Abstract
Mammalian physiological processes, and likely any organism with a biliary tree, can distinguish between dietary cholesterol and non-cholesterols, retaining very little of the non-cholesterol in their bodies. Historically, the distinction between plant sterols and cholesterol has been known about for a century or more. That plants sterols were not ‘absorbed’ has been investigated for almost half a century. Indeed, the oral of plant sterols in gram quantities was shown to interfere with cholesterol absorption and is one of the oldest pharmacological therapies for hypercholesterolemia. Although the basis for the latter was shown to be caused by exclusion of cholesterol from intestinal micelles by plant sterols, it was not until the identification of the a rare genetic disease, sitosterolemia, first described in 1974, that led to the hypothesis that specific molecular mechanism(s) governed both the entry and excretion of sterols by the body. This talk will cover the physiology of dietary sterol metabolism, genetics and pathophysiology of sitosterolemia. Additionally, the role of plant sterols in normal and abnormal metabolism in humans as well as selected animal models will be discussed.
Introduction
Dietary cholesterol absorption from the intestine is a critical component of cholesterol homeostasis. Under normal circumstances, the human diet contains approximately equal amounts of cholesterol and plant sterols. However, normal individuals retain on average approximately 50% of dietary cholesterol, but less than 5% of dietary plant sterols1. The normal physiologic process for handling dietary sterols by the gastrointestinal tract involves processing 200 to 500 mg of dietary cholesterol, but also 200 to 400 mg of phytosterols that are a component of plant sterols (Fig. 1). Almost all of the dietary phytosterols undergo fecal excretion along with 800 to 1500 mg of cholesterol plus its metabolic product, bile acids. The normal body is able to discriminate between cholesterol and non-cholesterol sterols2. This suggests that a molecular mechanism exists in the body that specifically retains cholesterol over non-cholesterol sterols 3. The exact molecular mechanisms by which preferential cholesterol absorption occurs, while non-cholesterol sterols are not absorbed has not been fully elucidated, but clues from a rare genetic disorder, as well as a novel drug have led to a major insights.
Figure 1. Normal physiology of dietary sterols.
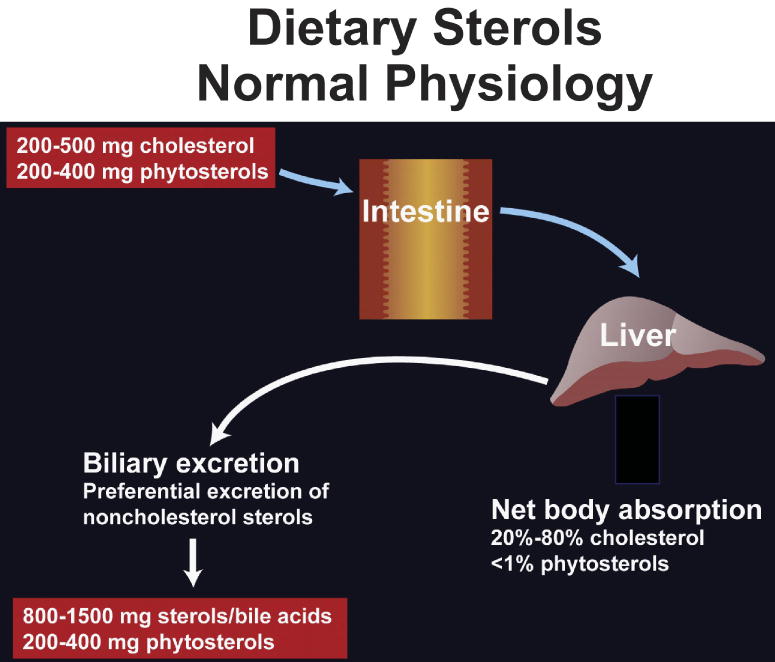
Although we ingest an equal amount of plant sterols and cholesterol, the net retention of the plant sterol is <5% on a daily basis, whereas an average of 55% of cholesterol may be absorbed. In addition, the liver excretes a significant amount of sterols, resulting in a net output of more than 1g per day loss in the feces.
The purpose of this review is to provide and overview of plant sterols and their role in health and disease. Included will be a discussion of the genetics of sitosterolemia, the role of plant sterols as markers for premature atherosclerosis, and potential treatment options for lowering plant sterol levels in the body.
Metabolism of Cholesterol and Plant Sterols
The enterohepatic metabolism of cholesterol and plant sterols is complex (Fig. 2). The metabolic process occurs within the intestinal lumen, where dietary cholesterol (and plant sterols) is reduced to free sterols by esterases, and transferred into micelles (a mixture of bile salts, phospholipids, free sterols and some fatty acids). These micelles interact with the enterocyte apical membrane (a process that has not been characterized at the molecular level) and allow entry of the sterols into the enterocyte. Cholesterol enters the metabolic pool, where it is esterified by ACAT-24, and incorporated into chylomicrons that are secreted at the basolateral surface into the lacteals that eventually drain into the venous circulation. Chylomicrons are acted upon by lipoprotein lipase in the capillary beds of all the organs, which allows for the dietary triglycerides as well as some fat-soluble vitamins to be delivered to these tissues. Sterols in these particles are not transferred out and remain as part of the remnant particles which are now recognized by receptors on the liver and cleared. Hence the bulk of the dietary sterols are delivered to the liver. Cholesterol enters the metabolic pool and can be re-packaged into VLDL and secreted back into the circulation, whereas non-cholesterol sterols are excreted into the biliary tree and thus back into the lumen of the intestine.
