Abstract
Safety assurance for diagnostic ultrasound in obstetrics began with a tacit assumption of safety allowed by a federal law enacted in 1976 for then-existing medical ultrasound equipment. The implementation of the 510(k) pre-market approval process for diagnostic ultrasound resulted in the establishment of guideline upper limits for several examination categories in 1985. The obstetrical category has undergone substantial evolution from initial limits (I. e., 46 mW/cm2 spatial peak temporal average (SPTA) intensity) set in 1985. Thermal and mechanical exposure indices, which are displayed on-screen according to an Output Display Standard (ODS), were developed for safety assurance with relaxed upper limits. In 1992, with the adoption of the ODS, the allowable output for obstetrical ultrasound was increased both in terms of the average exposure (e. g. to a possible 720 mW/cm2 SPTA intensity) and of the peak exposure (via the Mechanical Index). There has been little or no subsequent research with the modern obstetrical ultrasound machines to systematically assess potential risks to the fetus using either relevant animal models of obstetrical exposure or human epidemiology studies. The assurance of safety for obstetrical ultrasound therefore is supported by three ongoing means: (I) review of a substantial but uncoordinated bioeffect research literature, (ii) the theoretical evaluation of diagnostic ultrasound exposure in terms of thermal and nonthermal mechanisms for bioeffects, and (iii) the skill and knowledge of professional sonographers. At this time, there is no specific reason to suspect that there is any significant health risk to the fetus or mother from exposure to diagnostic ultrasound in obstetrics. This assurance of safety supports the prudent use of diagnostic ultrasound in obstetrics by trained professionals for any medically indicated examination.
Introduction
Since pulse-echo A mode ultrasound devices entered medical practice over 40 years ago, diagnostic ultrasound has become ubiquitous in the modern hospital, at satellite service providers, and even in ambulances and doctors offices. Diagnostic ultrasound has become an essential tool in obstetrics. The original analogue imaging devices have switched to digital two dimensional (2-D) imagers, and even into 3-D (4-D with motion). Massive ultrasound systems mounted on wheeled carts have evolved into laptop imagers. However, the fundamental imaging principles with pulses of ultrasound waves penetrating into patients’ tissues, and returning echos displaying anatomical information, have remained essentially the same. The tremendous growth in usage and the acceptance of diagnostic ultrasound has been fueled in part by its accumulating history of apparent safety, which now is almost taken for granted. However, this assurance of safety has not always been such a general consensus and derives for the most part from decades of scholarly investigation. The future persistence of this exceptional safety standing among imaging modalities requires continued scrutiny of the safety issues of diagnostic ultrasound, which is particularly crucial for ultrasound in obstetrics.
When ultrasonic waves in the megahertz frequency range became available about 80 years ago, the new penetrating energy beams rapidly attracted research interest into therapeutic applications. By 1965, simple hand-held applicators of continuous wave ultrasound were well developed and still find widespread use in physical therapy departments. In addition, high power devices were studied for use in trackless brain surgery, [1] or other applications. Diagnostic ultrasound was limited mostly to pulse-echo A-mode with single beams, which also could show movement of structures, such as the heart, in M mode. [2] However, work on actual 2-D image formation to view the fetus for obstetrical diagnosis was already under development. [3] The existence of biological effects, particularly occult but dangerous effects, was assumed to be a possible risk in diagnostic ultrasound (reminiscent of the earlier experience with such bioeffects which had emerged from the use of xrays for imaging). Research on bioeffects of ultrasound was pursued in several laboratories seeking to identify potential risks in diagnostic medical usage, as well as therapeutic applications, and to delineate boundaries to separate the two antithetical applications. The concern over the possibility of bioeffects grew as the acceptance and use of diagnostic ultrasound in medicine rapidly increased. This led to a comprehensive assessment of the problem in an international workshop on “The Interaction of Ultrasound and Biological Tissues” in 1971. [4]
Although there has not been a concerted, comprehensive research program to evaluate possible risks in diagnostic ultrasound, there has been a substantial pursuit of independent research projects. This research has produced a diverse collection of published studies, which requires careful interpretation and evaluation. An important early assessment of possible risks of diagnostic ultrasound imaging in pregnancy was conducted by a National Institutes of Health Consensus Development Conference. [5] The assessment of research developments has been pursued diligently in scholarly conferences conducted by several organizations including the American Institute of Ultrasound in Medicine,[6–8] and the World Federation of Ultrasound in Medicine and Biology.[9–12] The National Council on Radiation Protection (USA) established a long-standing committee to review the problem of medical ultrasound safety and provide regulatory recommendations, which produced three comprehensive reports. [13–15] In addition, numerous other reviews and evaluations have been completed by other organizations. [16] The general topic of diagnostic ultrasound safety has been reviewed previously in this journal.[17] The most recent organized assessments of ultrasound bioeffects were the 2005 conference sponsored jointly by the Radiation Protection Division of the Health Protection Agency (UK) and the International Commission on Nonionizing Radiation Protection and the 2005 bioeffects consensus conference sponsored by the AIUM. [18,19] The AIUM conference included five working groups on specific topics including two addressing potential thermal and nonthermal bioeffects of obstetrical ultrasound on the fetus.
In contrast to the periodic scholarly assessment of possible risks in diagnostic ultrasound, the actual regulation of the ultrasound exposure of patients was set in the USA essentially by government decree. The Medical Device Amendments of 1976 enacted by the US Congress allowed for simplified pre-market approval of new devices if these were substantially equivalent in safety and effectiveness to devices sold for the same applications before May 28, 1976. This law was implemented by the Food and Drug Administration (USA) through the 510(k) pre-marketing notification process. This process provides a tacit assumption of safety for diagnostic ultrasound devices without further demonstration of safety. The ultrasound exposure of patients is controlled by upper limits on selected parameters, which are determined from measurements of pre-enactment devices by standard methods. The AIUM and the National Equipment Manufacturers Association (NEMA) have been instrumental in developing standards for ultrasound field measurements.[20,21] The diagnostic ultrasound clearance process is governed by the latest 510(k) guidance document.[22]
The purpose of this communication is to provide a brief overview of the evolution of safety assurance in obstetrical ultrasound. First, a description of the nature of the ultrasound exposure to patients, the important parameters used to describe it and regulatory limits on obstetrical ultrasound exposures are presented. The potential risks of adverse effects are then described in the context of physical mechanisms responsible for bioeffects of ultrasound and of the exposure response relationships as they are presently understood. In addition, the problems posed by lingering doubts and concerns about the absolute safety of diagnostic ultrasound in obstetrics are noted. Finally, reasonable safety assurance viewpoints for the sonographer are discussed.
