.W., Walter P., Ottensmeyer F.P. Evidence for an extended 7SL RNA structure in the signal recognition particle. EMBO J. 1987;6:3471–3477. doi: 10.1002/j.1460-2075.1987.tb02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batey R.T., Rambo R.P., Lucast L., Rha B., Doudna J.A. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science. 2000;287:1232–1239. doi: 10.1126/science.287.5456.1232. [DOI] [PubMed] [Google Scholar]
- Bhuiyan S.H., Gowda K., Hotokezaka H., Zwieb C. Assembly of archaeal signal recognition particle from recombinant components. Nucleic Acids Res. 2000;28:1365–1373. doi: 10.1093/nar/28.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen J., Egebjerg J., Larsen N., Garrett R.A. Analysis of rRNA structure: Experimental and theoretical considerations. In: Speding G., editor. Ribosomes and protein synthesis: A practical approach. Oxford University Press; New York: 1990. pp. 229–252. [Google Scholar]
- Diener J.L., Wilson C. Role of SRP19 in assembly of the Archaeoglobus fulgidus signal recognition particle. Biochemistry. 2000;39:12862–12874. doi: 10.1021/bi001180s. [DOI] [PubMed] [Google Scholar]
- Doudna J.A., Batey R.T. Structural insights into the signal recognition particle. Annu. Rev. Biochem. 2004;73:539–557. doi: 10.1146/annurev.biochem.73.011303.074048. [DOI] [PubMed] [Google Scholar]
- Hainzl T., Huang S., Sauer-Eriksson A.E. Structure of the SRP19 RNA complex and implications for signal recognition particle assembly. Nature. 2002;417:767–771. doi: 10.1038/nature00768. [DOI] [PubMed] [Google Scholar]
- Halic M., Becker T., Pool M.R., Spahn C.M., Grassucci R.A., Frank J., Beckmann R. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature. 2004;427:808–814. doi: 10.1038/nature02342. [DOI] [PubMed] [Google Scholar]
- Halic M., Beckmann R. The signal recognition particle and its interactions during protein targeting. Curr. Opin. Struct. Biol. 2005;15:116–125. doi: 10.1016/j.sbi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Henry K.A., Zwieb C., Fried H.M. Purification and biochemical characterization of the 19-kDa signal recognition particle RNA-binding protein expressed as a hexahistidine-tagged polypeptide in Escherichia coli . Protein Expr. Purif. 1997;9:15–26. doi: 10.1006/prep.1996.0667. [DOI] [PubMed] [Google Scholar]
- Iakhiaeva E., Yin J., Zwieb C. Identification of an RNA-binding domain in human SRP72. J. Mol. Biol. 2005;345:659–666. doi: 10.1016/j.jmb.2004.10.087. [DOI] [PubMed] [Google Scholar]
- Iakhiaeva E., Bhuiyan S.H., Yin J., Zwieb C. Protein SRP68 of human signal recognition particle: Identification of the RNA and SRP72 binding domains. Protein Sci. 2006;12:467–468. doi: 10.1110/ps.051861406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuglstatter A., Oubridge C., Nagai K. Induced structural changes of 7SL RNA during the assembly of human signal recognition particle. Nat. Struct. Biol. 2002;9:740–744. doi: 10.1038/nsb843. [DOI] [PubMed] [Google Scholar]
- Larsen N., Zwieb C. SRP-RNA sequence alignment and secondary structure. Nucleic Acids Res. 1991;19:209–215. doi: 10.1093/nar/19.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H., Prehn S., Ashford A.J., Remus M., Frank R., Dobberstein B. Assembly of the 68- and 72-kDa proteins of signal recognition particle with 7S RNA. J. Cell Biol. 1993;121:977–985. doi: 10.1083/jcb.121.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichelli E., Isel C., Oubridge C., Nagai K. Protein-induced conformational changes of RNA during the assembly of human signal recognition particle. J. Mol. Biol. 2007;367:187–203. doi: 10.1016/j.jmb.2006.12.056. [DOI] [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Kuglstatter A., Menichelli E., Isel C., Jovine L. Structure, function and evolution of the signal recognition particle. EMBO J. 2003;22:3479–3485. doi: 10.1093/emboj/cdg337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oubridge C., Kuglstatter A., Jovine L., Nagai K. Crystal structure of SRP19 in complex with the S domain of SRP RNA and its implication for the assembly of the signal recognition particle. Mol. Cell. 2002;9:1251–1261. doi: 10.1016/s1097-2765(02)00530-0. [DOI] [PubMed] [Google Scholar]
