Abstract
Nitric oxide (NO) synthase-2 (NOS-2), a key source of NO at sites of neuroinflammation, is induced in astrocyte cultures treated with lipopolysaccharide (LPS) plus interferon-γ (IFNγ). A recent study examining the regulation of astrocytic NOS-2 expression demonstrated that transforming growth factor-β1 (TGFβ1) potentiated LPS plus IFNγ-induced NOS-2 expression via expansion of the pool of astrocytes that express NOS-2. Results in the current report indicate that this population-based mechanism of increasing NOS-2 expression is not restricted to TGFβ1, since it also accounts for the potentiation of NO production in astrocyte cultures by tumor necrosis factor-α (TNFα). In contrast to TGFβ1, which required 24 hr preincubation for optimal potentiation of NO production, TNFα was maximally effective when added concurrently with LPS plus IFNγ. Nevertheless, under conditions that optimally potentiated NO production, both cytokines recruited similar numbers of astrocytes to express NOS-2 (% NOS-2-positive cells after LPS plus IFNγ alone or with TNFα or TGFβ1 was 9.5 ± 1.2, 25.3 ± 2.9, and 32.4 ± 3.0, respectively). Interestingly, stimulation of astrocytes in the presence of both TGFβ1 and TNFα additively increased the number of astrocytes that expressed NOS-2 protein (% NOS-2- positive cells was 61.0 ± 4.2) relative to each cytokine alone. Potentiation of NO production by either TNFα or TGFβ1 was not ablated by neutralizing antibodies to TGFβ1 or TNFα, respectively. Thus, the two cytokines act independently to recruit separate pools of astrocytes to express NOS-2. These results are consistent with the notion that astrocytes possess an innate heterogeneity with respect to responsiveness to these cytokines.
Keywords: LPS, cytokines, heterogeneity, NO, iNOS, nitric oxide synthase
Nitric oxide (NO) is an endogenously derived free radical gas that affects a spectrum of homeostatic and physiologic processes, including blood flow and coagulation, normal brain and heart function, and innate immunity (Belge et al. 2005; Ignarro et al. 1999; MacMicking et al. 1997; Yun et al. 1996). However, it can be harmful under certain pathophysiological conditions. Within the CNS, aberrant NO production has been linked to cerebrovascular dysfunction (Thiel and Audus 2001), pathogenesis of autoimmune-mediated demyelination (Smith and Lassmann 2002), and neurodegeneration associated with cerebral ischemia as well as Parkinson and Alzheimer diseases (Iadecola et al. 1997; Liberatore et al. 1999; Nathan et al. 2005). The cytotoxic actions of NO involve oxidative mechanisms that are precipitated by reactive metabolites, such as s-nitrosothiol and/or peroxynitrite (Moncada and Bolanos 2006), which can cause functional alterations in the cytoplasm (Chung et al. 2005; Chung et al. 2004), endoplasmic reticulum (Uehara et al. 2006), mitochondria (Barsoum et al. 2006; Moncada and Bolanos 2006), and nucleus (Dawson and Dawson 2004). Whether NO is beneficial or detrimental depends on the context in which it is produced and on the isoform of nitric oxide synthase (NOS; EC 1.14.13.39) that catalyze its formation (Iadecola 1997).
NO is derived from L-arginine by the catalytic action of three different NOS isoforms (Stuehr 1999). NOS-1 and -3 are constitutively expressed in neurons and endothelial cells, respectively, and thus were initially termed nNOS and eNOS. In contrast, NOS-2 is not constitutively expressed but can be induced in many cell types by inflammatory mediators and, as such, was initially termed inducible NOS or iNOS. NO production by NOS-1 and -3 is coupled to elevated intracellular calcium by transient binding of calmodulin. However, calmodulin is tightly associated with NOS-2 at basal intracellular calcium levels, leading to sustained catalytic activity. The copious amount of NO derived from this isozyme, while an important component of the armamentarium against invading pathogens (MacMicking et al. 1997), accounts for much of the pathophysiology attributed to NO. This notion is supported by results from numerous studies demonstrating that selective inhibition of NOS-2 or NOS-2 gene deletion attenuates the neuropathological sequelae seen in several models of neurological diseases/disorders (Cross et al. 1994; Iadecola et al. 1997; Iadecola et al. 1995; Liberatore et al. 1999; MacMicking et al. 1995; Nathan et al. 2005; Thiemermann et al. 1993).
Astrocytes are among the resident cells of the CNS that express NOS-2 under inflammatory conditions. Inflammatory mediators induce NOS-2 expression in astrocyte cultures in vitro (Galea et al. 1992; Hewett et al. 1993; Simmons and Murphy 1992) and NOS-2 has been detected in astrocytes in several CNS inflammatory conditions in vivo, including cerebral ischemia and Alzheimer and Parkinson diseases (Acarin et al. 2002; Endoh et al. 1994; Garcion et al. 1998; Katsuse et al. 2003; Kwak et al. 2005; Luth et al. 2002). Importantly, NO from glial NOS-2 has been shown to disrupt the blood-brain barrier (Boveri et al. 2006) and promote or exacerbate excitotoxic neuronal cell death (Bal-Price and Brown 2001; Hewett et al. 1994; Hewett et al. 1996; Vidwans and Hewett 2004), an underlying cause of neuropathology in several neurological diseases (Choi 1988; Coyle and Puttfarcken 1993; Meldrum and Garthwaite 1990). Thus, perturbation of astrocyte function under neuroinflammatory conditions associated with CNS disease or trauma may seriously impinge on the normal CNS function and survival of neurons. These results underscore the need to understand the regulation of NOS-2 expression and function in astrocytes.
Lipopolysaccharide (LPS) in combination with interferon-γ (IFNγ) is a pro-inflammatory stimulus that is frequently used to study the regulation of NOS-2 protein expression and function in cultures of cells, including cortical astrocytes (Bal-Price and Brown 2001; Hewett et al. 1993). Interestingly, previous results demonstrated that only 5–10% of astrocytes in culture could be induced to express NOS-2 protein following exposure to LPS plus IFNγ indicating that these cells were not uniformly responsive to the stimulus (Hamby et al. 2006). Paradoxically, transforming growth factor-β1 (TGFβ1), a cytokine with anti-inflammatory properties, enhanced NO production from astrocytes treated with LPS plus IFNγ and this correlated with a 6-fold increase in the fraction of cells that expressed NOS-2 protein (Hamby et al. 2006). Since TGFβ1 alone did not induce NOS-2, this suggested that TGFβ1 enabled additional astrocytes to respond to LPS plus IFNγ. The question remained whether this population-based upregulation of NOS-2 expression was specific to TGFβ1, or rather, represented a more general mechanism by which NOS-2 expression could be enhanced. Hence, the present study sought to determine if the classical pro-inflammatory cytokine tumor necrosis factor-α (TNFα) also potentiated NO production by a similar population-based mechanism. The results raise the possibility that the extent to which astrocytes contribute to NO production at sites of neuroinflammation may be dictated not only by the cytokines that stimulate NOS-2 expression but also by an intrinsic heterogeneity among astrocytes in their ability to respond to these cytokines.
EXPERIMENTAL PROCEDURES
Cell Culture
Purified primary astrocyte cultures were prepared from postnatal day 1–3 CD-1 mice as described previously (Hamby et al. 2006) or similarly derived from TNFα gene targeted mutant mice or its recommended wild-type control strain (Stock #s 003008 and 101045, respectively, The Jackson Laboratory). Cerebral cortices were dissected and dissociated aseptically and cells plated in 15 mm 24-well (1–1.5 hemispheres/10 mL/plate; Falcon Primaria, BD Biosciences) or 35 mm 6-well (1.2–1.6 hemispheres/12 mL/plate; Falcon Primaria, BD Biosciences) plates. Plating media consisted of media stock (MS) supplemented with 10% fetal bovine serum (FBS), 10% iron-supplemented calf serum (CS), 50 IU/mL penicillin, 50 µg/mL streptomycin, and 10 ng/mL epidermal growth factor. MS was comprised of modified Eagle’s medium (Earle’s salt) containing L-glutamine, glucose, and sodium bicarbonate at final concentrations of 2 mM, 25.7 mM, and 28.2 mM, respectively. After ~7 days at 37°C in humidified air containing 6% CO2, contact-inhibited cell monolayers were cultured with 8 µM cytosine β-D-arabinofuranoside for 5–6 days to antagonize proliferation of microglia. Thereafter, cultures were maintained in MS containing 10% CS, 50 IU/mL penicillin, 50 µg/mL streptomycin and 2 mM L-glutamine and used in studies between 14–31 days after plating. All cultures were treated with 75 mM L-leucine methyl ester for 60 min one day prior to experimentation to further reduce microglia contamination (Hamby et al. 2006). All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Connecticut Health Center.
