Abstract
Prepulse inhibition (PPI), a form of sensorimotor gating, is reduced in a number of psychiatric disorders. Two experiments were conducted to determine whether corticotropin-releasing factor (CRF), which decreases PPI, does so via effects on serotonin (5-HT). Wistar Kyoto (WKY) and Brown Norway (BN) rats were used in both experiments in order to examine whether strain-dependent differences would be apparent in response to manipulations of the CRF and 5-HT systems. In the first experiment, WKY and BN rats received a subcutaneous injection of the 5-HT2A/C receptor antagonist, ketanserin (2.0 mg/kg). Ten minutes later, rats received an intracerebroventricular (ICV) infusion of either 6.0 μl saline or CRF (0.3 μg or 3.0 μg). CRF decreased PPI despite blockade of 5-HT2A/C receptors with ketanserin. In the second experiment, WKY and BN rats received an intraperitoneal injection of the 5-HT synthesis inhibitor, p-chlorophenylalanine (PCPA, 150 mg/kg), 48 and 24 hours prior to testing. On testing day, rats received an ICV infusion of either 6.0 μl saline or CRF (0.3 μg or 3.0 μg). CRF decreased PPI despite 5-HT depletion. These findings suggest that CRF does not decrease PPI via effects on 5-HT, since neither blockade of 5-HT2A/C receptors nor 5-HT depletion attenuated this decrease.
Keywords: Acoustic Startle Response, Corticotropin-Releasing Factor, Ketanserin, p-Chlorophenylalanine, Prepulse Inhibition, Serotonin
1. Introduction
Prepulse inhibition (PPI) of the acoustic startle response, a form of sensorimotor gating, is the decrease in startle amplitude caused by brief presentation of a non-startling stimulus shortly prior to a startling stimulus (Graham, 1975; Hoffman and Ison, 1980; Hoffman and Searle, 1968). PPI is diminished in a number of psychiatric disorders that are characterized by a reduced ability to suppress or "gate" intrusive sensory, motor, or cognitive information (Braff et al., 2001). Schizophrenia is one of the most frequently studied disorders in which deficient PPI is observed (Braff et al., 1992; Braff et al., 1978; Grillon et al., 1992; Parwani et al., 2000) and reduced PPI has been linked to the symptoms of sensory overload and cognitive fragmentation characteristic of this disorder (Braff and Geyer, 1990; McGhie and Chapman, 1961).
Abnormalities in several neurotransmitter systems are associated with schizophrenia, including dopamine (DA), glutamate, and serotonin (5-HT) (Lyne et al., 2004). Pharmacological manipulations of these neurotransmitters reduce PPI and serve as animal models of sensorimotor gating deficits. For example, drugs that cause 5-HT release (Kehne et al., 1996; Mansbach et al., 1989; Martinez and Geyer, 1997) or are 5-HT1A (Rigdon and Weatherspoon, 1992), 5-HT1B (Sipes and Geyer, 1994), or 5-HT2 receptor agonists (Johansson et al., 1995; Padich et al., 1996; Sipes and Geyer, 1994) reduce PPI.
Since stress exacerbates the symptoms of schizophrenia (Gispen-de Wied, 2000; Walker and Diforio, 1997), an additional cause of deficient PPI may involve corticotropin-releasing factor (CRF), one of the most important hormones and neurotransmitters involved in endocrine, autonomic, and behavioral components of the stress response (Bale and Vale, 2004; Gray, 1993). CRF is a 41-residue peptide that is synthesized in the paraventricular nucleus of the hypothalamus (Vale et al., 1981). Additionally, CRF is synthesized in extra-hypothalamic regions, including the cortex, hippocampus, central nucleus of the amygdala, and dorsal raphe nucleus (Swanson et al., 1983) and is released as a neurotransmitter (Gabr et al., 1994; Van Bockstaele et al., 1998). CRF acts at two different G-protein coupled receptors, CRF1 and CRF2 (Chang et al., 1993; Lovenberg et al., 1995), which are widely expressed throughout the brain (Chalmers et al., 1995; Van Pett et al., 2000). Importantly, CRF receptors are expressed in regions of the brain known to modulate PPI, including the prefrontal cortex, hippocampus, basolateral amygdala, and nucleus accumbens (Swerdlow et al., 2001).
We have shown that intracerebroventricular (ICV) infusion of CRF reduces PPI in both Wistar-Kyoto (WKY) and Brown Norway (BN) rats (Conti, 2005; Conti et al., 2002), two inbred rat strains. Interestingly, BN rats exhibit diminished PPI under basal conditions compared to WKY rats (Conti et al., 2002; Palmer et al., 2000) and may represent a good genetic model for the PPI deficits observed in schizophrenia. Additionally, WKY and BN rats exhibit different sensitivities to the effect of CRF on PPI. For example, a low dose of CRF (0.3 μg) decreases PPI in BN rats while WKY rats require a high dose of CRF (3.0 μg) to decrease PPI (Conti, 2005; Conti et al., 2002). It is intriguing to speculate that this difference in sensitivities to CRF may be due to BN rats having a greater density of CRF receptors in the cortex and hippocampus compared to WKY rats (Lahmame et al., 1997).
ICV infusion of CRF also reduces PPI in mice (Risbrough et al., 2004) and transgenic mice over-expressing CRF show reduced PPI compared to wild-type controls (Dirks et al., 2002). Interestingly, both typical and atypical antipsychotics attenuate the effect of CRF on PPI (Conti et al., 2005) and improve PPI in CRF over-expressing mice (Dirks et al., 2003).
Although ICV infusion of CRF diminishes PPI, it remains unclear whether this effect of CRF depends on other neurotransmitters. CRF could alter PPI directly via CRF receptors located in regions of the brain important for mediating PPI, such as the prefrontal cortex, hippocampus, basolateral amygdala, or nucleus accumbens (Swerdlow et al., 2001). Alternatively, CRF could alter PPI indirectly via its effects on other neurotransmitters, such as 5-HT. CRF-immunoreactive fibers project to, and are found in, the dorsal raphe nucleus (DRN) (Kirby et al., 2000; Swanson et al., 1983; Valentino et al., 2001), a primary site of forebrain-projecting serotonergic neurons (Jacobs and Azmitia, 1992). The DRN expresses both types of CRF receptor mRNAs (Chalmers et al., 1995; Day et al., 2004; Van Pett et al., 2000) and CRF receptors (De Souza et al., 1985). CRF alters extracellular concentrations of 5-HT in brain regions receiving serotonergic input from raphe nuclei. For example, low doses of CRF decrease 5-HT concentrations in the lateral striatum while a high dose increases 5-HT concentrations in this brain region (Price et al., 1998). CRF administered ICV increases 5-HT concentrations in the hippocampus as well (de Groote et al., 2005; Kagamiishi et al., 2003; Linthorst et al., 2002). In the prefrontal cortex, CRF increases 5-HT utilization, as indicated by increased levels of the 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HIAA) (Lavicky and Dunn, 1993).
CRF can increase 5-HT release and drugs that cause 5-HT release, or are 5-HT1A, 5-HT1B, or 5-HT2 receptor agonists, reduce PPI. Thus, it is plausible that CRF decreases PPI indirectly via its effects on 5-HT. Two experiments were conducted to test this possibility. Pretreatment with ketanserin, a 5-HT2A/C receptor antagonist, blocks the decrease in PPI caused by DOI (2,5-dimethoxy-4-iodoamphetamine), a 5-HT2A/C agonist (Sipes and Geyer, 1994). Additionally, the atypical antipsychotic, clozapine, blocks several receptor types, including 5-HT2A/C receptors, (Brunello et al., 1995) and attenuates the CRF-induced decrease in PPI (Conti et al., 2005). Thus, the first experiment was conducted to examine the effects of ketanserin on the CRF-induced decrease in PPI.
Since 14 5-HT receptor subtypes exist (Nestler et al., 2001), the second experiment was conducted to investigate the effects of 5-HT depletion, and thus reduced 5-HT at all 5-HT receptors, using the 5-HT synthesis inhibitor, p-chlorophenylalanine (PCPA), on the CRF-induced decrease in PPI. Since there are strain-dependent differences in the effects of CRF on PPI, we also sought to examine whether strain-dependent differences would be apparent in response to manipulation of the 5-HT system.
2. Methods and materials
2.1. Experimental Animals
Male Wistar-Kyoto (Charles River Laboratories, Raleigh, NC) and Brown Norway rats (Harlan Sprague-Dawley Inc., Prattville, AL) were used. Rats (250–275 grams) were held in the vivarium for one week prior to undergoing stereotaxic surgery and maintained on a 12-hour light/dark cycle with food and water available ad libitum. Rats were housed two per cage until undergoing surgery and then housed separately. All facilities and procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Stereotaxic Surgery and ICV Infusion Procedure
Rats were anesthetized with a mixture of isoflurane-in-oxygen (2.0%) and placed in a Kopf stereotaxic instrument equipped with blunt ear bars. The incisor bar was set to -3.0 to hold the head level. A stainless steel guide cannula (22 gauge; Plastics One, Roanoke, VA) was placed into the lateral ventricle (AP -1.0 mm, ML 2.0 mm to bregma) for subsequent ICV infusion (Paxinos and Watson, 1986). Guide cannula extended 4.4 mm below the surface of the skull. Two jewelers’ screws were placed into the skull and dental cement was poured over the exposed skull to hold the screws and cannula in place and close the incision site. A dummy cannula was placed into the guide. Rats were allowed to recover for 5–7 days prior to testing. Cannula placement was assessed in random animals via ICV infusion of methylene blue dye and verification of dye in the ventricular system.