Figure 2. Pathway for intestinal cholesterol absorption.
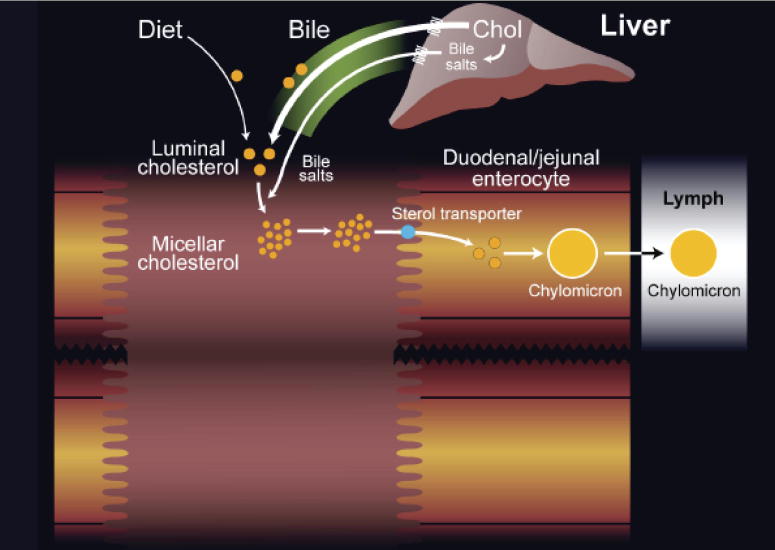
The process of cholesterol (and sterol) absorption begins with the formation of micelles that comprise bile salts and phospholipids present in bile, that allow for neutral sterols to partition into them. Once these micelles are formed, they interact with the apical surface of the enterocyte, leading to the entry of the cholesterol to join the metabolic pool and eventually get secreted as a component of chylomicron particles at the basolateral surface. Note that the bile salts do not enter in the proximal small intestine, but travel further down to the terminal ilieum for specific re-uptake.
How much dietary cholesterol do we absorb? The average person absorbs about 55% of normal dietary intake with a typical Gaussian distribution of rates; a small proportion of the population hyperabsorbs 90% of dietary cholesterol or underabsorbs less than 10%5. However, the dominant factor determining dietary cholesterol absorption for modern man is dietary intake rather than absorption efficiency. In contrast, intestinal absorption of plant sterols was thought to be negligible, although these different sterols are physically very similar (Fig. 3). The molecular mechanism underlying the exclusion of non-cholesterol sterols (xenosterols) was not considered until the discovery of a rare genetic disorder, sitosterolemia.
Figure 3. Structures of sterol molecules.
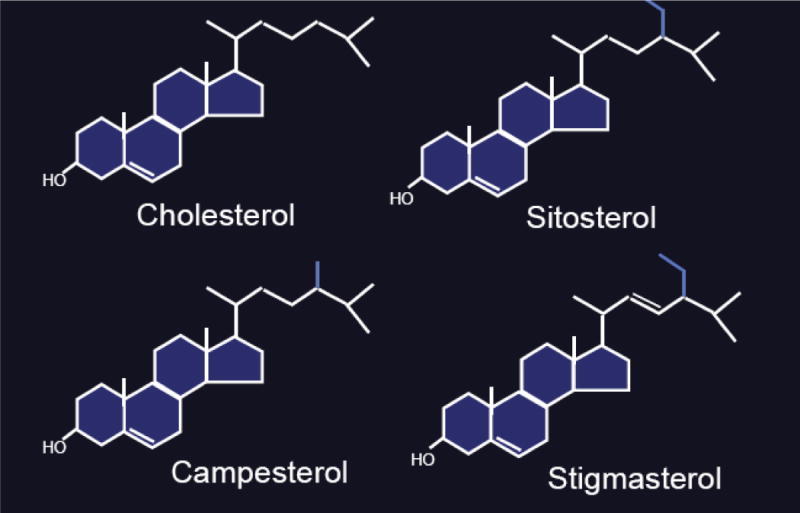
The various sterol structures are depicted and show that plant sterols differ primarily in the R tail of the sterol structure and these differences can range from only an extra methyl group (campesterol) to a more complex difference (stigmasterol).