Cell Stimulation
The combination of LPS (2 µg/mL; E. coli 0127:B8, Difco Laboratories, Inc.) plus IFNγ (3 ng/mL; recombinant mouse; R&D Systems) was used to induce expression of NOS-2 in mouse primary astrocyte cultures since either LPS or IFNγ alone are weak stimuli (Hamby et al. 2006; Hewett et al. 1993; Vidwans et al. 2001). These concentrations were empirically determined to provide a saturating response with respect to astrocytic NO production (Hamby et al. 2006). Stimulation medium consisted of DMEM supplemented with 5% CS, 2 mM L-glutamine, 50 IU/mL penicillin, and 50 µg/mL streptomycin. Some cultures of astrocytes were pre-treated for 24 hr with stimulation medium containing TGFβ1 (3 ng/mL; human recombinant; R&D Systems) or its vehicle (750 ng/mL BSA plus 3 µM HCl) prior to addition of LPS plus IFNγ. To assess the effects of TNFα on NOS-2 expression, cells were stimulated with LPS plus IFNγ concurrently with 0.01–3.0 µg/mL (0.39–116 nM) recombinant mouse TNFα (Alexis Biochemicals). In one study, 3 ng/mL TGFβ1 or 1.0 µg/mL TNFα were administered at various times between 0 and 24 hr prior to LPS plus IFNγ addition. Finally, to examine the possible contribution of endogenous TNFα or TGFβ1 in the potentiation of NO production by TGFβ1 or TNFα, respectively, cultures were treated in the presence of 100 or 30 µg/mL polyclonal anti-TNFα (rabbit IgG fraction, Calbiochem) or monoclonal anti-TGFβ (mouse IgG1, RD Systems) neutralizing antibodies or the appropriate control antibodies.
Assessment of NOS-2 Function
NOS-2-derived nitric oxide (NO) production was assessed indirectly by measurement of nitrite, a stable oxidative breakdown product of NO (Green et al. 1982). Cell culture medium (100 µL) was mixed with an equal volume of Griess Reagent prepared fresh by combining 1 part 0.1% (wt/vol) sulfanilamide in 60% (vol/vol) acetic acid with 1 part 0.1% (wt/vol) napthylenediamine dihydrochloride in distilled H2O. The absorbance at 550 nm was measured using a microtiter plate reader (Thermolabs).
Assessment of TNFα mRNA Expression
Total RNA was isolated (TRIzol, Life Technologies), purified, and resuspended in 20 µL H2O. First-strand cDNA was synthesize from 500 ng total RNA using reverse transcriptase and oligo(dT)(12–18) as described previously (Hamby et al. 2006; Hewett et al. 1999). cDNA samples (1 µL) were amplified for 27 (TNFα) or 23 cycles (β-actin) in a Biorad iCycler using Taq DNA polymerase (Invitrogen) and target-specific primers in a total volume of 25 µL. PCR amplimer pairs for mouse TNFα mRNA were 5’-CAGGTCTACTTTGGAGTCATTGC-3’ (sense) and 5’-CCAGCATCTTGTGTTTCTGAGTA-3’ (antisense). β-actin mRNA was also assessed to control for the amount and the integrity of RNA in each sample. Amplimers for analysis of β-actin mRNA were 5’-GTGGGCCGCTCTAGGCACCAA-3’ (sense) and 5’- CTCTTTGATGTCACGCACGATTTC-3’ (antisense). PCR products were separated by electrophoresis in 1% agarose along with 1 kb size markers (Gibco/BRL) and visualized by ethidium bromide using a UV transilluminator. Data was recorded using the Kodak Electrophoresis Documentation and Analysis System 120 and processed with Adobe Photoshop.
Quantification of the Percentage of Astrocytes Expressing NOS-2
The number of astrocytes expressing NOS-2 protein was determined by indirect immunofluorescence microscopy as described previously (Hamby et al. 2006). Cultures were fixed for 5 min with a freshly prepared solution of acetone/methanol and then incubated for 7 min with 0.25% Triton X-100 (vol/vol PBS). Cells were washed with PBS and non-specific binding sites blocked with 10% NGS in PBS (25°C, 1 hr). Rabbit polyclonal anti-NOS-2 antibody (2 µg/mL, Upstate Cell Signaling Solutions) was added in PBS containing 5% NGS (4°C overnight). Bound anti-NOS-2 antibody was visualized using Cy3-conjugated goat anti-rabbit IgG antibody (7.5 µg/mL; 25°C, 60 min; Jackson ImmunoResearch). DAPI (28 ng/mL; Molecular Probes) was included during the secondary antibody incubation to label nuclei. Images of three to five microscopic fields (40x magnification final) were acquired from each culture well using a 20x objective (Olympus, 0.4 NA) and a CRX digital camera (Digital Video Camera Co) mounted on an Olympus IX50 inverted microscope outfitted with epifluorescence and processed identically using Adobe Photoshop software. The number of DAPI-labeled nuclei/image was quantified using Scion NIH Image software, while the number of NOS-2 positive cells/image was determined by manual counting as described previously (Hamby et al. 2006). The percent of cells/image that expressed NOS-2 was calculated by dividing the number of NOS-2-positive cells by the total number of cells in each field (i.e. DAPI-labeled nuclei).
GFAP and NOS-2 Co-localization
To assess colocalization of NOS-2 protein with the astrocyte-specific marker, glial fibrillary acidic protein (GFAP), cultures were processed for immunofluorescence microscopy as described above except that cultures were incubated with anti-NOS-2 antibody together with rat monoclonal anti-bovine GFAP antibody (1 µg/mL, Zymed). Primary antibody binding was visualized with Alexa 488-conjugated goat anti-rabbit IgG antibody (10 µg/mL; Molecular Probes) and Cy3-conjugated goat anti-rat IgG antibody (15 µg/mL; Jackson ImmunoResearch), respectively. Nuclei were labeled with DAPI and images were acquired using a 40x objective (Olympus, 0.6 NA) and processed as described above. No cross-reactivity between primary and secondary antibodies from the different species was detected.
Statistical Analysis
Statistics were performed using GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego). Where necessary, analyses were performed on Log(Y) transformed data. For cell counts, % data were transformed using arcsine square root transformation prior to analysis (Steel and Torrie 1980). Specific tests are indicated in figure legends.
RESULTS
Comparison between TNFα- and TGFβ1-induced potentiation of NO production
Highly enriched primary mouse astrocyte monolayers did not produce NO when treated for 24 hr with 1.0 µg/mL TNFα by itself (nitrite = 0.39 ± 0.05 and 0.20 ± 0.06 µM in untreated and TNFα-treated cultures, respectively). However, when added concurrently with LPS plus IFNγ, TNFα potentiated NO production in a concentration-dependent manner (Figure 1A). Production of NO was enhanced more than 2-fold by 0.01 µg/mL TNFα, whereas 1.0 αg/mL TNFα (38 nM) potentiated NO production nearly to the same extent as a maximally effective concentration of TGFβ1 (3 ng/mL) [Figure 1A, (Hamby et al. 2006)]. The EC50 for TNFα was 0.064 ± 0.028 µg/mL (2.5 ± 1.1 nM), which was similar to that reported for C6 glioma cells (Feinstein et al. 1994).
Figure 1. Characterization of TNFα-induced potentiation of astrocytic NO production.