During infusions, rats were held in a towel and the dummy cannula were removed. The infusion cannula (28 gauge), attached to PE 20 tubing, was inserted into the guide cannula and extended 0.5 mm beyond the guide. A 10.0 μl Hamilton syringe was used to manually deliver saline or CRF over a one-minute period. The flow of infused CRF was monitored via introduction of an air bubble into the infusion line. The infusion cannula was kept in place for an additional 30 seconds following infusion. Rats were then placed back into their home cages.
2.3. Startle Chambers
Startle amplitude and PPI were measured in two identical startle chambers (SR-LAB, San Diego Instruments, San Diego, CA). Chambers consisted of a nonrestrictive Plexiglas cylinder (9 cm diameter, 18.5 cm length) mounted on a platform located inside a sound-and vibration-attenuating cabinet equipped with a 20-watt incandescent bulb and a fan for ventilation. A piezoelectric accelerometer, mounted under each cylinder, detected whole-body startle responses. Following the onset of each startle stimulus, output signals from the accelerometer were recorded once per msec for a period of 100 msec by the computer. Signals were rectified, digitized, and stored on the computer by the SR-LAB program (San Diego Instruments, San Diego, CA). On each testing day, startle response sensitivities were standardized to the same baseline value across chambers using a standard calibration tube (San Diego Instruments, San Diego, CA). White noise stimuli were delivered through a horn tweeter (Radio Shack) controlled by the SR-LAB program.
2.4. Startle and PPI Testing
The session consisted of 82 trials presented over a 70 dB white noise background. To begin each session, rats were exposed to a 5-minute acclimation period in which no auditory stimuli were presented. The first and last six trials of the session consisted of the startle stimulus alone (120 dB, 40 msec). Remaining trials occurred in a pseudorandom order and consisted of 12 startle alone trials (used to calculate % PPI and average startle amplitude), 10 prepulse + startle trials at each of 5 prepulse intensities (73, 76, 82, 85, 88 dB), and 8 no stimulus trials. Each prepulse stimulus was presented for 20 msec and preceded the startle stimulus by 100 msec. The inter-trial interval averaged 15 seconds. Testing was performed between 10 a.m. and 4 p.m.
2.5. Experimental Protocol
In the first experiment, WKY (n = 49) and BN (n = 63) rats received a subcutaneous (SC) injection of the 5-HT2A/C receptor antagonist, ketanserin (2.0 mg/kg) (Awouters, 1985), or saline, on testing day. Ten minutes later, the rats received an ICV infusion of either 6.0 μl saline or CRF (0.3 μg or 3.0 μg, in 6.0 μl saline). PPI was assessed 30 minutes after infusion.
In the second experiment, WKY (n = 67) and BN (n = 87) rats received an intraperitoneal (IP) injection of the 5-HT synthesis inhibitor, p-chlorophenylalanine, (PCPA, 150 mg/kg) (Koe and Weissman, 1966), or saline, 48 and 24 hours prior to testing. On testing day, rats received an ICV infusion of either 6.0 μl saline or CRF (0.3 μg or 3.0 μg, in 6.0 μl saline). PPI was assessed 30 minutes after infusion. General activity (including grooming, locomotion, burrowing, rearing, and chewing) was assessed visually as a secondary behavioral measure of CRF, beginning 15 minutes prior to PPI testing. Additionally, monoamine levels were assessed in the brain using high-performance liquid chromatography (HPLC) with electrochemical detection.
2.6. Determination of Monoamine Concentrations
Monoamine concentrations were determined to assess the effectiveness of the PCPA to inhibit 5-HT synthesis and decrease 5-HT levels. At the conclusion of each PPI testing day in the first experiment, rats were sacrificed by rapid decapitation and brains were removed immediately. The caudate putamen, frontal cortex (2–2.5 mm section, excluding olfactory bulbs, with the posterior edge located 3.0 mm anterior to bregma), entire hippocampus (bilateral), and entire hypothalamus (bilateral) were dissected and frozen on dry ice. Tissue samples were homogenized in 0.1 N perchloric acid with 100 μM EDTA (15 μl/mg tissue) using a tissue homogenizor according to previously published methods (Page et al., 1999). Samples were centrifuged at 15 000 rpm (23 143 g) for 15 minutes at 2–8°C. The supernatant was filtered through 0.45 μm nylon acrodisk syringe filters and divided for analysis of the monoamines, norepinephrine (NE), DA, and 5-HT as well as the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC). Two separate HPLC systems were used for analysis. Each consisted of an ESA solvent delivery system (ESA Inc., Chelmsford, MA) and an MD 150 reverse phase narrobore column (150 x 2 mm, 3 μm; ESA, Inc, Chelmsford, MA). For NE and DOPAC analysis, the mobile phase consisted of 60 mM sodium phosphate buffer (pH = 4.2) with 100 μM EDTA, 1.5 mM sodium octyl-sulfate, 3.5% (v:v) methanol. For 5-HT and DA analysis, the mobile phase consisted of 150 mM sodium phosphate buffer, 7.7 mM citric acid, 67 μM EDTA, 3.2 mM octyl sulfate, 15% acetonitrile (v:v) and 10% (v:v) methanol adjusted to a pH of 5.6. The flow rate through the system was 300 μl/min. The detection system consisted of an ESA Coulochem II electrochemical detector with a guard cell and a 5041 enhanced amperometric analytical cell (ESA Inc., Chelmsford, MA) with a glassy carbon in ceramic target electrode in series. The applied potential of the guard cell was -150 mV and the compounds of interest were quantified at the target electrode set at +200 mV (NE and DOPAC) and +500 mV (5-HT and DA). Peak heights were measured and compared to peak heights of 10−7 M standards.
2.7. Data Analysis
Percent PPI was calculated for each rat at each prepulse intensity using the following equation: % PPI = 100 – (100 X [prepulse/startle]). Prepulse was the average startle amplitude on trials in which a prepulse stimulus preceded the startle stimulus. Startle was the average amplitude on trials in which the startle stimulus was presented alone.
Initially, PPI data were analyzed using four-way analysis of variance (ANOVA), with strain, ketanserin or PCPA pretreatment, and CRF infusion as between-subjects factors, and prepulse intensity as a within-subjects factor. PPI data were also analyzed in each strain separately using three-way ANOVAs. Startle amplitude and activity were analyzed using three-way ANOVAs, with strain, ketanserin or PCPA (activity data only) pretreatment, and CRF infusion as between-subjects factors. For HPLC data, three-way ANOVAs were performed for each brain region and each monoamine, with strain, PCPA pretreatment, and CRF infusion as between-subjects factors. Additionally, the ratio of [DOPAC/DA] X 100 was calculated as an estimate of DA utilization in a subset of animals for which both DOPAC and DA values were available. Tukey’s post-hoc tests were performed if significant main effects or interactions were found. Where appropriate, specific treatment groups were compared using two-way ANOVAs or independent t-tests. The alpha level was set at 0.05. Trends are reported where p values range between 0.05 and 0.1. In each experiment, rats exhibiting a startle response greater or less than two standard deviations from the mean were removed from analysis, resulting in no more than one rat removed per group. For monoamine values, extreme outliers were removed from analysis, resulting in no more than two values removed per monoamine per brain region.
In order to demonstrate that CRF decreased PPI without increasing baseline startle amplitude, WKY rats from both experiments that received injection and ICV infusion of saline were combined into one group (SALINE, n = 22). Using a median split on the basis of startle amplitude, two groups were created from the saline injected rats infused with 3.0 μg CRF from both experiments. Thus, we created a CRF/LOW STARTLE group (n = 9) and a CRF/HIGH STARTLE group (n = 9). A one-way ANOVA was performed to examine startle amplitude. To examine PPI, a two-way ANOVA was performed, with group as a between-subjects factor and prepulse intensity as a within-subjects factor.
2.8. Peptides and Drugs
Rat/human CRF was kindly provided by Dr. Jean Rivier (The Salk Institute, La Jolla, CA). CRF was dissolved in 0.9 % saline and aliquots were frozen at –80°C until needed. PCPA and ketanserin (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9 % saline on each day they were needed.
3. Results
3.1. Experiment 1: Effect of Ketanserin on the CRF-Induced Decrease in PPI
A four-way ANOVA revealed a significant effect of rat strain on PPI [F(1,100) = 24.051, p < 0.001], with BN rats showing less PPI than WKY rats, and a significant effect of CRF infusion [F(2,100) = 12.207, p < 0.001] (Figure 1). There was also a significant strain X CRF interaction [F(2,100) = 4.087, p = 0.020]. No significant effect of ketanserin pretreatment, strain X ketanserin interaction, or three-way interaction was detected. There was a significant effect of prepulse intensity [F(4,400) = 227.492 , p < 0.001], indicating that percent PPI increased with increasing prepulse intensity. This main effect of prepulse intensity occurred in all subsequent analyses and experiments and is, therefore, not reported each time. There were significant interactions between prepulse intensity and strain [F(4,400) = 24.848, p < 0.001], prepulse intensity and ketanserin [F(4,400) = 2.653, p = 0.033], prepulse intensity and CRF [F(8,400) = 2.322, p 0.019], and among prepulse intensity, strain, and ketanserin [F(4,400) = 3.593, p = 0.007].