Sitosterolemia
A 22-year-old woman presented at the orthopedic clinic complaining of pain in both heels and knees. She had tendon xanthomas with a history of hand xanthomas beginning at age 8 years, which progressed to patellar, plantar, and Achilles tendon involvement. The patient has a sister who has a similar phenotype. This combination of arthralgia and tendon xanthomas is associated with the diagnosis of familial hypercholesterolemia. Familial hypercholesterolemia is characterized by hypercholesterolemia, tendon xanthoma, arthralgias and a family history of premature heart disease. It is relatively common and occurs in 1 in 200 to 500 of the general population. Diagnosis is typically confirmed by a lipid panel, which reveals an LDL-cholesterol level >200 mg/dL accompanied by a positive family history of premature heart disease and hypercholesterolemia. In the two sisters, the total cholesterol levels were borderline high at 195 mg/dL and 206 mg/dL. Serum triglyceride levels were 81 mg/dL and 63 mg/dL. Their parents had tendon xanthomas, but were otherwise normal with no family history of hyperlipidemia or cardiovascular disease for the previous two generations. The absence of a family history is not consistent with a diagnosis of familial hypercholesterolemia.
The original description of the rare disorder β-sitosterolemia and xanthomatosis was reported by Bhattacharyya and Connor in 19746. Sitosterolemia or phytosterolemia is an autosomal recessive disorder7. It is an exceedingly rare disorder that affects fewer than 1 patient per 1 million population, with about 20 cases reported so far in the US (though under-diagnosis is likely to be a factor). The underlying pathophysiology is increased absorption of dietary sterols, loss of discrimination between cholesterol and non-cholesterol sterols and reduced excretion of sterols (cholesterol or non-cholesterol sterols) into bile8, 9. Sitosterolemia is associated with tendon xanthomas, increased coronary atherosclerosis, and hemolysis7. Included in the differential diagnosis is cerebrotendinous xanthomatosis. Laboratory testing reveals elevated plasma levels of phytosterols that are typically >10mg/dL, of which sitosterol is the major species.
Segregation analyses in a larger Amish family showed that sitosterolemia was inherited as an autosomal recessive disease, suggesting a single locus was defective10. This locus is thus responsible for determining dietary sterol discrimination, cholesterol absorption, and biliary cholesterol (sterol) secretion. In healthy individuals, mechanisms exist that permit the body to distinguish between sterols. In sitosterolemia, either absorption of sterols is increased or excretion is decreased resulting in markedly elevated plasma non-cholesterol levels. A major consequence of sitosterolemia is increased risk of cardiovascular disease 11. Although the prevalence of premature atherosclerotic disease in patients with sitosterolemia has not been fully defined because of the rarity of the condition, it is estimated that more than half of patients have documented coronary artery disease (CAD). A greater understanding of the mechanisms involved in the metabolism of cholesterol might result in better treatment options for sitosterolemia, but also could provide a better understanding of cholesterol absorption and excretion.
Plasma Sterol Profiles In Sitosterolemia
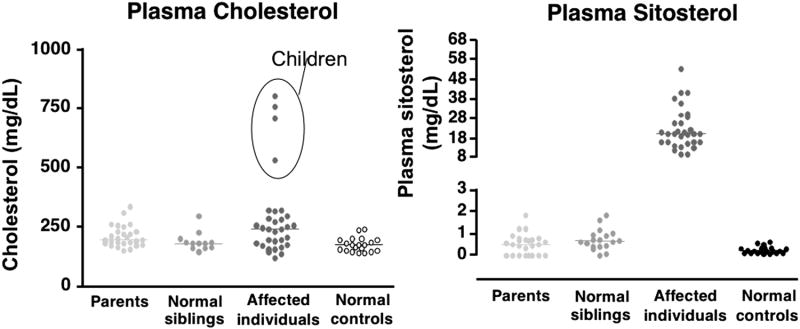
Early work was undertaken to elucidate the mechanisms of sitosterolemia in individuals and families affected by the disorder3, 12. Plasma sitosterol levels, measured by gas chromatography (GC) or high pressure liquid chromatography (HPLC) in affected individuals, their obligate heterozygous parents, unaffected siblings, and normal controls were compared13. Most unaffected individuals had plasma sitosterol levels <1 mg/dL (Fig. 4). Sitosterol levels >1 mg/dL in three parents and three siblings may have reflected the use of HPLC rather than GC in these six subjects. All individuals with sitosterolemia had plasma sitosterol values >8 mg/dL (some of these were on treatment). When plasma cholesterol levels were examined in the same groups, four subjects (all children) in the affected cohort had markedly elevated plasma cholesterol. All four of these subjects had been classified as pseudo-homozygous familial hypercholesterolemia (as all had responded to ‘dietary’ therapy), but in actuality all four subjects were sitosterolemic. Although a few rare cases of ‘pseudo-homozygous FH have been reported that do not have sitosterolemia, all such pediatric cases should be screened for this disease, especially as a therapy is now available (see below).
Figure 4. Plasma sitosterol and cholesterol in affected individuals with sitosterolemia and parents, normal siblings, and normal controls.
All sterols were determined by either GC or HPLC and show that cholesterol levels were not significantly different in patients with sitosterolemia, except for the 4 subjects (designated by circles in the right hand chart) all of whom had been clinically diagnosed as having ‘pseudohomozygous FH’ but all had sitosterolemia (right hand chart).