- Politz J.C., Yarovoi S., Kilroy S.M., Gowda K., Zwieb C., Pederson T. Signal recognition particle components in the nucleolus. Proc. Natl. Acad. Sci. 2000;97:55–60. doi: 10.1073/pnas.97.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalia M., Rosenblad M.A., Samuelsson T. Prediction of signal recognition particle RNA genes. Nucleic Acids Res. 2002;30:3368–3377. doi: 10.1093/nar/gkf468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal K.R., Wild K., Montoya G., Sinning I. Crystal structure of the complete core of archaeal signal recognition particle and implications for interdomain communication. Proc. Natl. Acad. Sci. 2003;100:14701–14706. doi: 10.1073/pnas.2436132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T. A Mycoplasma protein homologous to mammalian SRP54 recognizes a highly conserved domain of SRP RNA. Nucleic Acids Res. 1992;20:5763–5770. doi: 10.1093/nar/20.21.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K.L., Pollard K.S., Baertsch R., Pohl A., Lowe T.M. The UCSC Archaeal Genome Browser. Nucleic Acids Res. 2006;34:D407–D410. doi: 10.1093/nar/gkj134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoulica E., Krause E., Meese K., Dobberstein B. Disassembly and domain structure of the proteins in the signal-recognition particle. Eur. J. Biochem. 1987;163:519–528. doi: 10.1111/j.1432-1033.1987.tb10899.x. [DOI] [PubMed] [Google Scholar]
- Shan S.O., Walter P. Co-translational protein targeting by the signal recognition particle. FEBS Lett. 2005;579:921–926. doi: 10.1016/j.febslet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Siegel V., Walter P. Each of the activities of signal recognition particle (SRP) is contained with a distinct domain: Analysis of biochemical mutants of SRP. Cell. 1988a;52:39–49. doi: 10.1016/0092-8674(88)90529-6. [DOI] [PubMed] [Google Scholar]
- Siegel V., Walter P. Binding sites of the 19-kDa and 68/72-kDa signal recognition particle (SRP) proteins on SRP RNA as determined in protein-RNA “footprinting.”. Proc. Natl. Acad. Sci. 1988b;85:1801–1805. doi: 10.1073/pnas.85.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu F.Y., Spanggord R.J., Doudna J.A. SRP RNA provides the physiologically essential GTPase activation function in cotranslational protein targeting. RNA. 2007;13:240–250. doi: 10.1261/rna.135407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanggord R.J., Siu F., Ke A., Doudna J.A. RNA-mediated interaction between the peptide-binding and GTPase domains of the signal recognition particle. Nat. Struct. Mol. Biol. 2005;12:1116–1122. doi: 10.1038/nsmb1025. [DOI] [PubMed] [Google Scholar]
- Tozik I., Huang Q., Zwieb C., Eichler J. Reconstitution of the signal recognition particle of the halophilic archaeon Haloferax volcanii . Nucleic Acids Res. 2002;30:4166–4175. doi: 10.1093/nar/gkf548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Disassembly and reconstitution of signal recognition particle. Cell. 1983;34:525–533. doi: 10.1016/0092-8674(83)90385-9. [DOI] [PubMed] [Google Scholar]
- Weichenrieder O., Wild K., Strub K., Cusack S. Structure and assembly of the Alu domain of the mammalian signal recognition particle. Nature. 2000;408:167–173. doi: 10.1038/35041507. [DOI] [PubMed] [Google Scholar]
- Yin J., Yang C.H., Zwieb C. Assembly of human signal recognition particle (SRP): Overlap of regions required for binding of protein SRP54 and assembly control. RNA. 2001;7:1389–1396. [PMC free article] [PubMed] [Google Scholar]
- Yin J., Yang C.H., Zwieb C. Two strategically placed base pairs in helix 8 of mammalian signal recognition particle RNA are crucial for the SPR19-dependent binding of protein SRP54. RNA. 2004;10:574–580. doi: 10.1261/rna.5232404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Iakhiaeva E., Menichelli E., Zwieb C. Identification of the RNA binding regions of SRP68/72 and SRP72 by systematic mutagenesis of human SRP RNA. RNA Biol. 2007;4:154–159. doi: 10.4161/rna.4.3.5428. [DOI] [PubMed] [Google Scholar]
- Zwieb C. Interaction of protein SRP19 with signal recognition particle RNA lacking individual RNA-helices. Nucleic Acids Res. 1991;19:2955–2960. doi: 10.1093/nar/19.11.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C. Recognition of a tetranucleotide loop of signal recognition particle RNA by protein SRP19. J. Biol. Chem. 1992;267:15650–15656. [PubMed] [Google Scholar]
- Zwieb C. Site-directed mutagenesis of signal recognition particle RNA: Identification of the nucleotides in helix 8 required for interaction with protein SRP19. Eur. J. Biochem. 1994;222:885–890. doi: 10.1111/j.1432-1033.1994.tb18936.x. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Eichler J. Getting on target: The archaeal signal recognition particle. Archaea. 2002;1:27–34. doi: 10.1155/2002/729649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Van Nues R.W., Rosenblad M.A., Brown J.D., Samuelsson T. A nomenclature for all signal recognition particle RNAs. RNA. 2005;11:7–13. doi: 10.1261/rna.7203605. [DOI] [PMC free article] [PubMed] [Google Scholar]