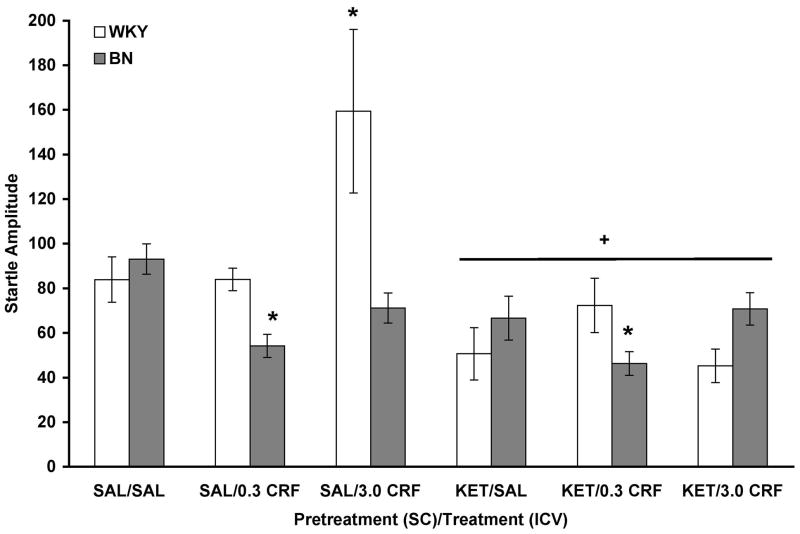
Figure 1.
(A and B). Ketanserin did not attenuate the effect of CRF on PPI in WKY (A) and BN (B) rats. Values are shown as mean ± SEM. WKY rats, n = 6–11/group; BN rats, n = 8–13/group. Rats received one SC injection of ketanserin (KET; 2.0 mg/kg) ten minutes prior to receiving a single ICV infusion of either 6.0 μl saline (SAL), 0.3 μg CRF, or 3.0 μg CRF (in 6.0 μl saline). PPI was assessed 30 minutes later. Prepulse intensities were 73, 76, 82, 85, and 88 dB. (A) * p < 0.01 comparing all 3.0 μg CRF (ICV) vs. all SAL (ICV), based on a Tukey’s test. (B) ** p < 0.05 comparing all 0.3 μg CRF (ICV) vs. all SAL (ICV), based on a Tukey’s test; + p < 0.03 comparing all 3.0 μg CRF (ICV) vs. all SAL (ICV), based on a Tukey’s test.
In WKY rats alone (Figure 1A), a three-way ANOVA revealed a significant effect of CRF infusion on PPI [F(2,43) = 9.936, p < 0.001], with a Tukey’s post hoc test showing that 3.0 μg CRF decreased PPI (p = 0.001). No significant effect of ketanserin pretreatment or ketanserin X CRF interaction was detected. Thus, 3.0 μg CRF decreased PPI in WKY rats despite blockade of 5-HT2A/C receptors with ketanserin. There was a significant prepulse intensity X ketanserin interaction [F(4,172) = 4.025, p = 0.004], which was likely due to ketanserin-pretreated rats having slightly decreased PPI at the 73 dB prepulse and slightly increased PPI at the 76, 82, 85, and 88 dB prepulses.
In BN rats alone (Figure 1B), a three-way ANOVA revealed a significant effect of CRF infusion on PPI [F(2,57) = 4.330, p = 0.018], with a Tukey’s post hoc testing showing that both 0.3 μg CRF (p = 0.043) and 3.0 μg CRF (p = 0.028) decreased PPI. There was no significant effect of ketanserin pretreatment and no ketanserin X CRF interaction. Therefore, both doses of CRF decreased PPI in BN rats despite blockade of 5-HT2A/C receptors with ketanserin, as supported by the lack of a ketanserin X CRF interaction.
Analysis of startle amplitude data indicated a significant effect of rat strain [F(1,100) = .644, p = 0.019] and CRF infusion [F(2,100) = 4.170, p = 0.018] (Figure 2). There was also a significant effect of ketanserin pretreatment [F(1,100) = 24.350, p < 0.001], indicating that ketanserin decreased startle amplitude. Significant interactions were detected between strain and ketanserin [F(1,100) = 10.043, p = 0.002], strain and CRF [F(2,100) = 4.502, p = 0.013], and ketanserin and CRF [F(2,100) = 4.655, p = 0.012], all of which can be attributed to the fact that 3.0 μg CRF increased startle amplitude in saline-pretreated WKY rats only. Additionally, the rat strain X CRF interaction shows that 0.3 μg CRF decreased startle amplitude in BN rats while not affecting startle in WKY rats. This conclusion is supported by the significant strain X ketanserin X CRF interaction [F(2,100) = 8.108, p = 0.001]. Thus, CRF-induced changes in startle amplitude were blocked by ketanserin pretreatment in WKY rats only.
Figure 2.
CRF did not increase startle amplitude in ketanserin-pretreated WKY rats. Values are shown as mean ± SEM. WKY rats, n = 6–11/group; BN rats, n = 8–13/group. + p < 0.001 vs. SAL (SC); * p < 0.02 vs. all SAL (ICV), based on main effects and interactions described in the text.
3.2. Experiment 2: Effect of PCPA on the CRF-Induced Decrease in PPI
In the analysis of PPI, a four-way ANOVA revealed a significant effect of rat strain [F(1,142) = 52.011, p < 0.001], with BN rats showing less PPI than WKY rats, and a significant effect of CRF infusion [F(2,142) = 6.534, p = 0.002] (Figure 3). No significant effect of PCPA pretreatment or PCPA X CRF interaction was detected. There were no interactions involving strain. The only significant interaction involving prepulse intensity was with strain [F(4,568) = 29.201, p < 0.001] due to the fact that increasing prepulse intensity had a greater effect in WKY rats than in BN rats.
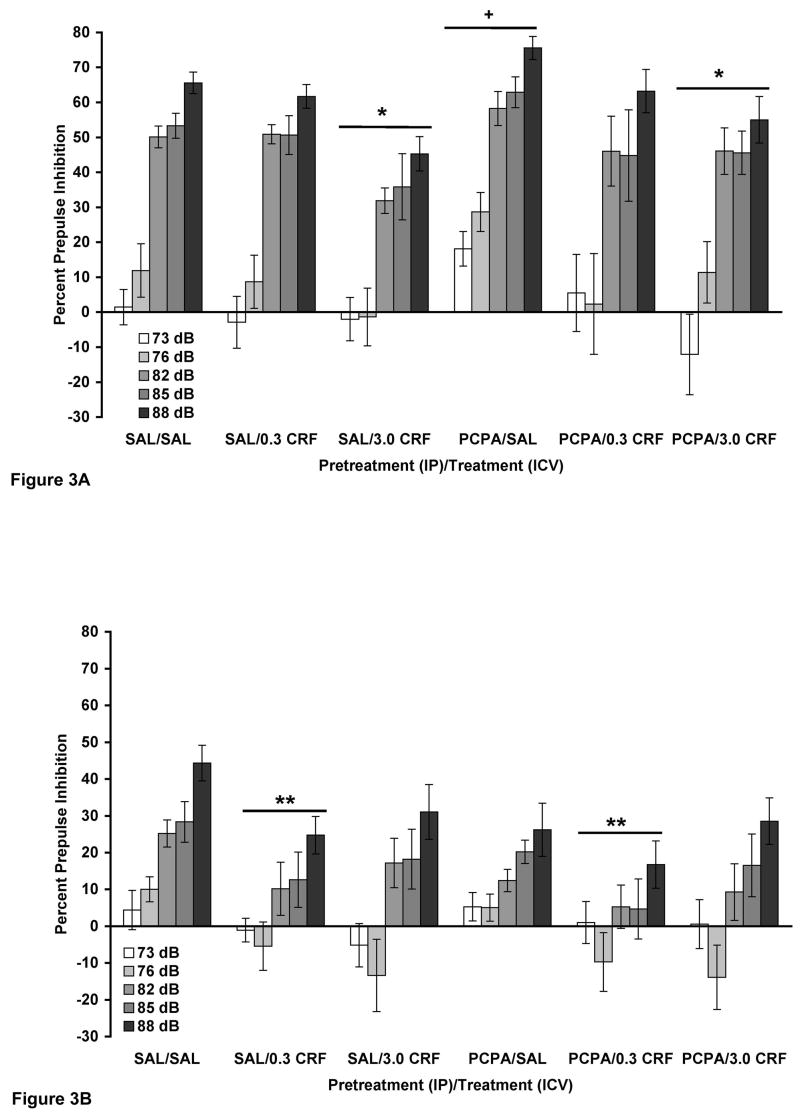
Figure 3.
(A and B). PCPA did not attenuate the effect of CRF on PPI in WKY (A) and BN (B) rats. Values are shown as mean ± SEM. WKY rats, n = 11–12/group; BN rats, n = 14–15/group. Rats received two IP injections of PCPA (150 mg/kg), 48 and 24 hours prior to PPI testing. On testing day, rats received a single ICV infusion of either 6.0 μl saline (SAL), 0.3 μg CRF, or 3.0 μg CRF (in 6.0 μl saline). PPI was assessed 30 minutes later. Prepulse intensities were 73, 76, 82, 85, and 88 dB. (A) * p < 0.01 comparing all 3.0 μg CRF (ICV) vs. all SAL (ICV), based on a Tukey’s test; + p < 0.03 vs. SAL/SAL. (B) ** p < 0.02 comparing all 0.3 μg CRF (ICV) vs. all SAL (ICV).
In WKY rats alone (Figure 3A), a three-way ANOVA revealed a significant effect of CRF infusion on PPI [F(2,61) = 5.145, p = 0.009], with a Tukey’s post hoc test showing that 3.0 μg CRF decreased PPI (p = 0.007). No significant effect of PCPA pretreatment or PCPA X CRF interaction was observed. Thus, 3.0 μg CRF decreased PPI despite PCPA pretreatment, as supported by the lack of a PCPA X CRF interaction. A separate two-way ANOVA comparing SAL/SAL vs. PCPA/SAL revealed that PCPA significantly increased PPI in this strain [F(1,20) = 5.796, p = 0.026].