Diagnostic Ultrasound Exposure
Obstetrical ultrasound is a means to monitor the development and well being of the fetus. The exposure of mother and fetus often occurs in routine examinations with minimal specific medical indication. First trimester scans or fetal heartbeat detection are performed to confirm pregnancy. Second and third trimester scans are performed to date the pregnancy and follow anatomical development. The scanning is generally performed trans abdominally and the examination can take up to an hour. The exposure of the fetus in trans abdominal scanning depends to some extent on factors such as body fat and the filling of the bladder, which can complicate the use of standard exposure models. Trans vaginal probes can be used, particularly for obese patients, to obtain improved fetal images, as shown in Fig. 1 for a first trimester fetus. This procedure also alters the fetal exposure due to the reduction in intervening tissues. Doppler modes are often used to evaluate blood flow in the heart or head. Color flow mapping in a 2-D Doppler window provides a qualitative image of flow, while the fixed beam pulsed Doppler method can quantify flow at specific vessels. These modes also modify the fetal exposure in terms of pulse durations and dwell times; spectral Doppler can involve especially long dwell times along a specific beam line because the beam is not scanned. The exposure to diagnostic ultrasound in obstetrics occurs for virtually every mother and fetus in the developed and developing world. The total exposure of each fetus is unique and is known only in general terms of the possible values of exposure parameters produced by the ultrasound machines.
1.
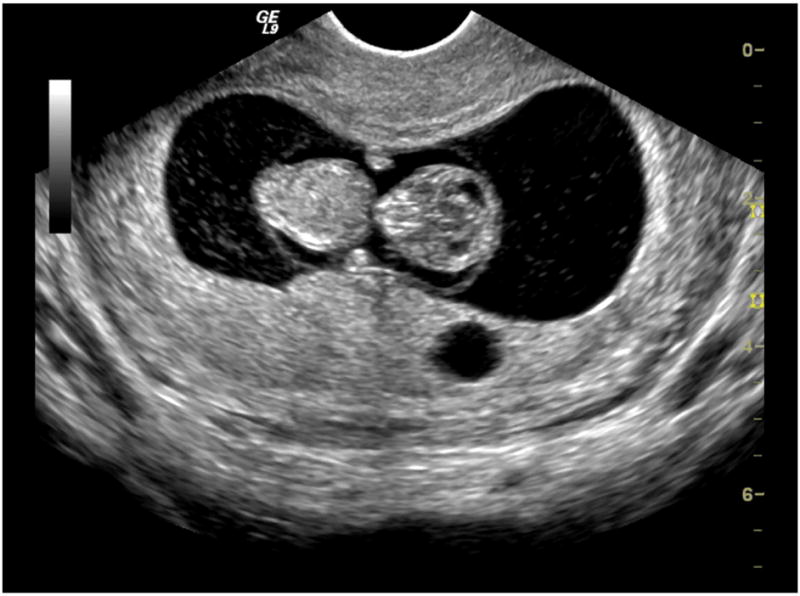
A diagnostic ultrasound image of a first trimester fetus obtained with a modern transvaginal probe. The clarity of such images allow the unambiguous verification of pregnancy and fetal viability. (courtesy GE Healthcare).
Diagnostic ultrasound imaging is accomplished by transmitting pulses of ultrasound waves into the body from a transducer, which converts electrical signals into ultrasonic vibration. Ultrasound does not penetrate far in air, so a liquid or gel coupling medium is normally used to connect the transducer to the patient. Transmitted beams of ultrasound pulses are focused at one or more depths and scanned to interrogate a plane or volume of tissue. The pulsatile nature of the exposure requires consideration of temporal peak and temporal average exposure metrics, which are associated with different physical mechanisms for bioeffects.
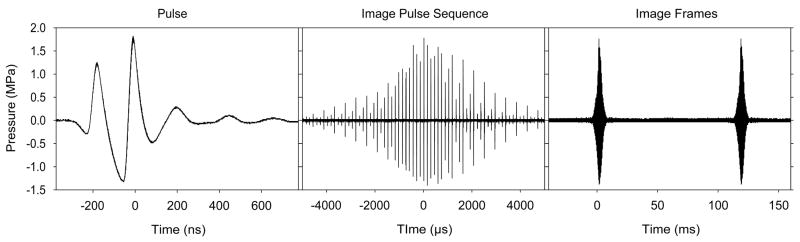
An ultrasound pulse is illustrated in Fig. 2a in terms of the pressure measured by a small hydrophone in a water bath, with a 2.5 cm thick tissue mimicking attenuator used to simulate intervening tissue. The wave duration was 172 ns (nanoseconds) for the pulse in Fig. 2a, corresponding to an approximate ultrasound frequency of 5.8 MHZ. The wave reaches a peak compressional (positive) and a peak rarefactional (negative) pressure amplitude (PRPA), which is an important temporal peak exposure parameter. Note that atmospheric pressure (0.1 MPa) was much smaller than the PRPA of 1.3 MPa for the pulse shown in Fig. 2a. The pressure below zero represents a negative tension in the medium. The pulse carries energy, which is typically expressed in terms of the pulse-average intensity (power delivered per unit area averaged over the pulse duration) at its spatial peak on the beam axis near the focus. The pulse in Fig. 2a had a duration of 240 ns and a spatial peak pulse average (SPPA) intensity of 68 W/cm2, which is another important temporal peak parameter. Pulses intended for Doppler estimation of the velocity of tissue or blood motion are typically of longer duration, e. g. 3 or more waves, to allow accurate determination of the Doppler frequency shift of the echo relative to the pulse.
2.