Genetics of Sitosterolemia
We performed linkage analyses of 10 well-characterized pedigrees and mapped the sitosterolemia locus, STSL, to human chromosome 2p21, between microsatellite markers D2S1788 and D2S13523, 12, 14. The disease locus interval was narrowed further and a physical map of this region constructed that allowed for the positional cloning of the sitosterolemia ‘gene’14.
The surprise was that two highly homologous genes, ABCG5 and ABCG8, comprised the STSL locus and mutations in ether were responsible for the disease15-17.
ABCG5 and ABCG8 are ATP-Binding Cassette containing proteins related to the ‘G’ family, members of which contain the White proteins in Drosophila, involved in retinal pigment metabolism18. Each gene consists of 13 exons and encodes for a cytoplasmic N-terminal segment that contains the characteristic ABC signature motifs that can bind ATP, and a C-terminal segment that contains six putative membrane-spanning domains. The protein products of these genes were named sterolin-1 and sterolin-215, 18. Mutational analyses showed that both ABCG5 and ABCG8 are mutated in sitosterolemia. Interestingly, patients were either completely mutated for both copies of ABCG8 or both copies of ABCG5, but not a mutation of one in ABCG5 and one in ABCG818. This finding indicates that the two proteins probably work as obligate heterodimers to provide the same function. Work from the Hobbs' laboratory has provided key data to support both the heterodimerization, as well as showing that coexpression is necessary for normal maturation of these proteins within the cell19-23.
Based on this research, two models were proposed as to how sterolins function (Fig. 5). Model A proposed that the heterodimer functions to pump sterols out of the cell13. Compared with cholesterol, the sterolin complex exhibits a greater affinity for pumping sitosterol (xenosterols) out of the cell (enterocyte or hepatocyte), and the sterolin complex can pump cholesterol out in the absence of sitosterol. The pumping mechanism is likely to be limited by the availability of substrate, thus, heterozygous individuals would be predicted to be normal. Theoretically, the heterodimer complex of sterolin-1 and sterolin-2 could have a dual function - pumping cholesterol into the cells and pumping non-cholesterol sterols out of cells. This model is not supported by current data and model A is the favored mechanism.
Figure 5. Proposed models for sitosterol and cholesterol metabolism.
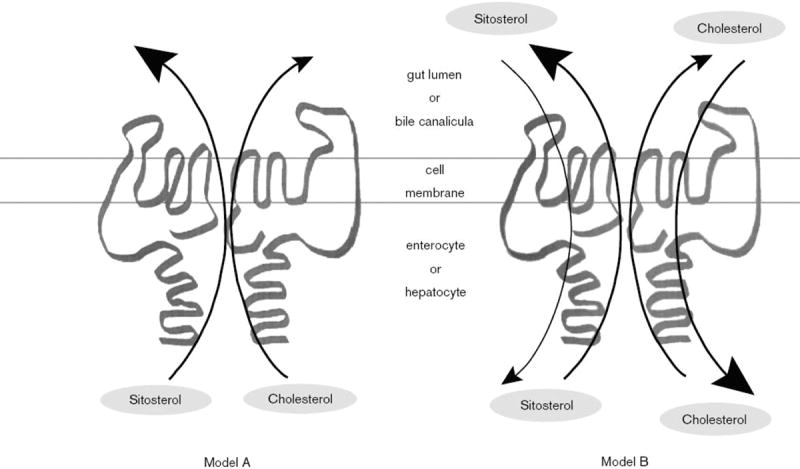
Based upon the genetics, sterolin 1 and -2 are proposed to function as heterodimers. They could act as extruders, pumping non-cholesterol sterols and cholesterol out (Model A), with a greater affinity to pump non-cholesterol sterol. Alternatively, they could act as channels, allowing sterols in and out of the cell (Model B), but favoring cholesterol in, and plant sterols out. Model A seems to be supported by current experimental evidence.
Pathophysiology of Sitosterolemia
Once the gene defects were identified, it became possible to dissect the pathophysiology, using in vitro as well as animal models methodology. Absence of ABCG5, ABCG8 or both results in an elevation of plant sterols in the tissues and blood and a failure to excrete sterols into bile24-31. Over expression of ABCG5 and ABCG8 in mice transgenic for the human genes led to a super saturation of cholesterol in bile and protected animals against atherosclerosis. Careful studies in ABCG8 knockout mice showed that there was increased intestinal absorption of both cholesterol and non-cholesterol sterols. Together with a failure to excrete cholesterol, even when forced, loss of sterolins showed that these proteins are the cholesterol and non-cholesterol transporters for excretion at the intestinal and biliary level. Interestingly, mice heterozygous for ABCG8 loss showed an intermediate phenotype for biliary sterol excretion.
To investigate whether humans who are heterozygous for mutations in ABCG8 (first degree relatives of patients with sitosterolemia), 12 heterozygotes were fed diets low in fat and cholesterol or enriched with plant sterols or stanols32. Blood levels of plant sterols increased significantly in the heterozygotes while on the enriched diet, and LDL-cholesterol levels decreased significantly while on the low fat diet. These studies confirmed earlier smaller studies by Salen and colleagues suggesting that heterozygous individuals may have a subtle phenotype33.