In BN rats alone (Figure 3B), a three-way ANOVA indicated a trend towards CRF decreasing PPI (p = 0.069), with a Tukey’s post hoc test showing that this trend was due to 0.3 μg CRF (p = 0.066). No significant effect of PCPA pretreatment or PCPA X CRF interaction was detected. There was a significant prepulse intensity X PCPA interaction [F(4,324) = 2.411, p = 0.049], which was likely due to PCPA reducing PPI at the four highest prepulse intensities only. A significant prepulse intensity X CRF interaction [F(8,324) = 2.403, p = 0.016] was likely due to a floor effect encountered at the lowest prepulse intensity. Since there was only a trend for 0.3 μg CRF to decrease PPI, a separate three-way ANOVA was performed with the 3.0 μg CRF groups removed from analysis. Here, 0.3 μg CRF significantly reduced PPI in BN rats [F(1,54) = 6.867, p = 0.011]. Once again, no significant effect of PCPA pretreatment or PCPA X CRF interaction was detected. However, it must be noted that PCPA/SAL and PCPA/0.3 μg CRF groups were not significantly different in a separate two-way ANOVA. Therefore, it cannot be conclusively stated that 0.3 μg CRF decreased PPI despite PCPA pretreatment in BN rats, since PCPA pretreatment alone decreased PPI at the three highest prepulse intensities.
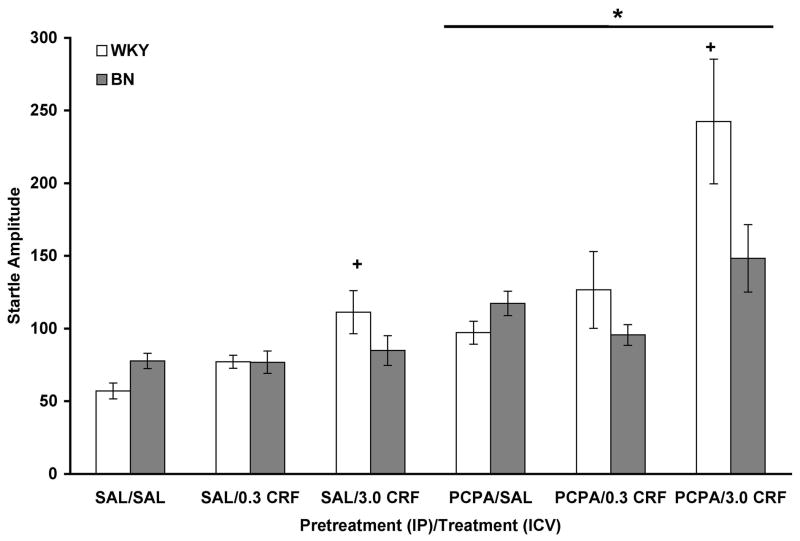
Analysis of startle amplitude data revealed a significant effect of PCPA pretreatment [F(1,142) = 33.857, p < 0.001], with PCPA increasing startle amplitude in both rat strains (Figure 4). There was a significant effect of CRF infusion [F(2,142) = 14.750, p < 0.001], with a Tukey’s post hoc test showing that 3.0 μg CRF increased startle amplitude (p < 0.001). A significant interaction between strain and CRF [F(2,142) = 5.657, p = 0.004] indicated that 3.0 μg CRF increased startle amplitude in WKY rats only. A significant interaction between PCPA and CRF [F(2,142) = 4.276, p = 0.016] revealed that 3.0 μg CRF increased startle amplitude to a greater extent in PCPA-pretreated rats compared to saline-pretreated rats. No significant effect of strain, strain X PCPA interaction, or three-way interaction was detected.
Figure 4.
PCPA enhanced the effect of CRF on startle amplitude in WKY rats. Values are shown as mean ± SEM. WKY rats, n = 11–12/group; BN rats, n = 14–15/group. * p < 0.001 vs. SAL (IP); + p < 0.001 vs. all SAL (ICV), based on a main effect, Tukey’s test, and interactions described in the text.
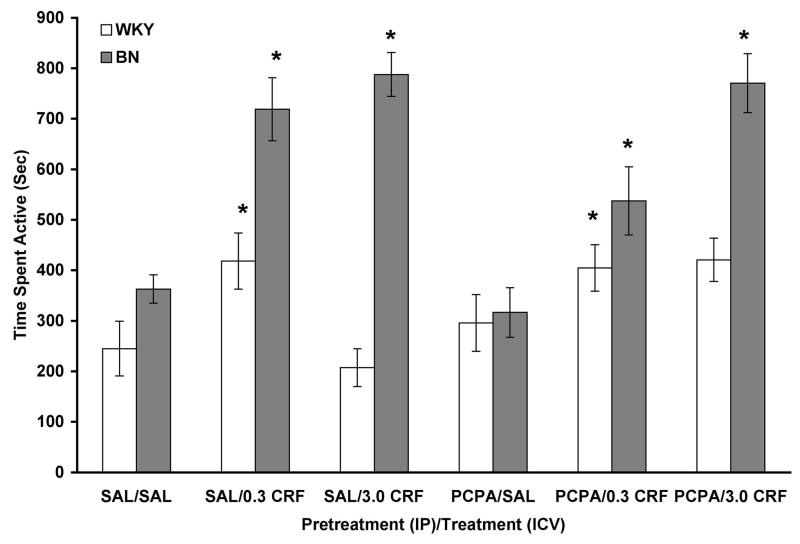
Analysis of general activity data showed a significant effect of rat strain [F(1,144) = 70.291, p < 0.001], with BN rats showing more activity (Figure 5). There was a significant effect of CRF infusion [F(2,144) = 26.404, p < 0.001], with a Tukey’s post hoc test showing that, overall, both doses of CRF increased activity (p < 0.001). There was no significant effect of PCPA pretreatment. A significant rat strain X CRF interaction [F(2,144) = 14.926, p < 0.001] indicated that both doses of CRF increased activity in BN rats while only the 0.3 μg dose increased activity in WKY rats. A significant rat strain X PCPA interaction [F(1,144) = 7.681, p = 0.006] showed that PCPA pretreatment tended to increase activity in WKY rats and decrease activity in BN rats. A significant PCPA X CRF interaction [F(2,144) = 3.553, p = 0.031] revealed that 0.3 μg CRF increased activity to a greater extent in saline-pretreated rats while 3.0 μg CRF increased activity to a greater extent in PCPA-pretreated rats. There was no three-way interaction.
Figure 5.
CRF dose-dependently increased activity in WKY (n = 11–12/group) and BN (n = 14–15/group) rats. Values are shown as mean ± SEM. Activity was assessed 15 minutes prior to PPI testing. * p < 0.001 vs. all SAL (ICV), based on a main effect, Tukey’s test, and interactions described in the text.
Results from the analysis of DA, DOPAC, 5-HT, and NE levels using HPLC are shown in Table 1. For the sake of brevity, only significant main effects and interactions are reported in the text. In the caudate putamen, PCPA pretreatment significantly reduced 5-HT levels by 78% [F(1,98) = 107.485, p < 0.001]. There were significant effects of rat strain on DA levels [F(1,99) = 11.269, p = 0.001] and DOPAC levels [F(1,75) = 16.294, p < 0.001], with BN rats exhibiting 32% more DA and 67% more DOPAC than WKY rats. When the ratio of DOPAC/DA was calculated for each strain, WKY rats had a ratio of 30.15 ± 4.55 and BN rats had a ratio of 42.88 ± 4.69. An independent t-test revealed a trend for BN rats to exhibit increased DA utilization [t(63) = −1.940, p = 0.057].
Table 1.
DA, DOPAC, 5-HT, and NE levels in the caudate putamen, frontal cortex, hippocampus, and hypothalamus of WKY and BN rats after PCPA pretreatment, CRF infusion, and PPI testing.