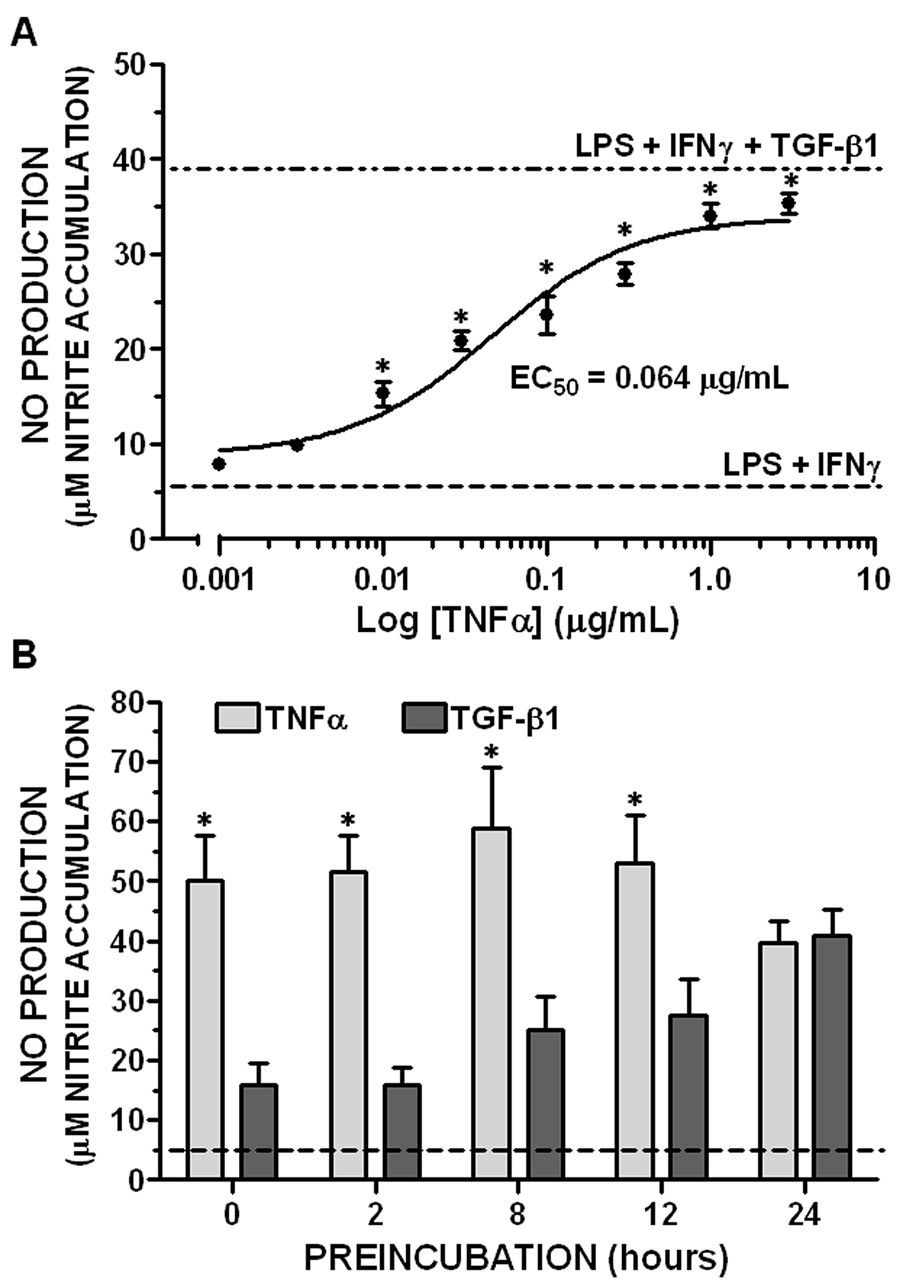
A. TNFα concentration response. Primary astrocyte monolayers were treated concurrently with LPS plus IFNγ (2 µg/mL and 3 ng/mL, respectively) and the indicated concentrations TNFα. NO production was assessed by nitrite accumulation 24 hr later as described in “Experimental Procedures” and results expressed as the mean ± SEM; n = 4–5 cultures from 2 separate dissections. Dashed line (“LPS + IFNγ”) = NO production induced by LPS plus IFNγ in the absence of TNFα. Dash-dot line (“LPS + IFNγ + TGFβ1”) = parallel cultures treated with 3 ng/mL TGFβ1 for 24 hr prior to stimulation with LPS plus IFNγ.
*, significantly different from “LPS + IFNγ” treatment group (one-way ANOVA, Dunnet’s post-test, p<0.05).
B. Effect of preincubation. Astrocyte monolayers were treated with TNFα (1 µg/mL) or TGFβ1 (3 ng/mL) for the indicated times prior to addition of LPS plus IFNγ. 0 = concurrent administration with LPS plus IFNγ. NO production (nitrite accumulation) was measured 36 hr after LPS plus IFNγ. Results are expressed as mean + SEM; n = 9 cultures from 3 separate dissections. Dashed line = stimulation with LPS plus IFNγ alone.
*, significant difference between cytokine treatment groups (two-way ANOVA, Bonferroni post-test, p<0.05).
Previous results indicated that optimal potentiation of astrocytic NO production by TGFβ1 required preincubation with the cytokine for 24 hr prior to LPS plus IFNγ administration (Hamby et al. 2006); this was recapitulated herein for the purpose of comparison with TNFα (Figure 1B). In contrast to TGFβ1, potentiation of LPS plus IFNγ-induced NO production by TNFα did not require preincubation. Thus, maximal potentiation was observed when TNFα was added concurrently with LPS plus IFNγ. However, TNFα persisted to effectively potentiate NO production even when administered for up to 24 hr prior to LPS plus IFNγ (Figure 1B).
TNFα expands the pool of astrocytes that express NOS-2
The effect of TNFα on NOS-2 protein expression in astrocyte monolayers was assessed by immunofluorescence microscopy under conditions that optimally potentiated LPS plus IFNγ-induced NO production (identified in Figure 1). TNFα increased the pool of astrocytes that Figure 1 expressed NOS-2 in a manner that closely approximated that elicited by TGFβ1 (Figure 2), in spite of the different exposure conditions for each cytokine (concurrent vs. 24 hr pretreatment, respectively). In this analysis, LPS plus IFNγ induced NOS-2 expression in ~10% of astrocytes and this was augmented to ~25 and 30% by either TNFα or TGFβ1, respectively.
Figure 2. Quantification of the number of astrocytes expressing NOS-2 protein.
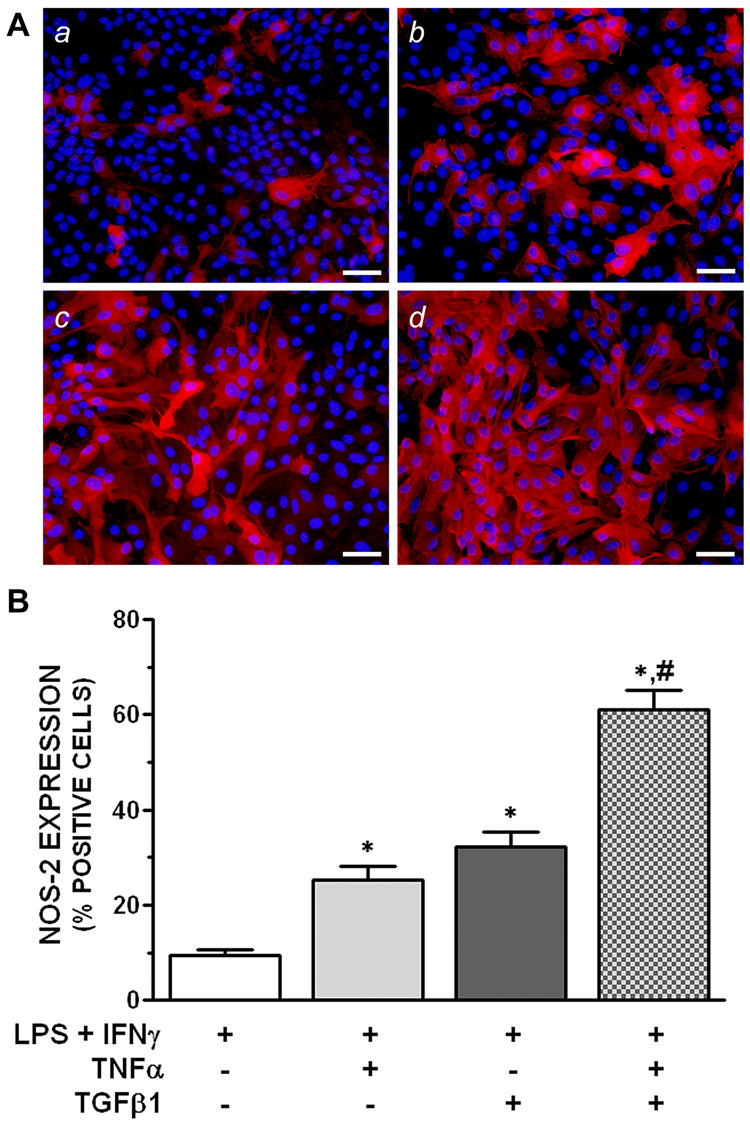
A. Representative fluorescent images. Astrocyte cultures were treated with LPS plus IFNγ (2 µg/mL and 3 ng/mL, respectively) without or with 1 µg/mL TNFα and/or 3 ng/mL TGFβ1. NOS-2 protein expression was assessed by immunofluorescence microscopy 12 hr after LPS plus IFNγ as described in “Experimental Procedures”. TNFα and TGFβ1 were added concurrently with or 24 hr prior to LPS plus IFNγ, respectively. (a) LPS + IFNγ alone, (b) LPS + IFNγ + TNFα, (c) LPS + IFNγ + TGFβ1, (d) LPS + IFNγ + TGFβ1 + TNFα. NOS-2 (red) and DAPI (blue) images were obtained from the same microscopic fields and superimposed using Adobe Photoshop. Scale bar = 50 µm.