Are There Other Clinical Features That May Indicate Sitosterolemia?
A case was reported of a 19-year-old male who presented with chronic active liver disease and after an extensive evaluation was found to have sitosterolemia34. Liver disease has not been reported to be feature in sitosterolemia. In contrast, the diagnosis of ‘idiopathic cirrhosis’ is not an uncommon diagnosis in people presenting with progressive liver failure. Since sitosterolemia requires analyses of blood by GC or HPLC for these sterols, it is possible that such cases are not diagnosed. Interestingly, our patient underwent orthotopic liver transplantation, which reduced his plant sterols to near-normal values, suggesting that restoration of normal sterolin activity in the liver alone is sufficient to effect a cure34.
Rees and colleagues reported five pedigrees with Mediterranean stomatocytosis or Mediterranean macrothrombocytopenia, which combines stomatocytic hemolysis with the presence of very large platelets35. All patients had grossly elevated plasma levels of phytosterols and all showed mutations in the ABCG5 and ABCG8 transporters. The authors concluded that Mediterranean stomatocytosis/macrothrombocytopenia is caused by an excess of phytosterols, that phytosterolemia can be diagnosed based on the distinctive hematology findings including unexplained hemolysis and macrothrombocytopenia, and that phytosterolemia should be considered in the differential diagnosis of all patients with large platelets35. A subsequent follow up study of one of these families showed that sitosterolemia was also associated with adrenal and ovarian failure, suggesting that build up of non-cholesterol sterols can lead to disruption of steroid hormone pathway synthesis, although it should be emphasized that these features are not classically present in most cases of sitosterolemia36.
Plant Sterol Levels and Normal Physiology
The normal function of ABCG5 and ABCG8 genes is to limit intestinal absorption and promote the biliary excretion of neutral sterols, and in particular limit non-cholesterol sterols (xenosterols). Small, but detectable levels of plant sterols can be found in all normal individuals and these levels are very strongly influenced by genetic inheritance37, 38. Common variations in ABCG5 or ABCG8 may contribute to wide inter-individual variation in plasma concentrations of plant sterols among subjects consuming similar amounts of dietary sitosterol. Typically, a 5-fold variation in plasma concentrations of cholesterol precursor sterols and plant sterols can be observed among individuals with normal lipid levels37, 38. The effect of genetic and environmental factors on variations in plasma concentrations and sterol-cholesterol ratios of five noncholesterol sterols (cholestanol, desmosterol, lathosterol, campesterol, and sitosterol) was examined38. Regression analysis indicated that plasma levels of all five noncholesterol sterols were highly heritable, with the greatest heritable index for sitosterol. Twin studies have confirmed this heritability37.
Despite this strong heritability, not all of the variations at the STSL locus explain the variability in plasma plant sterol levels, suggesting many other loci are likely involved that can affect the low steady-state levels of plasma plant sterols39.
On the other hand this locus has been implicated in playing a role in susceptibility to gallstone formation in mice and men40-43, in lipoprotein kinetics in obese individuals44, in an association with metabolic syndrome marker prevalence45, in determining plasma LDL concentrations46, in responsiveness to atorvastatin47, in responsiveness to plant sterol-fortified margarines, in plasma plant sterol levels as well as cholesterol synthesis rates48, and in responsiveness to certain diets and plasma lipid levels49. The major drawback with the majority of these studies is the small sample size which restricts the power to test the hypothesis that the STSL locus plays a role in the measured phenotype (despite the reported significant ‘P’ values). Another potential problem is that there seems to be no purported mechanistic connection made between the putative function of the sterolins and the phenotype that is studied; for example what is the connection between lutein levels and the function of the sterolins49, or why is the relationship of STSL variation seen only in obese persons (which itself is an ‘acquired’ phenotype)44, 50? Better designed and well-powered studies that also take into account the haplotype structure of this locus, as well as test mechanistically plausible phenotypes are needed to explore the role of the STSL locus in some of these pathways. To date, the strongest data may be for gallstone susceptibility, even though these are still not the level to provide definitive evidence.
Are Plant Sterols a Marker for Premature Atherosclerosis?
Glueck and colleagues were the first to report an association between serum phytosterol levels with a family history of CHD in 595 hypercholesterolemic subjects51. In a case-control study conducted in postmenopausal women, those with CAD had elevated ratios of squalene (p<0.001), desmosterol (p=0.005), campesterol (p=0.028) and sitosterol (p=0.022) to cholesterol, but had lower respective lathosterol values (p=0.041) compared with the controls52. After adjustment for risk factors, the ratios of squalene, lathosterol, campesterol, and sitosterol were significantly (p<0.05) associated with an increased risk of CAD. A positive family history of CHD was present among 27 patients but absent among 26 patients undergoing elective coronary artery bypass grafting (Sudhop et al, 2002). Patients with a positive family history for CHD had significantly (p<0.05) higher plasma levels of campesterol and sitosterol. Further analysis showed no influence of sex, age, triglycerides, total-, LDL-cholesterol, and HDL-cholesterol, but confirmed a strong influence of plant sterols53.