Oscilloscope traces illustrating the exposure to ultrasound at a point in tissue from a transvaginal probe (E8C, GE Healthcare Logiq 9) operating at 6 MHz. The measurements were made with a hydrophone in a water bath, with a tissue mimicking attenuator between the probe and the hydrophone. The minimum unit of exposure is the pulse (left), which oscillates in pressure at approximately 5.8 MHz for a duration of 240 ns. The peak rarefactional pressure amplitude, a parameter used in calculating the Mechanical Index was 1.3 MPa. The pulses are repeated each 144 μs and form an image pulse sequence (center) at a point as the scanned beam passes by. The imaging mode had three focal zones to improve the apparent depth of focus, and the overall image pulse sequence is made up of three groups displaced slightly in time (one for each focal depth). Finally, the image pulse sequence repeats for each image frame at 9 frames per second (right).
Echos are returned from structures within the body and normally are placed on an image according the time needed for the echo return at the known speed of ultrasound, and by the aim of the transducer. The resolution in a 2-D image in the depth direction (z axis) is related to the pulse duration, with shorter pulses generally better. Since the shortest pulse duration obtainable is approximately one wave, higher ultrasound frequencies yield better resolution. The resolution in the perpendicular direction (x axis) is normally best at the focal depth and worse near the transducer or beyond the focus into the tissue. Pulses are typically repeated at a repetition period sufficient to allow echos to return or die out from the deepest tissue. For A-mode, M mode and spectral Doppler, the ultrasound exposure consists of the pulses repeated at the pulse repetition period (PRP) limited to the tissue along the beam line direction. The average intensity over time, including on and off periods, is an important temporal average parameter. For a simple pulse train, this is given by the product of the SPPA intensity and the ratio of the pulse duration to the PRP.
For B mode, color flow Doppler and other imaging modes, the beam is scanned through the tissue. This originally was produced by physically moving the transducer for analogue imaging, but now is accomplished by phasing the pulses for elements in a multi-element transducer. Multi-element arrays can take a variety of forms, such as linear or curved arrays, or phased arrays designed to produce a sector scan of the beam. For a 3-D image, a 2-D scan plane typically is itself scanned in the perpendicular direction with computer processing of the resulting 2-D image series creating a 3-D display. A 3D display may show motion in time, called a 4-D display. For scanning, the minimum exposure is the amount ultrasound transmitted to form an image. At a point in tissue, this typically represents one passage of the scanned beam, which generates a sequence of pulses of different amplitudes. An image pulse sequence, which was produced by pulses like that shown in Fig. 2a delivered to a point at the focal depth, is shown in Fig. 2b. The pulses are separated by the PRP, which was 144 μs, and increase from a very small pressure amplitude as the beam approaches the point, to a maximum when the beam is aimed directly at the point, and then decreases as the beam passes by. This repeats for each image, as shown in Fig. 2c for a 9 Hz frame rate. The duration of the image pulse sequence is related to the beam size and the scan frame rate. This rate is important in determining the overall temporal average intensity incident on the tissue present in the image, because scanning reduces the SPTA intensity relative to a fixed beam.
The parameters called for in the 510(k) guide are determined by performing measurements in a waterbath. However, water measurements do not reflect the true ultrasound field present inside the body owing to the diminution of the pulse intensity caused by attenuative processes, primarily absorption. To obtain an approximation of the tissue values of needed parameters, the water values are derated by a calculated exponential decay rate with depth. The attenuation rate normally assumed is given by the logarithmic decay constant 0.3 dB/cm/MHz, which can be substantial for higher frequencies. For example, at 2.5 MHz and 4 cm depth the ultrasound pulse is reduced by 3 dB, cutting the intensity in half. At 10 cm depth and 6.6 MHz, the reduction is about 20 dB, or a factor of 100 in intensity. Due to time-gain compensation built into ultrasound machines, which is often adjustable with depth by a series of sliding controls, the effects of attenuation with depth are removed from the image presented on the screen.
The medical device amendments were enacted in 1976. After developing suitable measurement methods and accumulating data on pre-enactment devices, the FDA issued a 510(k) Guide for Measuring and Reporting Acoustic Output of Diagnostic Ultrasound Medical Devices.[22] This included values of the derated SPTA and SPPA intensities for four use categories, which served as guideline upper limits as given in Table 1. For fetal imaging the values were 46 mW/cm2 SPTA and 65 W/cm2 SPPA. These values seemed low to interested parties, and, in fact, a pre-enactment device was found with higher output values, which had been overlooked [see comments by Harris, GR in reference 23]. In 1987, the guideline values were increased to 94 mW/cm2 SPTA and 190 W/cm2 SPPA for fetal imaging. These values still serve as the guideline limits for the “Track One” approval process applied to ultrasound machines without on-screen output indicators specified in the “Output Display Standard” described in the next section. The SPTA intensity limits were much higher for cardiac and peripheral vascular categories.
Table 1.
| Original 1985 | Present Track 1 | Present Track 3 | ||||
|---|---|---|---|---|---|---|
| Ispta.3 | Isppa.3 | Ispta.3 | Isppa.3 | Ispta.3 | MI | |
| Application | mW/cm2 | W/cm2 | mW/cm2 | W/cm2 | mW/cm2 | none |
| Peripheral vessel | 720 | 65 | 720 | 190 | 720 | 1.9 |
| Cardiac | 430 | 65 | 430 | 190 | 720 | 1.9 |
| Fetal imaging* | 46 | 65 | 94 | 190 | 720 | 1.9 |
| Ophthalmic | 17 | 28 | 17 | 28 | 50 | 0.23 |
and other general imaging such as pediatric, intra-operative, and cephalic.
Physical Mechanisms and Adverse Bioeffects
The stochastic bioeffects of ionizing radiation, for which harmful effects only decline in probability with dose, remain possible for any low dose.[24] Ultrasound is a non-ionizing form of transmitted energy, and therefore does not have an ionization dose, as defined for x-rays or other ionizing radiations. The concept of dose was omitted from the discussion of ultrasound exposure, above, because no universal dose quantity has been defined for ultrasound. For ultrasound, bioeffects occur only above thresholds and increase from zero severity with increasing exposure above the threshold with certainty (not probability) for specific conditions. The key concept for safety assurance in diagnostic ultrasound is that an exposure level is expected, under a given set of conditions, for which there is no risk of harm. Ideally, the conditions existing for diagnostic ultrasound in obstetrics should yield exposures in the absolutely safe regime for all known bioeffects.