B. Percent cells expressing NOS-2. The number of cells positive for NOS-2 immunoreactivity is expressed as a % of DAPI-labeled nuclei (“Experimental Procedures”). Results are expressed as means + SEM; n = 9 cultures from 3 separate dissections.
*, significantly different from “LPS + IFNγ” treatment group.
#, significantly different from the effect of each cytokine alone (one-way ANOVA, Student-Newman-Keul’s, p<0.05).
TGFβ1 and TNFα additively increase the population of astrocytes that express NOS-2
Since TGFβ1 and TNFα affect LPS plus IFNγ-induced NO production by a similar population-based mechanism (i.e., augmenting the pool of cells expressing NOS-2), it was of interest to examine whether the cytokines targeted overlapping or separate populations of astrocytes. For example, if they targeted the same population, then treatment with both TGFβ1 and TNFα should not further augment the population of NOS-2-positive cells. On the other hand, if they targeted separate populations of astrocytes, then treatment with both cytokines should further enhance the population of cells expressing NOS-2 to a value that approximates the sum of the two cytokines alone (i.e., be additive). In fact, stimulation of cells with LPS plus IFNγ together with both TNFα and TGFβ1 further augmented the population of NOS-2-positive cells to ~60%. Thus, the effect of the cytokines together was additive relative to each cytokine alone (Figure 2).
While great care was taken to ensure that astrocyte cultures consisted of highly purified GFAP-positive cells (Hamby et al. 2006), it was nevertheless important to confirm that NOS-2 expression after combined treatment with LPS, IFNγ, TGFβ1 and TNFα was localized to GFAP-positive astrocytes. Results in Figure 3 demonstrate that NOS-2 immunoreactivity in cultures indeed colocalized with GFAP. This, together with the additive nature of TGFβ1 and TNFα, suggests that the two cytokines target different populations of astrocytes within the same monolayer culture.
Figure 3. Analysis of colocalization of NOS-2 and GFAP immunoreactivity.
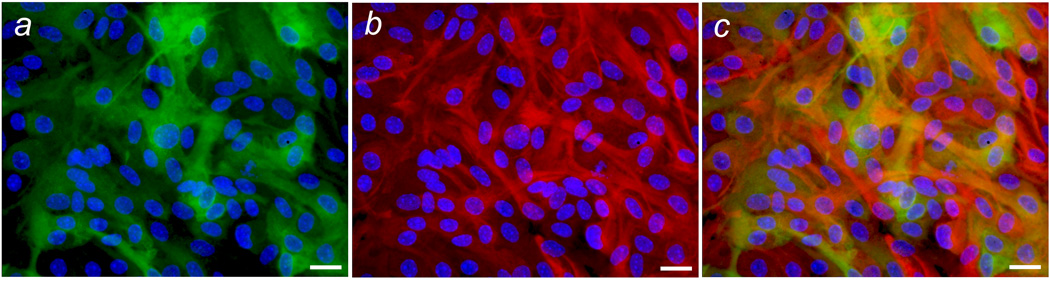
Astrocyte cultures were treated with both TNFα and TGFβ1 as described in Figure 2 and exposed to LPS plus IFNγ for 14 hr, after which NOS-2 and GFAP immunoreactivity was assessed by immunofluorescence microscopy. Representative fluorescent images for NOS-2 (green), GFAP (red) and DAPI (blue) were obtained from the same microscopic field and superimposed using Adobe Photoshop. (a) NOS-2 and DAPI; (b) GFAP and DAPI; (c) NOS-2,GFAP, and DAPI. Scale bar = 20 µm.
TNFα and TGFβ1 affect astrocytic NO production independently
The notion that TNFα and TGFβ1 recruit separate populations of astrocytes to respond to LPS plus IFNγ presupposes that TNFα acts independent of endogenous TGFβ1 and visa versa. wever, TNFα has been shown to induce expression of TGFβ1 (Churg et al. 2002; Sullivan et al. 2005). Therefore, it was necessary to determine the extent to which endogenous release of TGFβ1 contributed to the potentiation of LPS plus IFNγ-induced NO production by TNFα. This was assessed using an anti-TGFβ antibody that effectively blocked the potentiation of LPS plus IFNγ-induced NO production by 3 ng/mL exogenous TGFβ1 (nitrite = 42.2 ± 2.75 and 8.24 ± 0.59 µM in the presence of 30 µg/ml control and anti-TGFβ antibodies, respectively). The anti-TGFβ antibody significantly reduced NO production induced by LPS plus IFNγ in either the absence or presence of exogenous TNFα (Figure 4A). Moreover, a slight but significant difference remained after subtracting the responses elicited by LPS plus IFNγ alone from the respective antibody groups in the presence of TNFα (inset, Figure 4A). Although these results suggest a modest contribution of endogenous TGFβ under both stimulation conditions, the potentiation of LPS plus IFNγ-induced NO production by TNFα is largely independent of endogenous TGFβ1.
Figure 4. Effect of cytokine neutralizing antibodies on potentiation of NO.