The PROCAM Study evaluated the risks of cardiovascular disease associated with sitosterol54. In this case-control cohort study, 159 men who had experienced an acute myocardial infarction or sudden death during a 10-year follow-up were matched with controls. Plasma sitosterol concentrations were elevated in cases compared with controls (p=0.028). Elevated levels of sitosterol were associated with a 1.8-fold increase in risk (p<0.05). Among men at high risk, high sitosterol concentrations were associated with an incremental 3-fold increase in the incidence of coronary events (p=0.032), and a significant (p=0.03) association between high sitosterol/cholesterol ratio and coronary risk. Plasma sitosterol concentrations were independently predictive of cardiovascular disease. The hazard ratio for sitosterol (1.77) was comparable to that for HDL-cholesterol (1.82) and less than that for LDL-cholesterol (2.90). It was concluded that elevated sitosterol concentrations were associated with an increased risk for major coronary events in high risk men54.
Not all such studies confirm an association between non-cholesterol sterols and CAD. Results from the Dallas Heart Study were used to determine whether plasma levels of plant sterols were associated with coronary atherosclerosis, as judged by coronary artery calcium measured by EBCT55. In 2542 patients aged 30 to 67 years, plasma levels of cholesterol were significantly (p<0.05) higher in patients with elevated coronary calcium levels. But plasma levels of sitosterol or campesterol were not associated with elevated coronary calcium. No relationship was observed between a family history of CHD and plant sterol levels, and a similar lack of association was found in a subset of patients with hypercholesterolemia55. The EPIC-Norfolk Population study in a prospective nested case-control study consisting of 373 cases and 758 controls, also failed to show a relation ship between sitosterol and CAD56.
Can We Lower Plant Sterol Levels?
A multicenter, double-blind, randomized, placebo-controlled study examined the effect of ezetimibe on reducing plant sterol levels in patients with sitosterolemia57. Among 37 patients who were randomized to placebo or ezetimibe 10 mg/d for 8 weeks, sitosterol plasma levels decreased significantly (p<0.001) with ezetimibe (-21% vs. 4% increase with placebo). Campesterol levels also decreased significantly (p<0.001) with ezetimibe (-24% with ezetimibe vs. 3% increase with placebo). Total sterol and apolipoprotein B levels also were reduced. In non-sitosterolemic subjects, who have very low levels of plant sterols (<1mg/dL), ezetimibe also led to significant lowering of plant sterols, suggesting that if the entry of plant sterols is blocked via NPC1L1, this can lead to a further lowering in normal as well as sitosterolemia subjects58.
Summary
Increased knowledge of the defective genes in sitosterolemia expands our understanding of this disease and has shed light into how we regulate dietary sterol absorption, sterol excretion, and keep xenosterols from accumulating in our bodies. This disease has led to the identification of the molecular players in a key physiological step and led to the identification of a previously unknown biochemical pathway that is essential to understanding whole-body sterol balance. Expanding knowledge of the effects of plant sterols and their interplay with cholesterol should aid the identification of better dietary and pharmacological approaches to manage elevated lipid and lipoprotein levels. In addition, it may also focus attention on how diet, and particular xenosterols, may affect the risk of cardiovascular morbidity and mortality.
Footnotes
There are no significant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu K, Lee M, Patel SB. Dietary cholesterol absorption; more than just bile. Trends Endocrin Metab. 2001;12:314–20. doi: 10.1016/s1043-2760(01)00433-7. [DOI] [PubMed] [Google Scholar]
- 2.Schoenheimer R. Uber die Bedeutung der Pflanzensterine fur den tierischen Organismus. Hoppe-Seyler's Z für physiol Chem. 180:1–5. 129. [Google Scholar]
- 3.Patel SB, Honda A, Salen G. Sitosterolemia: exclusion of genes involved in reduced cholesterol biosynthesis. J Lipid Res. 1998;39:1055–61. [PubMed] [Google Scholar]
- 4.Rudel LL, Lee RG, Cockman TL. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr Opin Lipidol. 2001;12:121–7. doi: 10.1097/00041433-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bosner MS, Lange LG, Stenson WF, Ostlund RE., Jr Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res. 1999;40:302–308. [PubMed] [Google Scholar]
- 6.Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorkhem I, Boberg KM, Leitersdorf E. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease--OMMBID. Ch 123. New York: McGraw-Hill Inc; Sept 23, 2005. http://www.ommbid.com/ [Google Scholar]
- 8.Salen G, Shefer S, Nguyen L, et al. Sitosterolemia. J Lipid Res. 1992;33:945–55. [PubMed] [Google Scholar]
- 9.Salen G, Shore V, Tint GS, et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res. 1989;30:1319–30. [PubMed] [Google Scholar]
