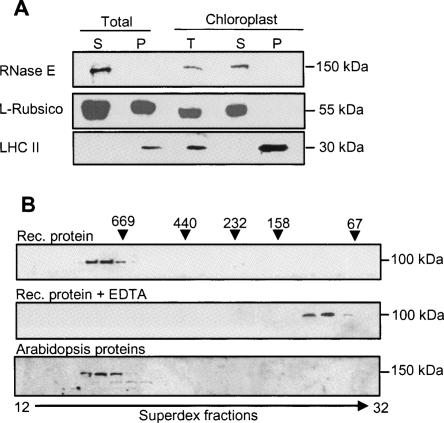
FIGURE 3.
Arabidopsis RNase E is located in the chloroplast in a high molecular weight complex. (A) Total Arabidopsis protein extract (soluble [S] and insoluble [P] fractions), together with purified chloroplasts, was separated on a 10% SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and probed with polyclonal antibodies against Arabidopsis RNase E (upper panel), Rubisco large subunit (middle panel), or LHC II (lower panel). (B) Purified recombinant truncated protein (amino acids 284–996) was fractionated on a Superdex 200 size-exclusion column, and the proteins of each fraction were analyzed by immunoblot with His6-specific antibodies (Rec. protein, upper panel). In the middle panel, 10 mM EDTA was added to the purified recombinant protein prior to fractionation on the same column (Rec. protein + EDTA). (C) Total Arabidopsis proteins were fractionated on the same column, and the proteins of each fraction analyzed by immunoblot using antibodies against RNase E. The elution profile of the following molecular weight markers is indicated along the top: thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), aldolase (158 kDa), and bovine serum albumin (67 kDa).