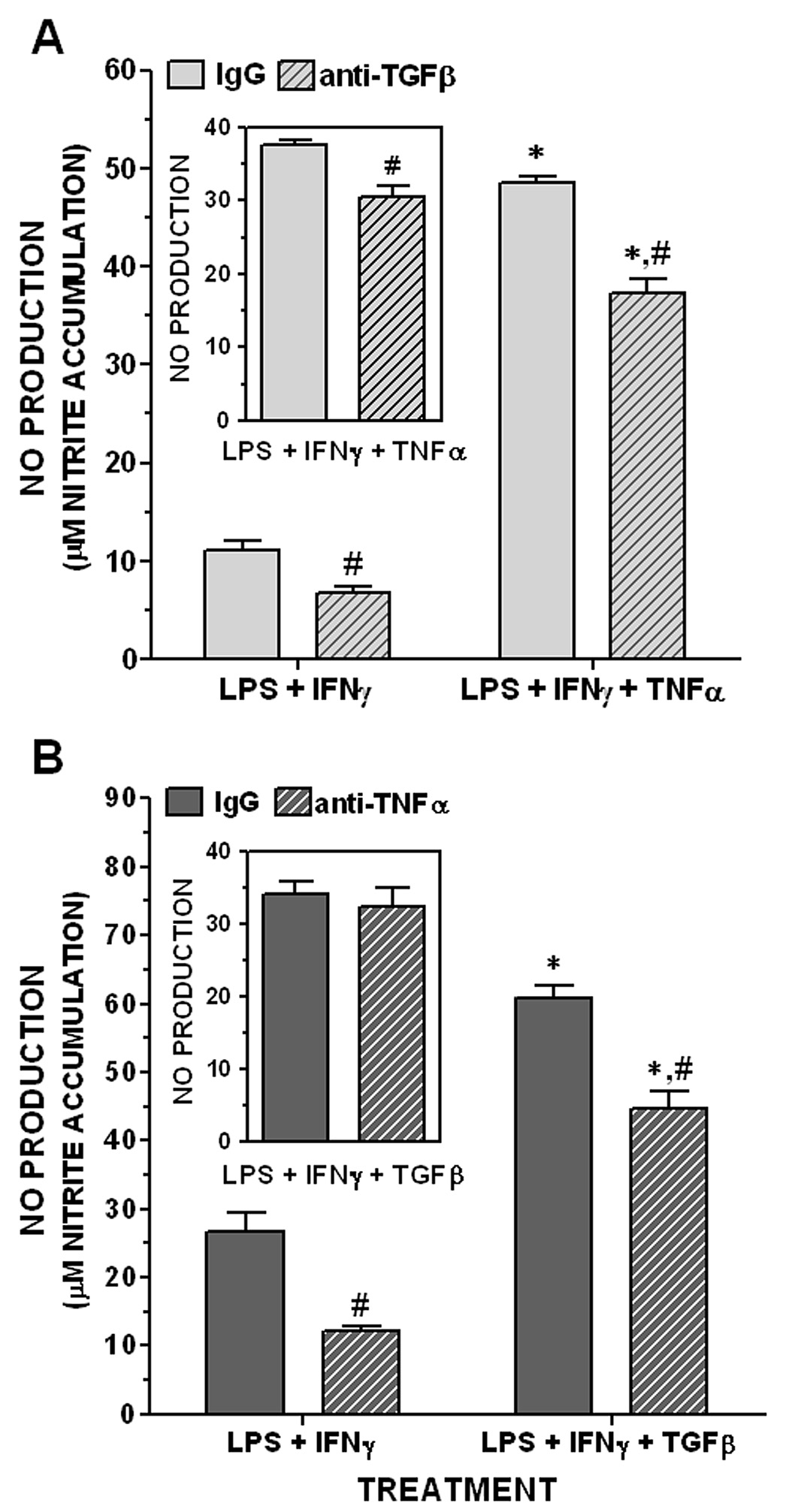
A. Effect of TGFβ1 neutralization on TNFα potentiation. Astrocyte cultures were stimulated with LPS plus IFNγ (2 µg/mL and 3 ng/mL, respectively) without or with 1 µg/mL TNFα as described in Figure 2. Stimulation was performed in the presence of 30 µg/mL anti-TGFβ antibody or its non-immune control (“IgG”) and NO production was assessed 24 hr later by nitrite accumulation. Inset, values in the presence of TNFα (“LPS + IFNγ + TNFα”) after subtracting the respective values in the absence of TNFα (“LPS + IFNγ”). Results are expressed as means + SEM; n = 4 cultures from 2 separate dissections.
*, significant potentiation over respective “LPS + IFNγ” treatment group (two-way ANOVA and Bonforroni post-test, p<0.05);
#, significant difference between IgG and anti-TGFβ treatment groups (t-test, p<0.05).
B. Effect of TNFα neutralization on TGFβ1 potentiation. Astrocyte cultures were pretreated for 24 hrs without or with 3 ng/mL TGFβ1 in the presence of 100 µg/mL of anti-TNFα antibody or its isotype-matched control (“IgG”). Cultures were stimulated 24 hr later with LPS plus IFNγ (2 µg/mL and 3 ng/mL, respectively) and NO production was assessed as in A. Inset, values in the presence of TGFβ1 (“LPS + IFNγ + TGFβ”) after subtracting the respective values in the absence of TGFβ1 (“LPS + IFNγ”). Results are expressed as means + SEM; n = 8 cultures.
*, significant potentiation over respective “LPS + IFNγ” treatment group (two-way ANOVA and Bonforroni post-test, p<0.05).
#, significant difference between IgG and anti-TNFα treatment groups (t-test, p<0.05).
Conversely, although TGFβ1 can negatively regulate TNFα production both in vitro and in vivo, perhaps via induction of tristetraprolin expression (Espevik et al. 1987; Ogawa et al. 2003), it can induce expression of TNFα in certain cell types (Chantry et al. 1989; Grammas and Ovase 2002). Therefore, it was also necessary to determine the extent to which endogenous release of TNFα contributed to the potentiation of LPS plus IFNγ-induced NO production by TGFβ1. This was assessed using an anti-TNFα antibody that effectively neutralized the potentiation of LPS plus IFNγ-induced NO production by 30 ng/ml exogenous TNFα (nitrite = 19.3 ± 1.81 and 3.96 ± 0.44 µM in the presence of 100 µg/mL control IgG and anti-TNFα antibody, respectively). Even though the anti-TNFα antibody attenuated NO production stimulated by LPS plus IFNγ without or with TGFβ1 (Figure 4B), no significant difference in the TGF-β1-induced potentiation of NO production was observed between the antibody treatment groups after the respective values for LPS plus IFNγ alone were subtracted (inset, Figure 4B). Thus, endogenous TNFα does not contribute to the potentiation by TGFβ1. In agreement with this result, TGFβ1 potentiation of LPS plus IFNγ-induced NO production and NOS-2 expression was fully retained in astrocyte cultures derived from TNFα null mice (Table 1). These results are consistent with the observation that TGFβ1 did not induce TNFα mRNA in astrocytes cultured from wild-type CD-1 mice (Figure 5). This latter effect was not due to the lack of ability of these cultures to express endogenous TNFα, since LPS plus IFNγ stimulated a time-dependent increase in TNFα mRNA expression (Figure 5). This likely explains the attenuation of LPS plus IFNγ-induced NO production by the anti-TNFα antibody (Figure 4B).
Table 1. Effect of TNFα gene deletion on TGFβ1-induced potentiation.
Astrocytes cultured from mice lacking TNFα (TNFα −/−) or their wild-type controls (TNFα +/+) were treated with or without 3 ng/mL TGFβ1 for 24 h prior to stimulation with LPS plus IFNγ (2 µg/mL and 3 ng/mL, respectively). NO production (nitrite accumulation) was quantified and the number of cells positive for NOS-2 immunoreactivity (%) was determined 16 hr later (see “Experimental Procedures”). Data are expressed as the TGFβ1-induced increase in NO production or NOS-2-positive cells relative to LPS plus IFNγ alone (Means ± SEM); n = 3 from 3 separate dissections. Significant differences in NO production or NOS-2 expression between genotypes were assessed using an unpaired t-test on data transformed by log or arcsin square root, respectively. No significant differences were detected (p<0.05).
| Mouse strain | NO PRODUCTION (fold increase by TGF-β1 ± SEM) | NOS-2 Positive Cells (fold increase by TGF-β1 ± SEM) |
|---|---|---|
| TNFα +/+ | 6.18 ± 1.27 | 9.99 ± 2.08 |
| TNFα −/− | 4.79 ± 1.33 | 13.13 ± 5.35 |
Figure 5. Effect of TGF²1 on TNF± mRNA expression in astrocytes.
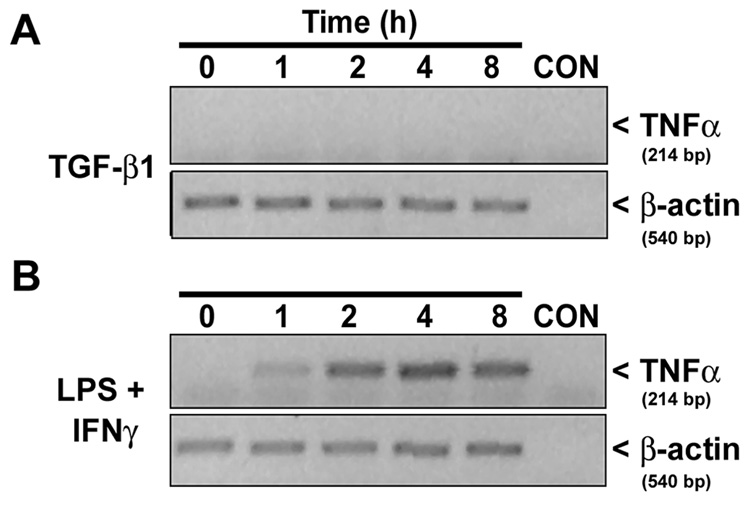
Astrocyte cultures were treated with either 3 ng/mL TGFβ1 alone (A) or 2 µg/mL LPS plus 3 ng/mL IFNγ (B) and total RNA was harvested at the times indicated. TNFα and β-actin mRNA levels were assessed via RT-PCR (see “Experimental Procedures”). CON = PCR reaction mix minus cDNA. Results are representative of two separate experiments on cultures from independent dissections.