- 10.Beaty TH, Kwiterovich P, Jr, Khoury MJ, et al. Genetic analysis of plasma sitosterol, apoprotein B, and lipoproteins in a large Amish pedigree with sitosterolemia. Am J Hum Genet. 1986;38:492–504. [PMC free article] [PubMed] [Google Scholar]
- 11.Salen G, Horak I, Rothkopf M, et al. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J Lipid Res. 1985;26:1126–33. [PubMed] [Google Scholar]
- 12.Patel SB, Salen G, Hidaka H, et al. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J Clin Invest. 1998;102:1041–4. doi: 10.1172/JCI3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipidol. 2001;12:141–9. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu K, Lee MH, Carpten JD, Sekhon M, Patel SB. High-resolution physical and transcript map of human chromosome 2p21 containing the sitosterolemia locus. Eur J Hum Genet. 2001;9:364–74. doi: 10.1038/sj.ejhg.5200627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MH, Lu K, Hazard S, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu K, Lee MH, Yu H, et al. Molecular cloning, genomic organization, genetic variations, and characterization of murine sterolin genes Abcg5 and Abcg8. J Lipid Res. 2002;43:565–78. [PMC free article] [PubMed] [Google Scholar]
- 17.Berge KE, Tian H, Graf GA, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–5. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 18.Lu K, Lee MH, Hazard S, et al. Two genes that map to the STSL locus cause sitosterolemia: Genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8 respectively. Am J Hum Genet. 2001;69:278–90. doi: 10.1086/321294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf GA, Li WP, Gerard RD, et al. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110:659–69. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graf GA, Yu L, Li WP, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–82. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 21.Graf GA, Cohen JC, Hobbs HH. Missense mutations in ABCG5 and ABCG8 disrupt heterodimerization and trafficking. J Biol Chem. 2004;279:24881–8. doi: 10.1074/jbc.M402634200. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Sun F, Zhang DW, et al. Sterol transfer by ABCG5 and ABCG8: In vitro assay and reconstitution. J Biol Chem. 2006;281:27894–904. doi: 10.1074/jbc.M605603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang DW, Graf GA, Gerard RD, Cohen JC, Hobbs HH. Functional asymmetry of nucleotide-binding domains in ABCG5 and ABCG8. J Biol Chem. 2006;281:4507–16. doi: 10.1074/jbc.M512277200. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Hammer RE, Li-Hawkins J, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99:16237–42. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu L, Li-Hawkins J, Hammer RE, et al. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–80. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klett EL, Lu K, Kosters A, et al. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2004;2:5. doi: 10.1186/1741-7015-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plosch T, Bloks VW, Terasawa Y, et al. Sitosterolemia in ABC-Transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 2004;126:290–300. doi: 10.1053/j.gastro.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 28.Wu JE, Basso F, Shamburek RD, et al. Hepatic ABCG5 and ABCG8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J Biol Chem. 2004;279:22913–25. doi: 10.1074/jbc.M402838200. [DOI] [PubMed] [Google Scholar]
- 29.Kosters A, Kunne C, Looije N, Patel SB, Oude Elferink RP, Groen AK. The mechanism of Abcg5/Abcg8 in biliary cholesterol secretion in mice. J Lipid Res. 2006;47:1959–66. doi: 10.1194/jlr.M500511-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basso F, Freeman LA, Ko C, et al. Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J Lipid Res. 2007;48:114–26. doi: 10.1194/jlr.M600353-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Wang HH, Patel SB, Carey MC, Wang DQ. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(-/-) mice. Hepatology. 2007;45:998–1006. doi: 10.1002/hep.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwiterovich PO, Jr, Chen SC, Virgil DG, Schweitzer A, Arnold DR, Kratz LE. Response of obligate heterozygotes for phytosterolemia to a low-fat diet and to a plant sterol ester dietary challenge. J Lipid Res. 2003;44:1143–55. doi: 10.1194/jlr.M200455-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler Thromb. 1992;12:563–8. doi: 10.1161/01.atv.12.5.563. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen TA, Klett EL, Gylling H, Isoniemi H, Patel SB. Liver transplantation in a patient with sitosterolemia and cirrhosis. Gastroenterology. 2006;130:542–7. doi: 10.1053/j.gastro.2005.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rees DC, Iolascon A, Carella M, et al. Stomatocytic haemolysis and macrothrombocytopenia (Mediterranean stomatocytosis/macrothrombocytopenia) is the haematological presentation of phytosterolaemia. Br J Haematol. 2005;130:297–309. doi: 10.1111/j.1365-2141.2005.05599.x. [DOI] [PubMed] [Google Scholar]
- 36.Mushtaq T, Wales JK, Wright NP. Adrenal insufficiency in phytosterolaemia. Eur J Endocrinol. 2007;157 1:S61–5. doi: 10.1530/EJE-07-0222. [DOI] [PubMed] [Google Scholar]