| CAUDATE PUTAMEN | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| WKY RATS TREATMENT | DA | DOPAC | 5-HT | NE | |||||
| Saline/Saline | 5688±1138 (7) | 1390±223 (8) | 289±35 (8) | 201±54 (8) | |||||
| Saline/0.3μg CRF | 5668±1074 (7) | 1981±365 (8) | 219±41 (8) | 135±34 (8) | |||||
| Saline/3.0μg CRF | 7645±1020 (9) | 2012±435 (6) | 203±44 (9) | 264±82 (9) | |||||
| PCPA/Saline | 6757±933 (6) | 1313±197 (6) | 64±12 (7) | * | 126±29 (6) | ||||
| PCPA/0.3μg CRF | 6610±1192 (9) | 1436±241 (7) | 62±14 (8) | 119±29 (9) | |||||
| PCPA/3.0μg CRF | 6732±831 (8) | 1810±421 (6) | 40±8 (8) | 141±45 (6) | |||||
| BN RATS TREATMENT | DA | DOPAC | 5-HT | NE | |||||
| Saline/Saline | 9916±1019 (12) | + | 3012±442 (10) | + | 289±50 (12) | 134±25 (12) | |||
| Saline/0.3μg CRF | 7617±1168 (10) | 3230±620 (7) | 312±34 (10) | 129±29 (12) | |||||
| Saline/3.0μg CRF | 9677±960 (10) | 3221±385 (8) | 250±52 (10) | 140±28 (11) | |||||
| PCPA/Saline | 7829±849 (11) | 3090±1013 (7) | 52±10 (10) | * | 145±39 (10) | ||||
| PCPA/0.3μg CRF | 8273±874 (11) | 1710±250 (7) | 56±7 (11) | 145±16 (8) | |||||
| PCPA/3.0μg CRF | 8328±1237 (11) | 2325±429 (7) | 59±14 (9) | 146±33 (8) | |||||
| FRONTAL CORTEX | |||||||||
| WKY RATS TREATMENT | DA | DOPAC | 5-HT | NE | |||||
| Saline/Saline | 77±35 (4) | 56±21 (8) | 362±34 (7) | 412±42 (10) | |||||
| Saline/0.3μg CRF | 40±4 (5) | 46±8 (6) | 365±45 (7) | 412±34 (9) | |||||
| Saline/3.0μg CRF | 93±36 (6) | 47±4 (6) | 388±34 (9) | 391±44 (9) | |||||
| PCPA/Saline | 39±9 (9) | * | 23±4 (4) | * | 65±17 (10) | * | 287±12 (9) | * | |
| PCPA/0.3μg CRF | 29±9 (7) | 16±2 (5) | 56±8 (9) | 311±22 (11) | |||||
| PCPA/3.0μg CRF | 47±11 (6) | 23±2 (4) | 60±10 (9) | 292±23 (9) | |||||
| BN RATS TREATMENT | DA | DOPAC | 5-HT | NE | |||||
| Saline/Saline | 76±42 (7) | 37±12 (11) | 426±19 (12) | 343±33 (12) | + | ||||
| Saline/0.3μg CRF | 141±51 (9) | 39±14 (8) | 385±43 (10) | 296±26 (11) | |||||
| Saline/3.0μg CRF | 57±15 (10) | 36±4 (12) | 397±24 (10) | 321±17 (11) | |||||
| PCPA/Saline | 24±4 (10) | * | 21±2 (7) | * | 76±10 (11) | * | 267±22 (11) | * | |
| PCPA/0.3μg CRF | 46±23 (7) | 23±7 (7) | 64±6 (10) | 253±36 (13) | |||||
| PCPA/3.0μg CRF | 23±5 (8) | 29±3 (7) | 105±19 (12) | 256±20 (14) | |||||
| HIPPOCAMPUS | |||||||||
| WKY RATS TREATMENT | DA | DOPAC | 5-HT | NE | |||||
| Saline/Saline | 57 (1) | 9±2 (5) | 93±13 (5) | 298±50 (8) | |||||
| Saline/0.3μg CRF | 61 (1) | 9 (2) | 130±32 (8) | 333±52 (8) | |||||
| Saline/3.0μg CRF | 22 (1) | 10±1 (4) | 106±14 (7) | 314±28 (10) | |||||
| PCPA/Saline | 52 (1) | 10±3 (4) | 22±9 (6) | * | 256±28 (9) | ** | |||
| PCPA/0.3μg CRF | 25 (2) | 9±2 (4) | 12±1 (6) | 261±38 (10) | |||||
| PCPA/3.0μg CRF | 35 (2) | 5 (2) | 19±3 (5) | 226±18 (9) | |||||
| BN RATS TREATMENT | DA | DOPAC | 5-HT | NE | |||||
| Saline/Saline | 22±10 (4) | 12±4 (6) | 228±74 (10) | 211±28 (12) | + | ||||
| Saline/0.3μg CRF | 27±11 (5) | 8±0.5 (3) | 170±43 (9) | 192±43 (9) | |||||
| Saline/3.0μg CRF | 32±9 (7) | 8±1 (3) | 95±13 (9) | 217±27 (13) | |||||
| PCPA/Saline | 23 (2) | 6±1 (3) | 20±4 (10) | * | 160±31 (11) | ||||
| PCPA/0.3μg CRF | 16 (2) | 8±0.5 (3) | 68±37 (10) | 202±36 (14) | |||||
| PCPA/3.0μg CRF | 15 (2) | 11 (2) | 64±29 (8) | 204±38 (13) | |||||
| HYPOTHALAMUS | |||||||||
| WKY RATS TREATMENT | DA | DOPAC | 5-HT | NE | |||||
| Saline/Saline | 274±40 (8) | 142±17 (9) | 312±40 (8) | 1680±205 (7) | |||||
| Saline/0.3μg CRF | 263±37 (5) | 133±23 (7) | 384±38 (5) | 1920±138 (8) | |||||
| Saline/3.0μg CRF | 314±37 (9) | 164±18 (8) | 326±24 (9) | 1647 49 (9) | |||||
| PCPA/Saline | 152±29 (7) | * | 75±8 (9) | * | 23±3 (8) | * | 1519±191 (8) | ||
| PCPA/0.3μg CRF | 223±28 (8) | 103±15 (9) | 23±2 (9) | 1674±122 (7) | |||||
| PCPA/3.0μg CRF | 163±19 (7) | 83±11 (9) | 33±4 (6) | 1766±140 (7) | |||||
| BN RATS TREATMENT | DA | DOPAC | 5-HT | NE | |||||
| Saline/Saline | 301±60 (11) | 208±33 (11) | 412±34 (11) | 1823±142 (12) | |||||
| Saline/0.3μg CRF | 245±44 (8) | 106±12 (8)& | 339±42 (9) | 1771±189 (10) | |||||
| Saline/3.0μg CRF | 269±40 (11) | 117±10 (7)& | 356±44 (12) | 1789±163 (13) | |||||
| PCPA/Saline | 160±31 (10) | * | 86±15 (8) | * | 37±7 (12) | * | 1486±83 (13) | * | |
| PCPA/0.3μg CRF | 158±16 (9) | 95±21 (9) | 35±5 (11) | 1382±64 (13) | |||||
| PCPA/3.0μg CRF | 163±15 (12) | 95±16 (9) | 51±11 (12) | 1287±71 (14) | |||||
Treatment (ex. Saline/Saline) refers to Pretreatment (IP)/Treatment (ICV). Values are given in pg/mg of tissue (± SEM). Number of samples per group is given in parenthesis (n) following monoamine values.
p < 0.01 vs. Saline (IP)
p < 0.05 vs. Saline (IP)
p < 0.001 vs. WKY rats
p < 0.05 vs. Saline/Saline.
In the frontal cortex, PCPA pretreatment significantly reduced 5-HT levels by 82% [F(1,104) = 502.865, p < 0.001]. PCPA pretreatment also decreased DA levels by 57% [F(1,76) = 8.935, p = 0.004], DOPAC levels by 48% [F(1,73) = 10.545, p = 0.002], and NE levels by 23% [F(1,117) = 25.094, p < 0.001]. A significant effect of rat strain on NE levels [F(1,117) = 13.138, p < 0.001] indicated that BN rats had lower levels of NE (17% reduction) in the frontal cortex compared to WKY rats.
In the hippocampus, PCPA pretreatment significantly reduced 5-HT levels by 77% [F(1,81) = 21.039, p < 0.001]. There was also a significant effect of PCPA pretreatment on NE levels [F(1,114) = 4.238, p = 0.042]. However, separate two-way ANOVAs conducted on each strain revealed that PCPA decreased NE levels by 21% in WKY rats only [F(1,48) = 5.086, p = 0.029], and not in BN rats. There was a trend for BN rats to exhibit higher levels of 5-HT in this brain region (p = 0.054). However, when a separate two-way ANOVA was conducted comparing WKY rats that received IP saline to BN rats that received IP saline, 5-HT levels in BN rats were not significantly different from WKY rats. A significant effect of strain on NE levels [F(1,114) = 16.239, p < 0.001] was observed, with BN rats exhibiting lower levels of NE (30% reduction) in the hippocampus compared to WKY rats.
In the hypothalamus, PCPA pretreatment significantly reduced 5-HT levels by 91% [F(1,100) = 383.376, p < 0.001]. PCPA pretreatment also decreased DA levels by 39% [F (1,93) = 24.037, p < 0.001] and DOPAC levels by 38% [F(1,91) = 25.093, p < 0.001]. For DOPAC, there was a significant PCPA X CRF interaction [F(2,91) = 3.826, p = 0.025] and a trend towards a rat strain X CRF interaction (p = 0.059), indicating that CRF decreased DOPAC in BN rats that received an IP injection of saline but not PCPA. There was also a significant effect of PCPA pretreatment on NE levels [F(1,109) = 10.281, p = 0.002] and a significant strain X PCPA interaction [F(1,109) = 3.963, p = 0.049], indicating that PCPA decreased NE levels by 23% in BN rats only.