DISCUSSION
The following conclusions can be drawn from the data reported herein. First, although TNFα has been shown to enhance NOS-2-derived NO production induced by LPS or LPS plus IFNγ (Feinstein et al. 1994; MacNaul and Hutchinson 1993; Pahan et al. 1998), results from the present study are the first to demonstrate that TNFα mediates this enhancement, like TGFβ1, by expanding the pool of astrocytes that express NOS-2. Moreover, these results indicate that this mode of regulating gene expression (i.e., population-based) may be a more general mechanism by which certain cytokines increase NO production from astrocytes. Second, the two cytokines additively increased the number of astrocytes that expressed NOS-2, suggesting that TGFβ1 and TNFα recruit different populations of astrocytes to express NOS-2. It is remarkable that the two cytokines produced an additive effect when administered together in the same culture preparations, given the well documented antagonism between TGFβ1 and TNFα intracellular signal transduction pathways (Bitzer et al. 2000; Schiffer et al. 2001). This further supports the contention that astrocyte monolayers are composed of two subpopulations that are exclusively responsive to only one of the two cytokines, at least with respect to induction of NOS-2 expression. Third, TGFβ1 and TNFα enhance NOS-2 expression by independent and distinct molecular mechanisms. Thus, neither cytokine affected NOS-2 expression via induction of endogenous production of the other, and TGFβ1 but not TNFα required a pretreatment period for optimal enhancement of NOS-2 expression. Take together, the present results raise the intriguing possibility that different populations of astrocytes may be recruited to produce NO at sites of neuroinflammation depending on the cytokines elaborated, and further, imply that the local milieu (i.e., cytokine composition) may determine the strength of the tissue reaction by controlling the number of astrocytes that respond (i.e., express NOS-2). Implicit in this hypothesis is the notion of astrocyte heterogeneity.
Early histochemical analyses of the CNS suggested that astrocytes could be classified by morphological criteria as radial, fibrous or protoplasmic. These anatomical distinctions indicated that astrocytes were structurally heterogeneous in vivo (Somjen 1988). Fibrous and protoplasmic astrocytes, which have since been referred to as type 1 and 2 astrocytes, respectively, also exhibit differences in distribution between white and gray matter and can be distinguished by growth characteristics and antigenic properties as well (Raff et al. 1983). It is now becoming clear that astrocytes from various functionally distinct regions of the CNS (e.g., hippocampus, cerebellum, spinal cord, etc.) differ phenotypically (Blomstrand et al. 1999; Cholewinski et al. 1988;Cholewinski and Wilkin 1988; Cholewinski and Wilkin 1988; Hosli and Hosli 1993; Morga et al. 1998; Morga et al. 1999; Porter and McCarthy 1997; Sousa Vde et al. 2004; Stadlin et al. 1998; Wink et al. 2003). For example, astrocytes in the hippocampus and cerebellum express different levels of glutamate transporter isoforms (Lehre et al. 1995). These results suggest that regional astrocyte heterogeneity may serve a functionally important role (Blomstrand et al. 1999; Hosli and Hosli 1993; Porter and McCarthy 1997; Song et al. 2002; Wilkin et al. 1990; Wink et al. 2003). This is supported by the demonstration that astrocytes contribute to the generation of new neurons in the adult hippocampus, where neurogenesis is sustained throughout life, but not in the adult spinal cord (Song et al. 2002), where it is not.
In addition to regional specialization, there is evidence for localized phenotypic diversity among astrocytes even within a given region of the CNS (e.g., within the hippocampus, cerebral cortex, etc.) (Bowman et al. 2003; Conti et al. 1994; Hamby et al. 2006; Hosli and Hosli 1993; Imura et al. 2006; Matthias et al. 2003; Miller and Szigeti 1991; Nikcevich et al. 1997; Porter and McCarthy 1995; Shao et al. 1994; St-Pierre et al. 2000; Swanson et al. 1997; Venance et al. 1998; Whitaker-Azmitia et al. 1993; Zhou and Kimelberg 2001). For example, both in vitro and in vivo studies show that the expression of glutamate transporter isoforms GLT-1 and GLAST segregate to different hippocampal GFAP-positive astrocytes (Conti et al. 1998; Perego et al. 2000; van Landeghem et al. 2006). Also, within a hippocampal slice culture, Matthias et al. reported the existence of two mutually exclusively subpopulations of astrocytes which exhibit morphologically and functionally distinct properties (Matthias et al. 2003). The results from the current report now suggest that astrocytes cultured from the cerebral cortex are also comprised of different subpopulations, as defined by the ability to express NOS-2 when exposed to either TGFβ1 or TNFα, thus providing additional evidence for the existence of localized astrocyte heterogeneity. The fact that these putative subpopulations accounted for only approximately twothirds of the total population suggests that there is a third population that is not recruited by either factor.
It has been argued that functional heterogeneity is not an innate property of astrocytes but instead reflects plasticity of a single astrocyte population that is tailored to suit the needs of different environments within the CNS (Walz 2000). In support of this, differences in astrocytic glutamate transporter expression are influenced by neurons, which appear to affect expression levels in an activity-dependent manner (Gegelashvili et al. 1997; Perego et al. 2000; Schlag et al. 1998; Swanson et al. 1997). On the other hand, regional differences in the neurogenic activity of astrocytes (Song et al., 2002), as well as the local differences in NOS-2 expression reported herein, were observed in monolayers of purified primary astrocytes cultured in a homogeneous environment devoid of neurons. These observations suggest that certain forms of astrocyte heterogeneity may indeed be innate or, in any case, are stable in culture.
How the heterogeneity in cytokine responsiveness arises within an astrocyte population remains to be determined. In culture, small numbers of seed cells expand clonally and coalesce to form confluent monolayers of astrocytes. Thus, it might be posited that different precursors could give rise to subpopulations of astrocytes with different phenotypic characteristics. However, preliminary results suggest that heterogeneity in response to cytokines occurs early in culture within individual colonies of astrocytes (unpublished observation). This argues against the role for phenotypic diversity between clonal subpopulations. Indeed, this result demonstrates that cells from individual precursors diverge during the proliferative growth phase in culture prior to becoming contact inhibited. It is possible that this phenotypic divergence within an astrocyte population is analogous to the heterogeneity in antibiotic resistance exhibited by bacteria [referred to as “persisters”, (Balaban et al. 2004)] and may be a manifestation of the postulated “extrinsic noise” by which phenotypic diversity may be generated within a population (Newman and Weissman 2006; Raser and O'Shea 2005). Regardless, further studies are necessary to identify the molecular determinants of this heterogeneity.
One obvious possible molecular explanation for the heterogeneity is the partitioning of TNFα and TGFβ1 cell surface receptors to non-overlapping subpopulations of astrocytes. TNFα signals through type-I (TNFR-I/CD120a) or type-II (TNFR-II/CD120b) cell surface receptors (Baud and Karin 2001). However, whereas rodent astrocytes express TNFR-I, they reportedly lack detectable TNFR-II (Aranguez et al. 1995; Dopp et al. 1997). Intracellular signaling by TGFβ1 in most cells is dependent on a heteromeric complex of type I and II receptors (Lebrin et al. 2005). A preliminary analysis using standard immunofluorescence microscopy found that TNFR-I and both TGFβ1 receptor chains were widely distributed among astrocytes of the monolayer cultures. Indeed, the distribution was much greater than the 25 to 30% of cells recruited to express NOS-2 by the respective cytokines (unpublished observations). This argues that the heterogeneity in cytokine responsiveness is not due simply to the segregation of signaling receptors to distinct astrocyte subpopulations and suggests that the molecular mechanism may be more complex, perhaps involving intracellular signal transduction pathways that enable or silence NOS-2 expression in a given subpopulation. The observation that optimal enhancement of LPS plus IFNγ-induced NO production by TGFβ1 required 24 hr preincubation with the cytokine, whereas TNFα was maximally effective when given simultaneously with the stimulus, is consistent with a fundamental difference in the signaling mechanisms by which these two cytokines recruit their respective populations of astrocytes to respond to LPS plus IFNγ. For example, it is possible that TGFβ1 requires de novo protein synthesis to enable a population of astrocytes to express NOS-2, whereas TNFβ does not.