- 37.Boomsma DI, Princen HM, Frants RR, Gevers Leuven JA, Kempen HJ. Genetic analysis of indicators of cholesterol synthesis and absorption: lathosterol and phytosterols in Dutch twins and their parents. Twin Res. 2003;6:307–14. doi: 10.1375/136905203322296674. [DOI] [PubMed] [Google Scholar]
- 38.Berge KE, von Bergmann K, Lutjohann D, et al. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res. 2002;43:486–94. [PubMed] [Google Scholar]
- 39.Sehayek E, Duncan EM, Lutjohann D, et al. Loci on chromosomes 14 and 2, distinct from ABCG5/ABCG8, regulate plasma plant sterol levels in a C57BL/6J × CASA/Rk intercross. Proc Natl Acad Sci U S A. 2002;99:16215–9. doi: 10.1073/pnas.212640599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wittenburg H, Lyons MA, Li R, et al. Association of a lithogenic Abcg5/Abcg8 allele on Chromosome 17 (Lith9) with cholesterol gallstone formation in PERA/EiJ mice. Mamm Genome. 2005;16:495–504. doi: 10.1007/s00335-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 41.Grunhage F, Acalovschi M, Tirziu S, et al. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 2007;46:793–801. doi: 10.1002/hep.21847. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Jiang ZY, Fei J, et al. ATP binding cassette G8 T400K polymorphism may affect the risk of gallstone disease among Chinese males. Clin Chim Acta. 2007;284:80–5. doi: 10.1016/j.cca.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Acalovschi M, Ciocan A, Mostean O, Tirziu S, Chiorean E, Keppeler H, Schirin-Sokhan R, Lammert F. Are plasma lipid levels related to ABCG5/ABCG8 polymorphisms? A preliminary study in siblings with gallstones. Eur J Intern Med. 2006;17:490–4. doi: 10.1016/j.ejim.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Chan DC, Watts GF, Barrett PH, Whitfield AJ, van Bockxmeer FM. ATP-binding cassette transporter G8 gene as a determinant of apolipoprotein B-100 kinetics in overweight men. Arterioscler Thromb Vasc Biol. 2004;24:2188–91. doi: 10.1161/01.ATV.0000143532.93729.d6. [DOI] [PubMed] [Google Scholar]
- 45.Gylling H, Hallikainen M, Pihlajamaki J, et al. Polymorphisms in the ABCG5 and ABCG8 genes associate with cholesterol absorption and insulin sensitivity. J Lipid Res. 2004;45:1660–5. doi: 10.1194/jlr.M300522-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Hubacek JA, Berge KE, Stefkova J, et al. Polymorphisms in ABCG5 and ABCG8 transporters and plasma cholesterol levels. Physiol Res. 2004;53:395–401. [PubMed] [Google Scholar]
- 47.Kajinami K, Brousseau ME, Nartsupha C, Ordovas JM, Schaefer EJ. ATP binding cassette transporter G5 and G8 genotypes and plasma lipoprotein levels before and after treatment with atorvastatin. J Lipid Res. 2004;45:653–6. doi: 10.1194/jlr.M300278-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Miwa K, Inazu A, Kobayashi J, et al. ATP-binding cassette transporter G8 M429V polymorphism as a novel genetic marker of higher cholesterol absorption in hypercholesterolaemic Japanese subjects. Clin Sci (Lond) 2005;109:183–8. doi: 10.1042/CS20050030. [DOI] [PubMed] [Google Scholar]
- 49.Herron KL, McGrane MM, Waters D, et al. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J Nutr. 2006;136:1161–5. doi: 10.1093/jn/136.5.1161. [DOI] [PubMed] [Google Scholar]
- 50.Santosa S, Demonty I, Lichtenstein AH, Ordovas JM, Jones PJH. Single nucleotide polymorphisms in ABCG5 and ABCG8 are associated with changes in cholesterol metabolism during weight loss. J Lipid Res. 2007;48:2607–13. doi: 10.1194/jlr.M600452-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Glueck CJ, Speirs J, Tracy T, Streicher P, Illig E, Vandegrift J. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metabolism. 1991;40:842–8. doi: 10.1016/0026-0495(91)90013-m. [DOI] [PubMed] [Google Scholar]
- 52.Rajaratnam RA, Gylling H, Miettinen TA. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J Am Coll Cardiol. 2000;35:1185–91. doi: 10.1016/s0735-1097(00)00527-1. [DOI] [PubMed] [Google Scholar]
- 53.Sudhop T, Gottwald BM, von Bergmann K. Serum plant sterols as a potential risk factor for coronary heart disease. Metabolism. 2002;51:1519–21. doi: 10.1053/meta.2002.36298. [DOI] [PubMed] [Google Scholar]
- 54.Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg F, Schulte H. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr Metab Cardiovasc Dis. 2006;16:13–21. doi: 10.1016/j.numecd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Wilund KR, Yu L, Xu F, et al. Plant sterol levels are not associated with atherosclerosis in mice and men. Arterioscler Thromb Vasc Biol. 2004;24:2326–32. doi: 10.1161/01.ATV.0000149140.00499.92. [DOI] [PubMed] [Google Scholar]
- 56.Pinedo S, Vissers MN, Bergmann K, et al. Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC-Norfolk Population Study. J Lipid Res. 2007;48:139–44. doi: 10.1194/jlr.M600371-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Salen G, von Bergmann K, Lutjohann D, et al. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–71. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sudhop T, Lutjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–8. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]