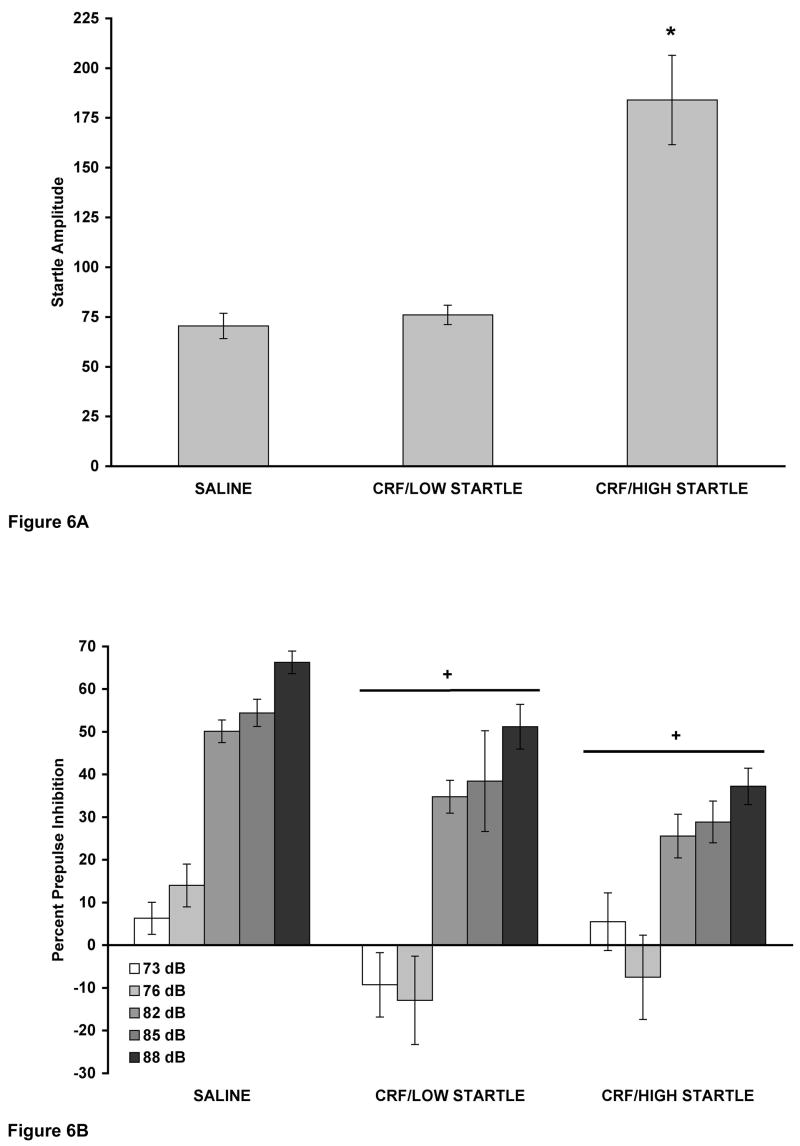
3.3. CRF-Induced Decreases in PPI Occur Without Increases in Startle Amplitude
CRF decreased PPI in WKY rats in which it did not increase baseline startle amplitude (Figure 6). Analysis of startle amplitude data comparing SALINE vs. CRF/LOW STARTLE vs. CRF/HIGH STARTLE showed a significant effect of group [F(2,37) = 28.530, p < 0.001], with a Tukey’s post hoc test showing that the CRF/LOW STARTLE group had comparable startle amplitude to SALINE (p > 0.05) while the CRF/HIGH STARTLE group had significantly greater startle amplitude compared to SALINE (p < 0.001) (Figure 6A). A two-way ANOVA revealed a significant effect of group on PPI [F(2,37) = 8.839, p = 0.001], with a Tukey’s post hoc test showing that both CRF/LOW STARTLE (p = 0.009) and CRF/HIGH STARTLE (p = 0.003) groups had significantly diminished PPI compared to SALINE (Figure 6B). Thus, 3.0 μg CRF decreased PPI in WKY rats whether a CRF-induced increase in startle was absent or present.
Figure 6.
(A and B). Mean (± SEM) startle amplitude (A) and percent PPI (B) in WKY rats from both experiments that received: 1) injection and infusion of saline (SALINE, n = 22); 2) saline injection and 3.0 μg CRF infusion in which CRF did not alter startle amplitude (CRF/LOW STARTLE, n = 9); or 3) saline injection and 3.0 μg CRF infusion in which CRF increased startle amplitude (CRF/HIGH STARTLE, n = 9). (A) * p < 0.001 vs. SALINE, based on a Tukey’s test. (B) + p < 0.01 vs. SALINE, based on a Tukey’s test. CRF decreased PPI in the group in which it did not increase startle.
4. Discussion
Since CRF can increase 5-HT release and drugs that cause 5-HT release, or are 5-HT1A, 5-HT1B, or 5-HT2 receptor agonists, reduce PPI, we tested the hypothesis that CRF decreases PPI indirectly via its effects on 5-HT in WKY and BN rats. Two experiments were conducted to test this possibility. The first experiment examined the effects of the 5-HT2A/C receptor antagonist, ketanserin, on the disruption in PPI caused by CRF. The second experiment investigated the effects of 5-HT depletion using the 5-HT synthesis inhibitor, PCPA, on the CRF-induced decrease in PPI. Additionally, monoamine content in the caudate putamen, frontal cortex, hippocampus, and hypothalamus, as well as time spent active, were assessed.
Results from the first experiment reveal that ICV CRF does not reduce PPI via its effects on 5-HT acting at 5-HT2A/C receptors since blockade of these receptors with ketanserin did not affect the CRF-induced decrease in PPI in either rat strain. Ketanserin treatment alone did not affect PPI in either strain, which is consistent with other studies in which rats were administered the same dose of ketanserin (Nanry and Tilson, 1989; Sipes and Geyer, 1994; Varty and Higgins, 1995). Interestingly, in a study by van der Elst and colleagues, ketanserin pretreatment did not block the decrease in PPI caused by cocaine, an indirect DA agonist (van der Elst et al., 2006). These two pieces of evidence suggest that neither CRF nor DA act indirectly via their effects on 5-HT acting at 5-HT2A/C receptors to modulate PPI.
The effects of ketanserin and CRF treatments on the acoustic startle response were also examined. In saline-injected WKY rats, 3.0 μg CRF increased startle amplitude, (Conti et al., 2002), and this effect was blocked by ketanserin pretreatment, indicating that a single dose of ketanserin (2.0 mg/kg) was sufficient to block a CRF-induced change in behavior. In saline-injected BN rats, 0.3 μg CRF decreased startle amplitude (Conti et al., 2006) and this effect was not blocked by ketanserin pretreatment. Thus, it may be that CRF-induced increases in startle amplitude depend on 5-HT acting on 5-HT2A/C receptors while CRF-induced decreases in startle amplitude do not, suggesting different pathways for CRF mediating increases and decreases in the startle response.
The dose of ketanserin used in this study (2.0 mg/kg) was chosen based on previously published reports demonstrating behavioral effects. For example, Sipes and Geyer (1994) observed that 2.0 mg/kg ketanserin blocks the decrease in PPI cause by a 5-HT2A/C agonist, demonstrating that this dose of ketanserin effectively antagonizes both 5-HT receptor subtypes. Additionally, a single dose of ketanserin (2.0 mg/kg) attenuates the reduction in PPI caused by dizocilpine, a non-competitive NMDA receptor antagonist (Varty and Higgins, 1995), suggesting an interaction between the glutamatergic system and 5-HT2A/C receptors in regulating PPI. In this same study, ketanserin reversed the dizocilpine-induced hyperactivity, indicating that 2.0 mg/kg ketanserin alters non-startle-related behaviors as well. The finding from our own study that ketanserin blocks the 3.0 μg CRF-induced increase in startle in WKY rats further validates our choice of ketanserin dose.
Since it appears that CRF does not reduce PPI via effects on 5-HT acting at 5-HT2A/C receptors, 5-HT levels were depleted in the second experiment to assess whether reduced activation at all 14 5-HT receptor subtypes would alter the ICV CRF-induced decrease in PPI. Results from this experiment reveal that it is unlikely that CRF reduces PPI via effects on 5-HT, as PCPA pretreatment did not attenuate the CRF-induced decrease in PPI in either WKY or BN rats. However, PCPA pretreatment alone decreased PPI at the three highest prepulse intensities (82, 85, and 88 dB) in BN rats. Although it appeared that 0.3 μg CRF further reduced PPI in the PCPA-pretreated BN rats, this decrease was not significant. This apparent lack of an effect of CRF is likely due to a floor effect caused by PCPA pretreatment, such that PPI could not be further reduced by CRF to such an extent as to be statistically significant. The issue of a floor effect also indicates that BN rats are not well-suited for use in experiments in which multiple treatments reduce PPI, since PPI under basal conditions is significantly reduced compared to WKY rats (Palmer et al., 2000).
Interestingly, 5-HT depletion increased PPI in WKY rats and decreased PPI at certain prepulse intensities in BN rats. PCPA decreases PPI in male Sprague-Dawley rats (Fletcher et al., 2001; Prinssen et al., 2002), similar to our findings in BN rats. We did observe that PCPA treatment decreased NE levels in the hippocampus of WKY rats only and this may help explain the differential effect of PCPA on PPI in the two rat strains. In addition to 5-HT, NE modulates PPI, with α1- adrenergic receptor agonists reducing PPI (Alsene et al., 2006; Carasso et al., 1998). Thus, reduction of NE in the hippocampus may have the opposite effect of an α1- adrenergic receptor agonist and cause the increase in PPI observed in WKY rats.
The effects of PCPA and CRF treatments on the startle response were also examined. High dose CRF (3.0 μg) increased startle amplitude in WKY rats and did not alter startle amplitude in BN rats, as previously shown (Conti et al., 2002). Interestingly, combined PCPA/3.0 μg CRF treatment greatly increased startle amplitude in WKY rats, suggesting that the two treatments had a synergistic effect on startle. Thus, it is possible that 5-HT serves to counteract an effect of CRF on startle. It is curious that in the present experiments, ketanserin, a 5-HT2A/C receptor antagonist, blocked the effect of CRF on startle while 5-HT depletion enhanced the effect. One possibility is that 5-HT, acting at receptors other than the 5-HT2A/C subtype, has an inhibitory effect on startle.