Research into the bioeffects of ultrasound has produced a large and varied data base of published studies over approximately 80 years. In general, this research has been directed toward producing bioeffects for study or for possible therapeutic applications, and therefore published studies may or may not have any relevance for ultrasound in obstetrics. The quality and credibility of reported results also has varied, with some of the most worrisome findings (which unfortunately tend to appear in news reports) gradually shown to be erroneous by extensive follow up work. Thus, the overall bioeffects data base must be sifted to glean useful insights for safety assurance. Useful information on bioeffects has come from in vitro, in vivo, theoretical, epidemiological and therapy research. An early assessment by the AIUM resulted in the landmark “Statement on Mammalian in Vivo Ultrasonic Biological Effects” of August 1976: “In the low megahertz frequency range there have been no demonstrated significant biological effects in mammalian tissues exposed to intensities below 100 mW/cm2. Furthermore, for ultrasonic exposure times less that 500 s and greater than 1 s, such effects have not been demonstrated even at higher intensities, when the product of intensity and exposure time is less than 50 joules/cm2.”[25] In this statement, the intensity was the SPTA value measured in water, the expose time included on and off times for pulsed exposure, and the energy unit joule is equal to Watts times seconds (W s). This statement was based primarily on exposures similar to unfocused physical therapy treatment and on focused exposure in ultrasound research for surgical therapy (i. e. not on results with clear relevance to diagnostic ultrasound exposure).
The 100 mW/cm2 statement value was well below the guideline upper limits (pre-enactment) values established in the 510(k) guidance of 1985. Even for obstetrics, the near coincidence between the 100 mW/cm2 in the AIUM statement and the 94 mW/cm2 upper bound found by the FDA was purely accidental, and not as fortuitous as it may seem. The FDA number was a derated value, and the AIUM number was a water value. A machine with the given derated value could have a much higher water value, thus exceeding the bioeffect statement level. Of course, diagnostic ultrasound exposures are generally quite brief at any given spot in tissue, so the 50 joules/cm2 number (allowing for 50 W/cm2 exposures of 1 s) presumably provided assurance of safety. Nevertheless, this safety assurance predicament for diagnostic ultrasound was generally felt to be unsatisfactory for such a rapidly growing and valuable medical tool. Medical imaging was rapidly becoming a routine, nearly universal source of human ultrasound exposure. An initial re-evaluation of the bioeffects literature in terms of the mechanisms of action of ultrasound lead to modification of the AIUM statement. A safe value of 1 W/cm2 was included for focused ultrasound (in addition to the 100 mW/cm2 for unfocused ultrasound), because focused ultrasound used for diagnostic imaging causes much lower temperature elevations over time, for a given SPTA intensity, than unfocused ultrasound.[6]
The re-evaluation process was continued to address several problems. First, there was a need to add a scientific footing to support the safety assurance framework, and to verify or replace the arbitrary 1976 pre-enactment guidelines. Second, there was a desire to liberalize the upper limit categories in some way to avoid inhibiting the development of diagnostic ultrasound and restricting the patient benefit which should follow. The rigid upper limit concept for safety assurance was questioned, and a new scheme was sought which would eliminate the hidden upper limits by providing on-screen exposure indicators, and providing bioeffects knowledge to the operators of diagnostic ultrasound machines.
The scientific approach to establish the new safety assurance scheme was to consider the likely physical mechanisms, which are responsible for biological effects of ultrasound. This would augment and support the phenomenological approach of trying to judge safe diagnostic exposures from published reports of often unrelated bioeffects research. The two primary mechanisms of ultrasonic bioeffects are heating, as the energy is absorbed, and ultrasonic cavitation, which occurs when a gaseous cavitation nuclei is driven above its cavitation threshold. Heating depends on the temporal average intensity. Cavitation can be very rapid and depends on temporal peak parameters. Thus, indices for the two mechanisms were sought, which related to the established temporal average and temporal peak exposure measurements. The result was the landmark Output Display Standard published by AIUM and NEMA in 1992, which specified the display of thermal indices and a “mechanical” index (based on considerations of cavitation) on the screens of diagnostic ultrasound machines.[21]
Thermal bioeffects depend on the temperature elevation and its duration. The temperature elevation produced by ultrasound depends on the SPTA intensity, the ultrasound frequency, the dwell time along the beam axis, the width of the beam, the tissue properties, the individual patient and other minor factors. In general, the temperature in a fixed beam will rise and level off asymptotically when the heat deposition is matched by the heat dissipation (through conduction or blood perfusion). Narrow focused beams will level off at lower temperatures for a given intensity, than unfocused beams, due to rapid conduction of heat from the small region. Scanned beams heat less than stationary beams due to the reduced SPTA intensity. Bone has a relatively high absorption, and heats quickly, while water as very low absorption and heats little. The problem of completely calculating the temperature profile for a diagnostic examination is quite difficult (if not impossible, due to unknowns for specific patients). Estimates of the reasonable worst case temperature elevation can be obtained by modeling possible exposures using a relatively low attenuation (e. g. the 0.3 dB/cm/MHz derating factor), durations sufficient to approach the asymptotic temperature elevation and the different exposure situations with respect to the presence of bone. The ODS includes three thermal indices (TI): one for regular soft tissues (TIS), one for bone at the focus (TIB), and one for bone at the surface(TIC) such as the skull. These indices are displayed on screen for most modern ultrasound imaging machines and provide an estimate of an asymptotic temperature elevation (not an actual real-time temperature estimate, hence the term index). For obstetrical ultrasound, the pertinent indices are the TIS for the first trimester, and the TIB for the 2nd and 3rd trimesters when bone development occurs. For the settings used for the measurements shown in Fig. 2, the on-screen TIS value was 0.5. That is, the temperature elevation produced in tissue would not be expected to exceed 0.5 °C for any examination duration.