Further studies to elucidate the molecular determinants that underlie the population-based changes in NOS-2 expression reported herein are needed. Such information should contribute to a better understanding of astrocyte biology and will likely lead to the identification of novel molecular markers that could be employed to distinguish subpopulations of astrocytes. It will also be important to determine whether such heterogeneity applies to inducible genes other than NOS-2 and to cell types other than astrocytes. Finally, although the functional significance of this heterogeneity remains to be clarified, it may have relevance at sites of neuroinflammation where NO contributes to neuropathology, including cerebrovascular dysfunction (Boveri et al. 2006; Mark et al. 2004; Mayhan 1998) and neurodegeneration (Hewett et al. 1994; Iadecola et al. 1997). In any case, the results reported herein from cultures of astrocytes may explain, at least in part, the apparent heterogeneous expression of NOS-2 in astrocytes in vivo under neuroinflammatory conditions (Iravani et al. 2005; Luth et al. 2001; Oleszak et al. 1998).
ACKNOWLEDGEMENTS
Grant sponsors: NINDS, National Institutes of Health, Grant # NS36812 (SJH and JAH) and American Heart Association (SJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acarin L, Peluffo H, Gonzalez B, Castellano B. Expression of inducible nitric oxide synthase and cyclooxygenase-2 after excitotoxic damage to the immature rat brain. J Neurosci Res. 2002;68(6):745–754. doi: 10.1002/jnr.10261. [DOI] [PubMed] [Google Scholar]
- Aranguez I, Torres C, Rubio N. The receptor for tumor necrosis factor on murine astrocytes: characterization, intracellular degradation, and regulation by cytokines and Theiler's murine encephalomyelitis virus. GLIA. 1995;13(3):185–194. doi: 10.1002/glia.440130305. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. Journal of Neuroscience. 2001;21(17):6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory Neurodegeneration Mediated by Nitric Oxide from Activated Glia-Inhibiting Neuronal Respiration, Causing Glutamate Release and Excitotoxicity. J Neurosci. 2001;21(17):6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305(5690):1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. Embo J. 2006;25(16):3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends in Cell Biology. 2001;11(9):372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Belge C, Massion PB, Pelat M, Balligand JL. Nitric oxide and the heart: update on new paradigms. Ann N Y Acad Sci. 2005;1047:173–182. doi: 10.1196/annals.1341.016. [DOI] [PubMed] [Google Scholar]
- Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Bottinger EP. A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA. Genes Dev. 2000;14(2):187–197. [PMC free article] [PubMed] [Google Scholar]
- Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92(1):255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- Boveri M, Kinsner A, Berezowski V, Lenfant AM, Draing C, Cecchelli R, Dehouck MP, Hartung T, Prieto P, Bal-Price A. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: role of pro-inflammatory cytokines and nitric oxide. Neuroscience. 2006;137(4):1193–1209. doi: 10.1016/j.neuroscience.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43(3):281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Chantry D, Turner M, Abney E, Feldmann M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989;142(12):4295–4300. [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Cholewinski AJ, Hanley MR, Wilkin GP. A phosphoinositide-linked peptide response in astrocytes: evidence for regional heterogeneity. Neurochem Res. 1988;13(4):389–394. doi: 10.1007/BF00972490. [DOI] [PubMed] [Google Scholar]
- Cholewinski AJ, Wilkin GP. Astrocytes from forebrain, cerebellum, and spinal cord differ in their responses to vasoactive intestinal peptide. J Neurochem. 1988;51(5):1626–1633. doi: 10.1111/j.1471-4159.1988.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Cholewinski AJ, Wilkin GP. Peptide receptors on astrocytes: evidence for regional heterogeneity. Biochem Soc Trans. 1988;16(4):429–432. doi: 10.1042/bst0160429. [DOI] [PubMed] [Google Scholar]
- Chung KK, Dawson VL, Dawson TM. S-nitrosylation in Parkinson's disease and related neurodegenerative disorders. Methods Enzymol. 2005;396:139–150. doi: 10.1016/S0076-6879(05)96014-X. [DOI] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304(5675):1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor Necrosis Factor-{alpha} Is Central to Acute Cigarette Smoke-induced Inflammation and Connective Tissue Breakdown. Am J Respir Crit Care Med. 2002;166(6):849–854. doi: 10.1164/rccm.200202-097OC. [DOI] [PubMed] [Google Scholar]
- Conti F, DeBiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate tranporter, is localized to astrocytes and gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb Cortex. 1998;8(2):108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A, Brecha NC. Cellular localization and laminar distribution of AMPA glutamate receptor subunits mRNAs and proteins in the rat cerebral cortex. J Comp Neurol. 1994;350(2):241–259. doi: 10.1002/cne.903500208. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Cross AH, Misko TP, Lin RF, Hickey WF, Trotter JL, Tilton RG. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994;93(6):2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Deadly conversations: nuclear-mitochondrial cross-talk. J Bioenerg Biomembr. 2004;36(4):287–294. doi: 10.1023/B:JOBB.0000041755.22613.8d. [DOI] [PubMed] [Google Scholar]
- Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. Journal of Neuroimmunology. 1997;75(1–2):104–112. doi: 10.1016/s0165-5728(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res. 1994;651(1–2):92–100. doi: 10.1016/0006-8993(94)90683-1. [DOI] [PubMed] [Google Scholar]
- Espevik T, Figari IS, Shalaby MR, Lackides GA, Lewis GD, Shepard HM, Palladino MA., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Roberts S, Berquist H, Wang H, Reis DJ. Induction of nitric oxide synthase in rat C6 glioma cells. J Neurochem. 1994;62(1):315–321. doi: 10.1046/j.1471-4159.1994.62010315.x. [DOI] [PubMed] [Google Scholar]
- Galea E, Feinstein DL, Reis DJ. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992;89(22):10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia. 1998;22(3):282–294. [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69(6):2612–2615. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer–s disease brain. Am J Pathol. 2002;160(5):1583–1587. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Hewett JA, Hewett SJ. TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia. 2006;54(6):566–577. doi: 10.1002/glia.20411. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150(1):128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hewett JA, Hewett SJ, Winkler S, Pfeiffer SE. Inducible nitric oxide synthase expression in cultures enriched for mature oligodendrocytes is due to microglia. J Neurosci Res. 1999;56(2):189–198. doi: 10.1002/(sici)1097-4547(19990415)56:2<189::aid-jnr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hewett SJ, Corbett JA, McDaniel ML, Choi DW. Interferon-gamma and interleukin-1 beta induce nitric oxide formation from primary mouse astrocytes. Neurosci Lett. 1993;164(1–2):229–232. doi: 10.1016/0304-3940(93)90898-u. [DOI] [PubMed] [Google Scholar]
- Hewett SJ, Csernansky CA, Choi DW. Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron. 1994;13(2):487–494. doi: 10.1016/0896-6273(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Hewett SJ, Muir JK, Lobner D, Symons A, Choi DW. Potentiation of oxygen-glucose deprivation-induced neuronal death after induction of iNOS. Stroke. 1996;27(9):1586–1591. doi: 10.1161/01.str.27.9.1586. [DOI] [PubMed] [Google Scholar]
- Hosli E, Hosli L. Receptors for neurotransmitters on astrocytes in the mammalian central nervous system. Prog Neurobiol. 1993;40(4):477–506. doi: 10.1016/0301-0082(93)90019-o. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20(3):132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. Journal of Neuroscience. 1997;17(23):9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17(23):9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995;268(1 Pt 2):R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34(6):879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- Imura T, Nakano I, Kornblum HI, Sofroniew MV. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53(3):277–293. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Leung CC, Sadeghian M, Haddon CO, Rose S, Jenner P. The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur J Neurosci. 2005;22(2):317–330. doi: 10.1111/j.1460-9568.2005.04220.x. [DOI] [PubMed] [Google Scholar]