CRF increases grooming, as well as general activity, in rats kept in a familiar environment (Dunn and Berridge, 1990; Jones et al., 1998). In the PCPA experiment, CRF-induced increases in activity (including grooming, locomotion, burrowing, rearing, and chewing) were examined. In saline-injected BN rats, doses of CRF that increased activity (0.3 and 3.0 μg) also diminished PPI. Interestingly, in saline-injected WKY rats, CRF increased activity at a dose that did not decrease PPI (0.3 μg) and decreased PPI at a dose that did not affect activity (3.0 μg), similar to our previous findings on grooming (Conti et al., 2002) and activity (Conti, 2005). Thus, the observation that WKY rats appear to be less sensitive to the PPI-reducing effects of CRF cannot be explained by an overall reduction in behavioral sensitivity to CRF.
After completion of PPI testing in the PCPA experiment, the caudate putamen, frontal cortex, hippocampus, and hypothalamus were removed. Levels of DA, DOPAC, 5-HT, and NE were analyzed by HPLC. Injection of the 5-HT synthesis inhibitor, PCPA, for two consecutive days prior to PPI testing greatly reduced 5-HT levels in all four brain regions. It must be noted that PCPA treatment did not completely abolish the presence of 5-HT in these brain regions. Thus, a certain amount of PPI regulation by 5-HT was possible. However, the fact that a roughly 80% reduction in brain 5-HT did not even slightly attenuate the CRF-induced decrease in PPI in our studies suggests that CRF reduced PPI independently of its effects on 5-HT. PCPA treatment also decreased other monoamine levels, albeit to a lesser extent than 5-HT, as previously observed (Koe and Weissman, 1966; Yang and Pan, 1999). However, it does not appear that the effects of PCPA on DA, DOPAC, and NE altered the effects of CRF on PPI.
Strain differences were also revealed with respect to monoamine levels. BN rats had higher DA and DOPAC levels, as well as increased DA utilization, in the caudate putamen compared to WKY rats. Since DA receptor agonists (Mansbach et al., 1988; Swerdlow et al., 1991) and drugs that increase extracellular DA concentrations (Byrnes and Hammer, 2000; Martinez et al., 1999) decrease PPI, perhaps increased DA levels and utilization in the caudate putamen contribute to the reduced PPI observed in BN rats under basal conditions (Conti et al., 2002; Palmer et al., 2000). However, administration of haloperidol, a DA receptor antagonist, did not enhance PPI in BN rats (Conti et al., 2005). BN rats also had less NE in the frontal cortex and hippocampus than WKY rats. It is also unlikely that reduced NE levels in these brain regions contribute to the diminished PPI in BN rats since α1-adrenergic receptor agonists reduce PPI (Alsene et al., 2006; Carasso et al., 1998) and lower NE levels may have the opposite effect of an α1-adrenergic agonist. Thus, the reasons for the BN strain exhibiting diminished baseline PPI remain unknown.
One of the problems inherent in studying PPI occurs when experimental treatments alter baseline startle amplitude. Davis demonstrated that apparent decreases in percent PPI may be due solely to drug-induced increases in baseline startle (Davis, 1988). One technique has been successfully employed to circumvent this issue and involves subjecting the data from drug-treated animals to a median split on the basis of startle amplitude. This results in the formation of two groups: one in which the drug treatment increases startle amplitude and one in which the drug treatment does not increase startle amplitude (Conti et al., 2006). In this study, we found that 3.0 μg CRF, when infused ICV into WKY rats, either elevated (CRF/HIGH STARTLE), or did not affect (CRF/LOW STARTLE), baseline startle amplitude when compared to a saline-treated control group (SALINE). Most importantly, both CRF/LOW STARTLE and CRF/HIGH STARTLE groups had significant reductions in PPI compared to the SALINE group. Thus, CRF decreased PPI in a group of rats without affecting startle amplitude. The ketanserin experiment offers further evidence that CRF decreases PPI without increasing startle amplitude. In WKY rats, 3.0 μg CRF reduced PPI despite the fact that ketanserin pretreatment blocked the CRF-induced increase in startle.
This is the first study to our knowledge to examine possible interactions between CRF and 5-HT in modulating PPI. However, other groups have studied this interaction with respect to non-startle-related behaviors, including performance in the elevated plus maze, acquisition of learned helplessness, and grooming. For example, ICV infusion of CRF increases anxiety-like behavior in the elevated plus maze, as indicated by a decrease in the time spent in the open arms of the maze and the number of open arm entries. Pretreatment with 8-OH-DPAT, a 5-HT1A receptor agonist, attenuates the anxiety-like behavior produced by CRF (Kagamiishi et al., 2003). Exposure to uncontrollable stress is essential for the development of learned helplessness (Maier and Watkins, 2005). Injection of a non-selective CRF receptor antagonist into the DRN, a primary site of forebrain-projecting serotonergic neurons (Jacobs and Azmitia, 1992), prior to uncontrollable stress exposure prevents the acquisition of learned helplessness (Hammack et al., 2002). Additionally, infusion of CRF into the DRN mimics the effects of uncontrollable stress, resulting in learned helplessness (Hammack et al., 2002) and this effect appears to be mediated by CRF2 receptors in the DRN (Hammack et al., 2003). Temel and colleagues found that 5-HT depletion does not affect CRF-induced grooming (Temel et al., 2003). Thus, it appears that CRF and 5-HT interact to mediate some behaviors (anxiety in the elevated plus maze, learned helplessness) and not others (grooming, PPI, general activity). The present results suggest that CRF and 5-HT interact to mediate the acoustic startle response in a manner that depends on the rat strain being examined, the dose of CRF being infused, and perhaps the 5-HT receptor subtype being affected.
Interestingly, WKY and BN rats differ in their behavior in the elevated plus maze and in their susceptibility to learned helplessness. For example, WKY rats exhibit greater anxiety-like behavior in the elevated plus maze, as they spend significantly less time in the open arms of the maze compared to BN rats (Berton et al., 1997; Ramos et al., 1997). WKY rats are also highly susceptible to learned helplessness while BN rats show a complete lack of susceptibility (Wieland et al., 1986). It would be interesting to examine whether CRF and 5-HT interact to mediate these behaviors in a manner similar to those observed in other rat strains, even though CRF and 5-HT do not interact to mediate PPI in WKY and BN rats.
In conclusion, our results show that ICV CRF decreases PPI in both WKY and BN rats and that neither blockade of 5-HT2A/C receptors nor 5-HT depletion attenuates this decrease. Thus, it appears that CRF does not decrease PPI indirectly via its effects on 5-HT in either of these two rat strains. However, it is important to keep in mind that 14 distinct 5-HT receptor subtypes exist (Nestler et al., 2001). Thus, examining whether other selective 5-HT receptor antagonists block the CRF-induced decrease in PPI would identify any possible interactions that may exist between CRF and 5-HT in modulating PPI. CRF and 5-HT do appear to interact to modulate the startle response in WKY rats, since ketanserin pretreatment blocked the CRF-induced increase in startle. We uncovered differences in monoamine levels between the two rat strains, with BN rats exhibiting higher levels of DA and DOPAC in the caudate putamen, and lower levels of NE in the frontal cortex and hippocampus compared to WKY rats. Importantly, we showed that CRF decreases PPI in the absence of an increased startle response in WKY rats, which is critical for proper interpretation of the data.
Acknowledgments
We would like to acknowledge the technical assistance of Ms. Jennifer Costill. These studies were supported by MH065467.