Another index was developed to account for mechanical (i. e. nonthermal) mechanisms for which ultrasonic cavitation was considered to be of primary concern. Ultrasonic cavitation can cause mechanical damage and can even generate free radicals and sonochemicals capable of DNA damage. For example, single strand breaks from hydrogen peroxide production and genetic mutation have been demonstrated in in vitro studies of cultured cells subjected to ultrasonic cavitation. [26, 27] The occurrence of cavitation for a given cavitation nucleus depends on the PRPA, which is a pulse-peak parameter thereby related to the SPPA intensity of the 510(k) guidelines (which is not displayed on screen), and the ultrasonic frequency. For optimum nucleation, cavitation activity decreases and cavitation thresholds increase with increasing ultrasonic frequency.[28] The Mechanical Index (MI) provides an estimate of the PRPA in an ultrasound beam in tissue, as measured in water but derated by a 0.3 dB/cm/MHz tissue attenuation factor, divided by the square-root of the ultrasonic frequency. For the settings used for the measurements shown in Fig. 2, the on-screen MI value was 0.7.
The guideline upper limits on diagnostic ultrasound were not removed after continued reevaluation of the safety problem. Some participants and other interested parties believed that the upper limits were needed to maintain the established and accepted safety record of diagnostic ultrasound.[29] Removal of upper limits would ensure uncertainty, rather than safety. The action taken by the FDA in 1992 was to allow for a new set of guidelines for new machines, which displayed the exposure indices, in addition to the older limits, known in the 510(k) process as Track 3 and Track 1, respectively.[30] The present upper limits are given in Table 1, which compares the original pre-enactment values to the newer numbers. The new limits were essentially made uniform across categories, except for opthalmological examinations, for which consideration of possible heating indicated exceptional risk to the eye lens.[31] The obstetrical ultrasound limit for the SPTA intensity was elevated to the highest 720 mW/cm2 pre-enactment value. The MI was limited to 1.9, which was found for a 2.25 MHz pre-enactment transducer.[23] Thus, the net effect of the 1992 change in guideline limits was to increase the SPTA intensity allowed for obstetrical ultrasound from 94 mW/cm2 to 720 mW/cm2 (the values of the TI are not limited specifically). The allowed pulse parameter values also increased. For example, an MI of 1.9 at 9 MHz allows for 5.7 MPa PRPA, which could correspond to SPPA intensities exceeding 1,000 W/cm2 in tissue, compared to the 190 W/cm2 track 1 limit. However, the actual increases have not been as dramatic as these numbers indicate, and measurements over several decades, while showing an upward trend in outputs,[32] indicate that modern ultrasound machines do not approach the highest PRPA values allowed by MI = 1.9.
Safety Assurance
Safety assurance in the ODS scheme is provided by comparing the appropriate on screen TI and MI to bioeffects thresholds relevant to the diagnostic examination at hand. Data on adverse effects of heating on the fetus, which include birth defects and other worrisome effects, have come from a variety of studies. The information can be summarized by a threshold line plotted as temperature elevation against its duration in Fig. 3.[33] This plot indicates temperature increases less than 6°C for 1 min would not induce adverse bioeffects on the fetus. For long times, increases of less than 1–1.5°C would be inconsequential for any conceivable duration of an ultrasound examination, because these are within the normal variation of body temperature. Nor does there appear to be a cumulative effect for slight tissue heating. Elevation to temperatures less than 1–1.5°C above normal for any conceivable number of repetitions of diagnostic ultrasound examinations would not be expected to increase risk. In obstetrical examinations, appropriate TI values less than this guideline (Fig. 3) imply that the examination would not pose a risk to the fetus based on the thermal mechanism. Most obstetrical examinations do not approach these TI values, except possibly for spectral Doppler mode (see below). A complex flow chart is provided by an NCRP report 14 to help decide when to withhold ultrasound based on the TI values.[14]
3.
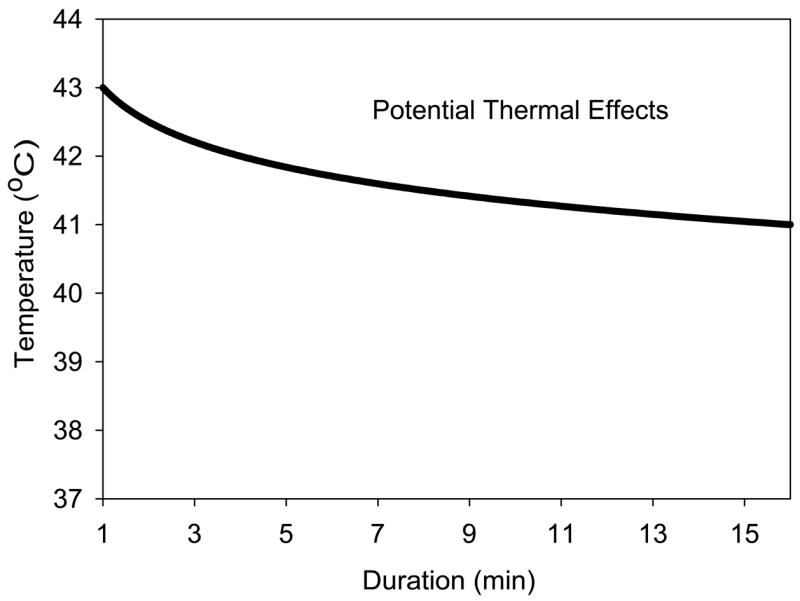
A plot of the boundary above which bioeffects, including teratological effects, have been detected in animal models of thermal exposure. The data indicate concern for a 6°C temperature elevation for 1 min declining to 4°C for 16 min, which might be within the range of somespectral Doppler exposures based on the Thermal Index display. Extending the boundary for small elevations above normal body temperature results in very long times (e. g., 4 h 16 min for 39°C) relative to any normal ultrasound examination durations at a fixed scan plane.