- Katsuse O, Iseki E, Kosaka K. Immunohistochemical study of the expression of cytokines and nitric oxide synthases in brains of patients with dementia with Lewy bodies. Neuropathology. 2003;23(1):9–15. doi: 10.1046/j.1440-1789.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- Kwak EK, Kim JW, Kang KS, Lee YH, Hua QH, Park TI, Park JY, Sohn YK. The role of inducible nitric oxide synthase following spinal cord injury in rat. J Korean Med Sci. 2005;20(4):663–669. doi: 10.3346/jkms.2005.20.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovascular Research. 2005;65(3):599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15(3 Pt 1):1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5(12):1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Luth HJ, Holzer M, Gartner U, Staufenbiel M, Arendt T. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res. 2001;913(1):57–67. doi: 10.1016/s0006-8993(01)02758-5. [DOI] [PubMed] [Google Scholar]
- Luth HJ, Munch G, Arendt T. Aberrant expression of NOS isoforms in Alzheimer's disease is structurally related to nitrotyrosine formation. Brain Res. 2002;953(1–2):135–143. doi: 10.1016/s0006-8993(02)03280-8. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81(4):641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94(10):5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993;196(3):1330–1334. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- Mark KS, Burroughs AR, Brown RC, Huber JD, Davis TP. Nitric oxide mediates hypoxiainduced changes in paracellular permeability of cerebral microvasculature. American Journal of Physiology - Heart & Circulatory Physiology. 2004;286(1):H174–H180. doi: 10.1152/ajpheart.00669.2002. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23(5):1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG. Effect of lipopolysaccharide on the permeability and reactivity of the cerebral microcirculation: role of inducible nitric oxide synthase. Brain Research. 1998;792(2):353–357. doi: 10.1016/s0006-8993(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Miller RH, Szigeti V. Clonal analysis of astrocyte diversity in neonatal rat spinal cord cultures. Development. 1991;113(1):353–362. doi: 10.1242/dev.113.1.353. [DOI] [PubMed] [Google Scholar]
- Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. Journal of Neurochemistry. 2006;97(6):1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97(6):1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Morga E, Faber C, Heuschling P. Cultured astrocytes express regional heterogeneity of the immunoreactive phenotype under basal conditions and after gamma-IFN induction. J Neuroimmunol. 1998;87(1–2):179–184. doi: 10.1016/s0165-5728(98)00099-x. [DOI] [PubMed] [Google Scholar]
- Morga E, Faber C, Heuschling P. Regional heterogeneity of the astroglial immun oreactivephenotype: effect of lipopolysaccharide. J Neurosci Res. 1999;57(6):941–952. [PubMed] [Google Scholar]
- Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, et al. Protection from Alzheimer's-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202(9):1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JR, Weissman JS. Systems biology: many things from one. Nature. 2006;444(7119):561–562. doi: 10.1038/nature05407. [DOI] [PubMed] [Google Scholar]
- Nikcevich KM, Gordon KB, Tan L, Hurst SD, Kroepfl JF, Gardinier M, Barrett TA, Miller SD. IFN-gamma-activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol. 1997;158(2):614–621. [PubMed] [Google Scholar]
- Ogawa K, Chen F, Kim Y-J, Chen Y. Transcriptional Regulation of Tristetraprolin by Transforming Growth Factor-{beta} in Human T Cells. J Biol Chem. 2003;278(32):30373–30381. doi: 10.1074/jbc.M304856200. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Zaczynska E, Bhattacharjee M, Butunoi C, Legido A, Katsetos CD. Inducible nitric oxide synthase and nitrotyrosine are found in monocytes/macrophages and/or astrocytes in acute, but not in chronic, multiple sclerosis. Clin Diagn Lab Immunol. 1998;5(4):438–445. doi: 10.1128/cdli.5.4.438-445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Namboodiri AM, Singh I. N-acetyl cysteine inhibits induction of no production by endotoxin or cytokine stimulated rat peritoneal macrophages, C6 glial cells and astrocytes. Free Radic Biol Med. 1998;24(1):39–48. doi: 10.1016/s0891-5849(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Bossi M, Massari S, Basudev H, Longhi R, Pietrini G. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J Neurochem. 2000;75(3):1076–1084. doi: 10.1046/j.1471-4159.2000.0751076.x. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. GFAP-positive hippocampal astrocytes in situ respond to glutamatergic neuroligands with increases in [Ca2+]i. Glia. 1995;13(2):101–112. doi: 10.1002/glia.440130204. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51(4):439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O'Shea EK. Noise in gene expression: origins, consequences, and control. Science. 2005;309(5743):2010–2013. doi: 10.1126/science.1105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Bottinger EP. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108(6):807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol. 1998;53(3):355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Shao Y, Porter JT, McCarthy KD. Neuroligand receptor heterogeneity among astroglia. Perspect Dev Neurobiol. 1994;2(3):205–215. [PubMed] [Google Scholar]
- Simmons ML, Murphy S. Induction of nitric oxide synthase in glial cells. J Neurochem. 1992;59(3):897–905. doi: 10.1111/j.1471-4159.1992.tb08328.x. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1(4):232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1988;1(1):2–9. doi: 10.1002/glia.440010103. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Sousa Vde O, Romao L, Neto VM, Gomes FC. Glial fibrillary acidic protein gene promoter is differently modulated by transforming growth factor-beta 1 in astrocytes from distinct brain regions. Eur J Neurosci. 2004;19(7):1721–1730. doi: 10.1111/j.1460-9568.2004.03249.x. [DOI] [PubMed] [Google Scholar]
- St-Pierre JA, Nouel D, Dumont Y, Beaudet A, Quirion R. Sub-population of cultured hippocampal astrocytes expresses neuropeptide Y Y(1) receptors. Glia. 2000;30(1):82–91. [PubMed] [Google Scholar]
- Stadlin A, Lau JW, Szeto YK. A selective regional response of cultured astrocytes to methamphetamine. Ann N Y Acad Sci. 1998;844:108–121. [PubMed] [Google Scholar]
- Steel R, Torrie J. Principles and Procedures of Statistics: A Biometrical Approach. New York: McGraw-Hill Book Co.; 1980. 633 pp. [Google Scholar]
- Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411(2–3):217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Sullivan DE, Ferris M, Pociask D, Brody AR. Tumor necrosis factor-alpha induces transforming growth factor-beta1 expression in lung fibroblasts through the extracellular signal-regulated kinase pathway. Am J Respir Cell Mol Biol. 2005;32(4):342–349. doi: 10.1165/rcmb.2004-0288OC. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17(3):932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel VE, Audus KL. Nitric oxide and blood-brain barrier integrity. Antioxidants & Redox Signaling. 2001;3(2):273–278. doi: 10.1089/152308601300185223. [DOI] [PubMed] [Google Scholar]
- Thiemermann C, Szabo C, Mitchell JA, Vane JR. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc Natl Acad Sci U S A. 1993;90(1):267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441(7092):513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- van Landeghem FK, Weiss T, Oehmichen M, von Deimling A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J Neurotrauma. 2006;23(10):1518–1528. doi: 10.1089/neu.2006.23.1518. [DOI] [PubMed] [Google Scholar]
- Venance L, Premont J, Glowinski J, Giaume C. Gap junctional communication and pharmacological heterogeneity in astrocytes cultured from the rat striatum. J Physiol. 1998;510(Pt 2):429–440. doi: 10.1111/j.1469-7793.1998.429bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidwans AS, Hewett SJ. Enhanced release of synaptic glutamate underlies the potentiation of oxygen-glucose deprivation-induced neuronal injury after induction of NOS-2. Exp Neurol. 2004;190(1):91–101. doi: 10.1016/j.expneurol.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Vidwans AS, Uliasz TF, Hewett JA, Hewett SJ. Differential modulation of prostaglandin H synthase-2 by nitric oxide-related species in intact cells. Biochemistry. 2001;40(38):11533–11542. doi: 10.1021/bi0108960. [DOI] [PubMed] [Google Scholar]
- Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31(2):95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Clarke C, Azmitia EC. Localization of 5-HT1A receptors to astroglial cells in adult rats: implications for neuronal-glial interactions and psychoactive drug mechanism of action. Synapse. 1993;14(3):201–205. doi: 10.1002/syn.890140303. [DOI] [PubMed] [Google Scholar]
- Wilkin GP, Marriott DR, Cholewinski AJ. Astrocyte heterogeneity. Trends Neurosci. 1990;13(2):43–46. doi: 10.1016/0166-2236(90)90065-i. [DOI] [PubMed] [Google Scholar]
- Wink MR, Braganhol E, Tamajusuku AS, Casali EA, Karl J, Barreto-Chaves ML, Sarkis JJ, Battastini AM. Extracellular adenine nucleotides metabolism in astrocyte cultures from different brain regions. Neurochem Int. 2003;43(7):621–628. doi: 10.1016/s0197-0186(03)00094-9. [DOI] [PubMed] [Google Scholar]
- Yun HY, Dawson VL, Dawson TM. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996;10(3–4):291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci. 2001;21(20):7901–7908. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