Footnotes
Disclosure/Conflict of Interest
The authors declare that, except for income received from our primary employers, no financial support or compensation has been received from any individual or corporate entity for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsene KM, Carasso BS, Connors EE, Bakshi VP. Disruption of prepulse inhibition after stimulation of central but not peripheral alpha-1 adrenergic receptors. Neuropsychopharmacology. 2006;31:2150–61. doi: 10.1038/sj.npp.1300989. [DOI] [PubMed] [Google Scholar]
- Awouters F. The pharmacology of ketanserin, the first selective serotonin S2-antagonist. Drug Development Research. 1985;6:263–300. [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual Review of Pharmacology and Toxicology. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Berton O, Ramos A, Chaouloff F, Mormede P. Behavioral reactivity to social and nonsocial stimulations: a multivariate analysis of six inbred rat strains. Behavior Genetics. 1997;27:155–66. doi: 10.1023/a:1025641509809. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer MA, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Archives of General Psychiatry. 1990;47:181–8. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenia patients. Archives of General Psychiatry. 1992;49:206–15. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Brunello N, Masotto C, Steardo L, Markstein R, Racagni G. New insights into the biology of schizophrenia through the mechanism of action of clozapine. Neuropsychopharmacology. 1995;13:177–213. doi: 10.1016/0893-133X(95)00068-O. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Hammer RP., Jr The disruptive effect of cocaine on prepulse inhibition is prevented by repeated administration in rats. Neuropsychopharmacology. 2000;22:551–54. doi: 10.1016/S0893-133X(99)00151-7. [DOI] [PubMed] [Google Scholar]
- Carasso BS, Bakshi VP, Geyer MA. Disruption of prepulse inhibition after alpha-1 adrenoreceptor stimulation in rats. Neuropharmacology. 1998;37:401–4. doi: 10.1016/s0028-3908(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. The Journal of Neuroscience. 1995;15:6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Pearse RV, II, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–95. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Conti LH. Characterization of the effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in Brown Norway and Wistar-Kyoto rats. European Journal of Pharmacology. 2005;507:125–34. doi: 10.1016/j.ejphar.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Conti LH, Berridge CW, Tayler JE. Both corticotropin-releasing factor and apomorphine reduce prepulse inhibition following repeated central infusion of corticotropin-releasing factor. Pharmacology, Biochemistry and Behavior. 2006;85:261–72. doi: 10.1016/j.pbb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Conti LH, Costill JE, Flynn S, Tayler JE. Effects of a Typical and an Atypical Antipsychotic on the Disruption of Prepulse Inhibition Caused by Corticotropin-Releasing Factor and by Rat Strain. Behavioral Neuroscience. 2005;119:1052–60. doi: 10.1037/0735-7044.119.4.1052. [DOI] [PubMed] [Google Scholar]
- Conti LH, Murry J, Ruiz M, Printz M. Effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in two rat strains. Psychopharmacology. 2002;161:296–303. doi: 10.1007/s00213-002-1025-2. [DOI] [PubMed] [Google Scholar]
- Davis M. Apomorphine, d-amphetamine, strychnine and yohimbine do not alter prepulse inhibition of the acoustic startle reflex. Psychopharmacology. 1988;95:151–6. doi: 10.1007/BF00174500. [DOI] [PubMed] [Google Scholar]
- Day HEW, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, et al. Differential expression of 5HT-1A, alpha1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. The Journal of Comparative Neurology. 2004;474:364–78. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groote L, Penalva RG, Flachskamm C, Reul JM, Linthorst AC. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor type 1 and type 2 agonists. Journal of Neurochemistry. 2005;94:45–56. doi: 10.1111/j.1471-4159.2005.03164.x. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. The Journal of Neuroscience. 1985;5:3189–203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Schipholt MI, van der Gugten J, Hijzen TH, Geyer MA, et al. Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biological Psychiatry. 2002;51:583–90. doi: 10.1016/s0006-3223(01)01323-3. [DOI] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Westphal KG, Olivier JD, Verdouw PM, van der Gugten J, et al. Reversal of startle gating deficits in transgenic mice overexpressing corticotropin-releasing factor by antipsychotic drugs. Neuropsychopharmacology. 2003;28:1790–98. doi: 10.1038/sj.npp.1300256. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress. Brain Research Reviews. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Selhi ZF, Azampanah A, Sills TL. Reduced brain serotonin activity disrupts prepulse inhibition of the acoustic startle reflex: effects of 5,7-dihydroxytryptamine and p-chlorophenylalanine. Neuropsychopharmacology. 2001;24:399–409. doi: 10.1016/S0893-133X(00)00215-3. [DOI] [PubMed] [Google Scholar]
- Gabr RW, Gladfelter WE, Birkle DL, Azzaro AJ. In vivo microdialysis of corticotropin-releasing factor (CRF): calcium dependence of depolarization-induced neurosecretion of CRF. Neuroscience Letters. 1994;169:63–7. doi: 10.1016/0304-3940(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Gispen-de Wied CC. Stress in schizophrenia: an integrative view. European Journal of Pharmacology. 2000;405:375–84. doi: 10.1016/s0014-2999(00)00567-7. [DOI] [PubMed] [Google Scholar]
- Graham F. The more or less startling effects of weak prestimuli. Psychophysiology. 1975;12:238–48. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways: Role in autonomic, neuroendocrine, and behavioral responses to stress. Annals of the New York Academy of Sciences. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Charney DS, Krystal J, Braff D. Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biological Psychiatry. 1992;32:939–43. doi: 10.1016/0006-3223(92)90183-z. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. The Journal of Neuroscience. 2002;22:1020–26. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, et al. Corticotropin-releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. The Journal of Neuroscience. 2003;23:1019–25. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological Review. 1980;87:175–89. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic and temporal factors in the evocation of startle. Journal of the Acoustical Society of America. 1968;43:269–82. doi: 10.1121/1.1910776. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological Reviews. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacology, Biochemistry and Behavior. 1995;52:649–54. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology. 1998;138:124–32. doi: 10.1007/s002130050654. [DOI] [PubMed] [Google Scholar]
- Kagamiishi Y, Yamamoto T, Watanabe S. Hippocampal serotonergic system is involved in anxiety-like behavior induced by corticotropin-releasing factor. Brain Research. 2003;991:212–21. doi: 10.1016/j.brainres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Padich RA, McCloskey TC, Taylor VL, Schmidt CJ. 5-HT modulation of auditory and visual sensorimotor gating: I. Effects of 5-HT releasers on sound and light prepulse inhibition in Wistar rats. Psychopharmacology. 1996;124:95–106. doi: 10.1007/BF02245609. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–62. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koe BK, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. The Journal of Pharmacology and Experimental Therapeutics. 1966;154:499–516. [PubMed] [Google Scholar]
- Lahmame A, Grigoriadis DE, De Souza EB, Armario A. Brain corticotropin-releasing factor immunoreactivity and receptors in five inbred rat strains: relationship to forced swimming behaviour. Brain Research. 1997;750:285–92. doi: 10.1016/s0006-8993(96)01368-6. [DOI] [PubMed] [Google Scholar]
- Lavicky J, Dunn AJ. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. Journal of Neurochemistry. 1993;60:602–12. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Linthorst ACE, Penalva RG, Flachskamm C, Holsboer F, Reul JMHM. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. European Journal of Neuroscience. 2002;16:2441–52. doi: 10.1046/j.1460-9568.2002.02400.x. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers T, De Souza EB, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:836–40. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyne J, Kelly BD, O'Connor WT. Schizophrenia: a review of neuropharmacology. Irish Journal of Medical Science. 2004;173:155–8. doi: 10.1007/BF03167931. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Braff DL, Geyer MA. Prepulse inhibition of the acoustic startle response is disrupted by N-ethyl-3,4-methylenedioxyamphetamine (MDEA) in the rat. European Journal of Pharmacology. 1989;167:49–55. doi: 10.1016/0014-2999(89)90746-2. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–14. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Martinez DL, Geyer MA. Characterization of the disruptions of prepulse inhibition and habituation of startle induced by alpha-ethyltryptamine. Neuropsychopharmacology. 1997;16:246–55. doi: 10.1016/S0893-133X(96)00240-0. [DOI] [PubMed] [Google Scholar]
- Martinez ZA, Ellison GD, Geyer MA, Swerdlow NR. Effects of sustained cocaine exposure on sensorimotor gating of startle in rats. Psychopharmacology. 1999;142:253–60. doi: 10.1007/s002130050887. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. The British Journal of Medical Psychology. 1961;34:103–16. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Nanry KP, Tilson HA. The role of 5HT1A receptors in the modulation of the acoustic startle reflex in rats. Psychopharmacology. 1989;97:507–13. doi: 10.1007/BF00439556. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, Malenka RC. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. New York: The McGraw-Hill Companies; 2001. [Google Scholar]
- Padich RA, McCloskey TC, Kehne JH. 5-HT modulation of auditory and visual sensorimotor gating: II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology. 1996;124:107–16. doi: 10.1007/BF02245610. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology. 1999;147:162–67. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Dulawa SC, Mottiwala AA, Conti LH, Geyer MA, Printz MP. Prepulse startle deficit in the Brown Norway rat: a potential genetic model. Behavioral Neuroscience. 2000;114:374–88. doi: 10.1037//0735-7044.114.2.374. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biological Psychiatry. 2000;47:662–9. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1986. [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Prinssen EPM, Assie MB, Koek W, Kleven MS. Depletion of 5-HT disrupts prepulse inhibition in rats: dependence on the magnitude of depletion, and reversal by a 5-HT precursor. Neuropsychopharmacology. 2002;26:340–7. doi: 10.1016/S0893-133X(01)00348-7. [DOI] [PubMed] [Google Scholar]
- Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behavioural Brain Research. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- Rigdon GC, Weatherspoon JK. 5-hydroxytryptamine1a receptor agonists block prepulse inhibition of acoustic startle reflex. The Journal of Pharmacology and Experimental Therapeutics. 1992;263:486–93. [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. The Journal of Neuroscience. 2004;24:6545–52. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–48. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine-corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Keith VA, Braff DL, Geyer MA. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. The Journal of Pharmacology and Experimental Therapeutics. 1991;256:530–36. [PubMed] [Google Scholar]
- Temel Y, Helmy A, Pinnock S, Herbert J. Effect of serotonin depletion on the neuronal, endocrine and behavioural responses to corticotropin-releasing factor in the rat. Neuroscience Letters. 2003;338:139–42. doi: 10.1016/s0304-3940(02)01392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. The Journal of Comparative Neurology. 2001;435:450–63. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the coordination of emotional and cognitive limbs of the stress response. Journal of Neuroendocrinology. 1998;10:743–57. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- van der Elst MCJ, Ellenbroek BA, Cools AR. Cocaine strongly reduces prepulse inhibition in apomorphine-susceptible rats, but not in apomorphine-unsusceptible rats: regulation by dopamine D2 receptors. Behavioural Brain Research. 2006;175:392–98. doi: 10.1016/j.bbr.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. The Journal of Comparative Neurology. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Reversal of dizocilpine-induced disruption of prepulse inhibition of an acoustic startle response by the 5-HT2 receptor antagonist ketanserin. European Journal of Pharmacology. 1995;287:201–5. doi: 10.1016/0014-2999(95)00660-5. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: A neural diathesis-stress model. Psychological Review. 1997;104:667–85. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Wieland S, Boren JL, Consroe PF, Martin A. Stock differences in the susceptibility of rats to learned helplessness training. Life Sciences. 1986;39:937–44. doi: 10.1016/0024-3205(86)90376-0. [DOI] [PubMed] [Google Scholar]
- Yang I, Pan J. Effects of serotonin depletion by p-chlorophenylalanine, p-chloroamphetamine or 5,7-dihydroxytryptamine on central dopaminergic neurons: focus on tuberoinfundibular dopaminergic neurons and serum prolactin. Journal of Biomedical Science. 1999;6:183–93. doi: 10.1007/BF02255902. [DOI] [PubMed] [Google Scholar]