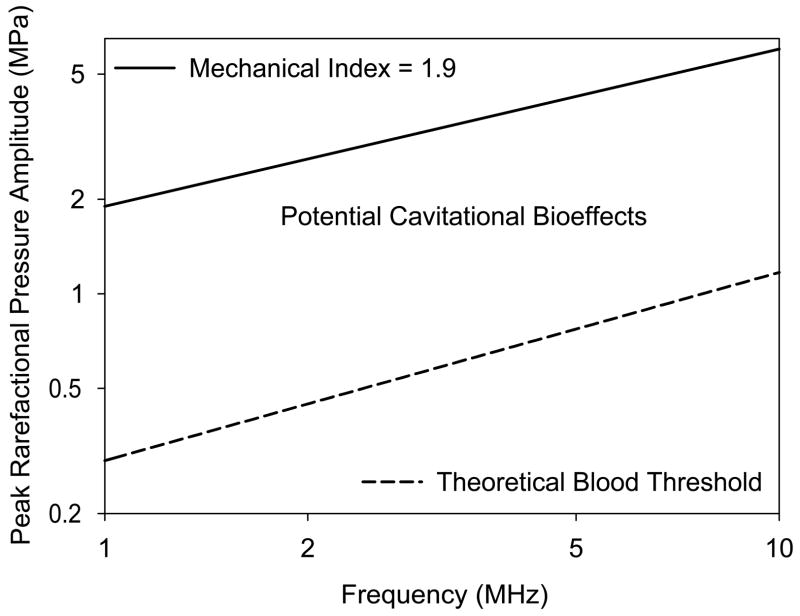
The use of the MI for safety assurance is less complex, but more confusing. The theoretical PRPA thresholds calculated for optimum cavitation nuclei in blood are plotted against frequency in Fig. 4.[28] For comparison the line representing MI=1.9 is also plotted, which lies well above the threshold line. However, this apparent indication of risk for the region between the lines appears to be insubstantial. There is no evidence that inertial cavitation occurs during diagnostic ultrasound exposure in the fetus (excluding the possible use of ultrasound contrast agents). This fortunate circumstance is due to the apparent lack of cavitation nuclei in mammalian tissues, which would be optimum or near optimum for cavitation nucleation by diagnostic ultrasound. The MI would be a relevant exposure index for adult examinations involving possible exposure of gas bodies within tissue, such as in intestine and lung or from injected ultrasound contrast agents. [8, 12, 15] However, no specific guidance can be given for the MI in obstetrical ultrasound, and any value (up to the upper limit) would be expected to be safe.
4.
A plot of the region bounded by the FDA upper limit of MI=1.9 (solid line), and a theoretical estimate of the cavitation threshold in blood for optimal nucleation (dashed curve). Within this region, cavitational bioeffects are presumably possible when suitable nuclei are present. Research results suggest that optimal nuclei are not normally present in mammalian blood, except for contrast enhanced diagnostic ultrasound with stabilized microbubbles. Therefore, the apparent zone of risk between the two curves, which otherwise might be a source of concern, is considered to be immaterial for obstetrical ultrasound.
For the measurements shown in Fig. 2, the TIS was 0.5 and the MI was 0.7. With these on-screen values, there is no expectation of bioeffects risk from the thermal or cavitational mechanisms. This conclusion exemplifies safety assurance in obstetrical ultrasound.
Shadows of Doubt
The present use of diagnostic ultrasound in obstetrics is expected to be essentially free of risk of ultrasound-induced bioeffects. However, it is not possible to prove this expectation (logically, absence of evidence of harm is not evidence that no harm is possible). Scientifically, only the opposite can be shown; that is, continuing research could elucidate a significant harmful effect. In fact, there several observations which cast some doubt on the present safety assurance framework. While these issues should not intrude on clinical judgement (many are circumstantial or purely speculative), some are worthy of mention to help frame the appropriate safety assurance philosophy for obstetrical ultrasound.
Due to the exemption received by diagnostic ultrasound through the 510(k) process, there has been no extensive and planned investigation by independent laboratories into potential bioeffects risks of diagnostic ultrasound. Safety assurance rests on (1) an assumption of safety for pre-1976 ultrasound devices, (2) theoretical consideration of important bioeffects mechanisms and (3) interpretation of published research studies which may or may not have any relation to obstetrical ultrasound.
There are scattered, difficult to interpret, reports of possibly relevant bioeffects. For example, one small study in monkeys using older equipment found statistically significant effects on some fetal parameters.[34] There have been no well planned and thorough studies of possible fetal effects in pregnant animals using modern ultrasound machines (e. g. post-ODS devices). Some human epidemiological studies involving older equipment have found odd effects, which are difficult to interpret, such as increased incidence of left handedness.[35] There have been no high-quality human epidemiological studies involving modern ultrasound machines (e. g. post-ODS devices). This lack of a solid research foundation leaves open opportunities for speculation about potential safety problems, which may or may not have any validity. Safety assurance rests most heavily on the consideration of bioffects mechanisms.
Thermal bioeffects are reasonably well known. However, the data used, for example, to derive the line in Fig. 3 did not involve heating of the fetus by ultrasound. Rather, most studies were conducted by placing a pregnant animal in a heated environment sufficient to overwhelm its thermoregulation (commonly called heat stroke) and had durations of hours. It is not known whether fetal heating by external focused, scanned ultrasound, without maternal heating, would produce less or more effects. Some detailed analyses of available data suggest that presently available ultrasonic energy delivered by modern ultrasound imagers may be very near the levels capable, under worst case conditions, of harming the fetus by the thermal mechanism.[36]
Cavitational bioeffects would depend on the presence of suitable cavitation nuclei, as well as suitable exposure. There are few nuclei available in mammalian tissue, but it is not clear how few. In the specific environment of the fetus, there could be a low probability of occurrence of cavitation, but this possibility or the possible consequences if cavitation did occur have not been studied in the mammalian fetus using modern diagnostic ultrasound machines.
In addition, it is not clear that other non-thermal mechanisms can be ignored relative to the cavitation problem, particularly since the cavitation mechanism is not expected to be important in obstetrics (without added cavitation nuclei). For example, pulsed ultrasound can generate audible sound and stimulate the fetus.[37] Beams of ultrasound can cause the mechanical distortion of an elastic medium, or fluid flow in liquids. The possible impact on the fetus of such physical mechanisms are largely unknown.[38] There are instances of observed bioeffects which may be relevant. Low intensity pulsed ultrasound has been shown to aid in bone healing for therapy, a clearly established but possibly not adverse bioeffect.[39] An unconfirmed study with diagnostic ultrasound exposure of mouse fetuses suggests a possible influence on migration of neuronal cells.[40] Such reports fuel speculation that the growth in routine use of diagnostic ultrasound in obstetrics may explain increases in some adverse fetal outcomes. For example, ultrasound has been suggest as a possible explanation for the apparently increasing incidence of autism in children,[41] even though there is no direct experimental or theoretical connection between diagnostic ultrasound and the incidence of this disease.[42]
Safety Assurance Viewpoints for the Sonographer
Diagnostic ultrasound in obstetrics has an expectation of safety. However, the safety assurance framework has evolved in a haphazard way, leaving room for some doubt about the absolute safety of ultrasound in obstetrics. The detailed consideration of basic science issues should not intrude into the clinical examination room, but the operating assumptions and viewpoint of safety are important for sonographers. Two different concepts have been suggested as appropriate for diagnostic ultrasound.
The radiation protection concept of “As Low As Reasonably Achievable” (ALARA) has been suggested for application to the problem of assuring the safety of diagnostic ultrasound in implementing the 510(k) guide,[30] and in a safety publication provided with diagnostic ultrasound machines.[43] However, this safety principle may not be congruous with diagnostic ultrasound. ALARA was developed for protection of radiation workers and the public for the nuclear industry and assumes the stochastic risk of harm appropriate for ionizing radiation.[44] For ultrasound, there is no defined dose which one could systematically lower. Furthermore, if the clinical exposures are below thresholds for significant bioeffects, then effort spent for reduction in exposure would be pointless and would distract from the primary goal of obtaining the best diagnostic result. Mandatory and diligent clinical application of ALARA to diagnostic ultrasound actually might create a risk of harm from missed or incorrect diagnostic information.
A more reasonable safety viewpoint for obstetrical ultrasound is one of the prudent use of this valuable medical tool. Prudent use implies that medially indicated diagnostic ultrasound examination in obstetrics should not be withheld or modified from the optimum imaging protocols based on safety considerations. However, the prudent use principle allows for the questioning of the wisdom of some uses of diagnostic ultrasound scanning of the fetus on the basis of the remnant shadow of doubt noted above and other issues. For example, the scanning of the fetus purely for the purpose of keepsake pictures or videos is clearly questionable, particularly if no medically useful evaluation is contemplated for the images. Medically indicated scans often are available for the purpose of “bonding” between mother and fetus. Repeated scans to get a good artistic portrait is of no medical value and should be considered an imprudent use. Likewise, scanning of the fetus purely for sales demonstrations purposes without any medical indication or expectation of medical benefit, also seems imprudent. Even if the safety issue is considered mute, the stress associated with repeated scanning on the product show floor of annual conventions seems to be inappropriate medical treatment of an expectant mother. However, scanning for medical educational benefit is acceptable, particularly for physician or sonographer training in a medical setting with defined goals (such as CME credit). Through the judgement and skill of professional sonographers, the prudent use principle provides further overall assurance of safety in obstetrical ultrasound.
Acknowledgments
Supported in part by National Institutes of Health grant EB000338.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fry WJ. Recent developments in ultrasound at the biophysical research laboratory and their application to basic problems in biology and medicine. In: Kelly E, editor. Ultrasonic Energy. Urbana, Il: University of Illinois Press; 1965. pp. 200–228. [Google Scholar]
- 2.Reid JM, Joyner CR. The use of ultrasound to record the motion of heart structure. In: Kelly E, editor. Ultrasonic Energy. Urbana, Il: University of Illinois Press; 1965. pp. 200–228. [Google Scholar]
- 3.Kossoff G, Garrett WJ, Robinson DE. An ultrasonic echoscope for visualizing the pregnant uterus. In: Kelly E, editor. Ultrasonic Energy. Urbana, Il: University of Illinois Press; 1965. pp. 200–228. [Google Scholar]
- 4.Reid JM, Sikov MR. Interaction of Ultrasound and BIlogical Tissues. Rockville, MD: USDHEW; 1972. [Google Scholar]
- 5.NIH. Diagnostic Ultrasound Imaging in Pregnancy, NIH Publication No. 84–667. Bethesda, MD: National Institutes of Health; 1984. [Google Scholar]
- 6.AIUM. Bioeffects considerations for the safety of diagnostic ultrasound. J Ultrasound Med. 1988;7(Suppl):S1–S38. [PubMed] [Google Scholar]
- 7.AIUM. Bioeffects and safety of diagnostic ultrasound. Laurel, MD: American Institute of Ultrasound in Medicne; 1993. [Google Scholar]
- 8.AIUM. Mechanical bioeffects from diagnostic ultrasound: AIUM consusus statements. J Ultrasound Med. 2000;19:67–170. doi: 10.7863/jum.2000.19.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WFUMB. First symposium on safety and standardization of ultrasound in obstetrics. Ultras Med Biol. 1986;12:673–723. [PubMed] [Google Scholar]
- 10.WFUMB. Second World Federation of Ultrasound in Medicine and Biology symposium on safety and standardization in medical ultrasound. Ultras Med Biol. 1989;15:1–111. [PubMed] [Google Scholar]
- 11.WFUMB. WFUMB symposium on safety and standardization in medical ultrasound. Ultras Med Biol. 1992;18:731–811. [PubMed] [Google Scholar]
- 12.WFUMB. WFUMB symposium on safety of ultrasound in medicine. Ultras Med Biol. 1998;24(Suppl 1):S1–S58. [Google Scholar]
- 13.NCRP. Biological Effects of Ultrasound: Mechanisms and Clinical Implications (Report No. 74) Bethesda, MD: National Council on Radiation Protection and Measurements; 1983. [Google Scholar]
- 14.NCRP. Exposure Criteria for Medical Diagnostic Ultrasound: I. Criteria Based on Thermal Mechanisms. (Report No. 113) Bethesda, MD: National Council on Radiation Protection and Measurements; 1992. [Google Scholar]
- 15.NCRP. Exposure Criteria for Medical Diagnostic Ultrasound: II. Criteria Based on All Known Mechanisms. (Report No. 140) Bethesda, MD: National Council on Radiation Protection and Measurements; 2002. [Google Scholar]
- 16.Barnett SB, Ter Haar GR, Ziskin MC, Rott HD, Duck FA, Maeda K, et al. International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound Med Biol. 2000;26:355–66. doi: 10.1016/s0301-5629(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 17.Nyborg WL. Safety of medical diagnostic ultrasound. Semin Ultrasound CT MR. 2002;23:377–386. doi: 10.1016/s0887-2171(02)90008-9. [DOI] [PubMed] [Google Scholar]
- 18.McKinlay Effects of ultrasound and infrasound relevant to human health. Prog Biophys Mol Biol. 2007;93:1–420. [Google Scholar]
- 19.AIUM. American Institute of Ultrasound in Medicine 2005 Bioeffects Consensus Conference. J Ultras Med. in press. [Google Scholar]
- 20.AIUM/NEMA. Safety standard for diagnostic ultrasound equipment Ul 1–1981. J Ultras Med. 1983;2(suppl):S1–S50. [PubMed] [Google Scholar]
- 21.AIUM/NEMA. Standard for Real-Time Display of Thermal and Mechanical Acoustic Output Indices on Diagnostic Ultrasound Equipment. Rockville, MD: American Institute of Ultrasound in Medicine and the National Electrical Manufactures Association; 1992. [Google Scholar]
- 22.USFDA. 510(k) Guide for Measuring and Reporting Acoustic Output of Diagnostic Ultrasound Medical Devices. Rockville, MD: Center for Devices and Radiological Health; 1985. [Google Scholar]
- 23.Nyborg WL. Bological effects of ultrasound: development of safety guidelines. Part I: personal histories. Ultrasound Med Biol. 2000;26:911–64. doi: 10.1016/s0301-5629(00)00243-x. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press; 2005. [PubMed] [Google Scholar]
- 25.AIUM. Communication from the Bioeffects Committee August 1976. J Clin Ultras. 1977;5:2–4. [Google Scholar]
- 26.Miller DL, Thomas RM, Buschbom RL. Comet assay reveals DNA strand breaks induced by ultrasonic cavitation in vitro. Ultrasound Med Biol. 1995;21:841–848. doi: 10.1016/0301-5629(95)00017-l. [DOI] [PubMed] [Google Scholar]
- 27.Doida Y, Brayman AA, Miller MW. Modest enhancement of ultrasound-induced mutations in V79 cells in vitro. Ultrasound Med Biol. 1992;18:465–469. doi: 10.1016/0301-5629(92)90086-p. [DOI] [PubMed] [Google Scholar]
- 28.Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol. 1991;17:179–185. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien WD, Jr, Abbott JG, Stratmeyer ME, et al. Acoustic output upper limits proposition: should upper limits be retained? J Ultrasound Med. 2002;21:1335–41. doi: 10.7863/jum.2002.21.12.1335. [DOI] [PubMed] [Google Scholar]
- 30.USFDA. Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. Rockville, MD: Center for Devices and Radiological Health; 1997. [Google Scholar]
- 31.Herman BA, Harris GR. Theoretical study of steady-state temperature rise within the eye due to ultrasound insonation. IEEE Trans UFFC. 1999;46:1566–1574. doi: 10.1109/58.808882. [DOI] [PubMed] [Google Scholar]
- 32.Whittingham TA. WFUMB Safety Symposium on Echo-Contrast Agents: exposure from diagnostic ultrasound equipment relating to cavitation risk. Ultrasound Med Biol. 2007;33:214–223. doi: 10.1016/j.ultrasmedbio.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Miller MW, Nyborg WL, Dewey WC, et al. Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: implications of new standards on tissue heating. Int J Hyperthermia. 2002;18:361–384. doi: 10.1080/02656730210146890. [DOI] [PubMed] [Google Scholar]
- 34.Tarantal AF, Hendrickx AG. Evaluation of the bioeffects of prenatal ultrasound exposure in the cynomolgus macaque (Macaca fascicularis): I. Neonatal/infant observations. Teratology. 1989;39:137–147. doi: 10.1002/tera.1420390206. [DOI] [PubMed] [Google Scholar]
- 35.Salvesen KA. Epidemiological prenatal ultrasound studies. Prog Biophys Mol Biol. 2007;93:295–300. doi: 10.1016/j.pbiomolbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Church CC, Miller MW. Quantification of risk from fetal exposure to diagnostic ultrasound. Prog Biophys Mol Biol. 2007;93:331–353. doi: 10.1016/j.pbiomolbio.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Fatemi M, Ogburn PL, Jr, Greenleaf JF. Fetal stimulation by pulsed diagnostic ultrasound. J Ultrasound Med. 2001;20:883–889. doi: 10.7863/jum.2001.20.8.883. [DOI] [PubMed] [Google Scholar]
- 38.Barnett SB. Routine ultrasound scanning in first trimester: what are the risks? Semin Ultrasound CT MR. 2002;23:387–391. doi: 10.1016/s0887-2171(02)90009-0. [DOI] [PubMed] [Google Scholar]
- 39.Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol. 2007;93:384–398. doi: 10.1016/j.pbiomolbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 40.Ang ES, Jr, Gluncic V, Duque A, et al. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc Natl Acad Sci U S A. 2006;103:12903–12910. doi: 10.1073/pnas.0605294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers C. Questions about prenatal ultrasound and the alarming increase in autism. Midwifery Today Int Midwife. 2006;80:16–19. 66–67. [PubMed] [Google Scholar]
- 42.Abramowicz JS. Prenatal exposure to ultrasound waves: is there a risk? Ultrasound Obstet Gynecol. 2007;29:363–367. doi: 10.1002/uog.3983. [DOI] [PubMed] [Google Scholar]
- 43.AIUM. Medical Ultrasound Safety. Laurel, MD: American Institute of Ultrasound in Medicine; 1994. [Google Scholar]
- 44.Walker JD. Permissible Dose: A History of Radiation Protection in the Twentieth Century. Berkeley, CA: University of California Press; 2000. [PubMed] [Google Scholar]