Abstract
In comparison with the NMR data of salvinorin A and its 8-epimer, the published structure of deacetyl-1,10-didehydrosalvinorin G was revised to its 8-epimer. The stereochemistry of 8-epi-deacetyl-1,10-didehydrosalvinorin G was further confirmed by NOESY and chemical synthesis.
Keywords: Salvinorin A, Epimer, NMR, Revised structure
Salvinorin A (1a), a non-nitrogenous neoclerodane diterpenoid, was isolated from the Mexican medicinal plant Salvia divinorum.1,2 1a was identified as a potent and selective kappa (κ) opioid receptor (KOR) agonist and the key ingredient for psychoactive effects.3–5 During the course of structure-activity relationship (SAR) studies,6–18 it was found that 1a and its derivatives readily underwent epimerization at C-8 under basic conditions. Using various inorganic bases, such as NaBH4,2,12,19 NaHCO3,20 Na2CO3,7,12 K2CO3,8 LiSEt10 and LiI,11 1a produced corresponding natural (8-Hβ) and unnatural (8-Hα, also called 8-epi-) mixtures. Surprisingly, treatment of 1a with excess strong base KOH or Ba(OH)2 gave a natural salvinorin derivative deacetyl-1,10-didehydrosalvinorin G that was claimed by different research groups to have structures 2a and 3.13,8 In general, the affinity and potency of natural salvinorin derivatives show much higher KOR binding activities than those of unnatural 8-epimers.8,12,15,21 Since the configuration of a molecule can significantly affect KOR binding, it is essential to establish a reliable method which would unambiguously determine the stereochemistry at C-8 of salvinorins.
This prompted us to conduct a comprehensive analysis of the 1H and 13C NMR data of natural and unnatural salvinorin derivatives. The differences of 1a and its 8-epimer (1b) in their 1H and 13C NMR spectra are discussed in this paper. Based on the summarized NMR data and chemical synthesis, the stereochemistry of 2a at C-8 is revised to its 8-epimer (2b) as shown in Figure 1.
Figure 1.
Conformational structures of 2a and 2b
In connection with our previous study related to determining the stereochemistry of betulinic acids,22 we recognized that the 1H and 13C NMR data of salvinorins and their corresponding 8-epimers have significant differences. A pair of 8-epimers (1a and 1b), isolated9 and synthesized8 in our group, were selected as standard compounds for NMR analyses. Using 2D NMR techniques, including COSY, NOESY, HMQC and HMBC, permitted the full assignments of all 1H and 13C NMR chemical shifts. The relative stereochemistry at the C-8 position of these compounds was unambiguously determined, based on their 1H and 13C NMR data. For instance, in the 1H NMR spectra, the C-8-H of 1a resonated at δ 2.07 (dd, J = 3.0 and 12.0 Hz), confirming axial orientation, while that of 1b resonated at δ 2.45 (d, J = 2.7 Hz) for equatorial orientation. The chemical shift change (Δδ) between 1a and 1b is about 0.38 ppm (Table 1). In addition, the C-12-H of 1b shifted upfield (Δδ 0.27 ppm) to δ 5.26 (dd, J = 1.8 and 12.0 Hz) in comparison with that of 1a at δ 5.53 (dd, J = 4.8 and 11.7 Hz). The coupling constants of H-12 revealed the axial orientation of H-12 in both 1a and 1b. The X-ray analyses showed that the lactone ring in 1a adopts a chair confirmation,1,2,17 while the one in 1b is a boat conformation.21 The different conformation may explain the J values differences of H-12 between 1a and 1b. On the other hand, the H-8 configuration also strongly affects the 13C NMR data in B- and C-ring carbons. For instance, the methylene carbon (C-11), carbonyl carbon (C-17) and axial methyl carbon (C-20) of 1b showed lower-field chemical shifts in comparison with those of 1a (Table 2), while the 13C resonances of C-6, C-8, C-12, C-13 and C-19 of 1b shifted to upper field. In summary, the characteristic carbon peaks of C-12, C-17 and C-20 can be employed readily for identification of natural and unnatural 8-epimers. In the previous published papers,10,13,21 8-epimers were mainly determined based on the coupling constants of H-8 and H-12, irradiation of H-12 for a NOE enhancement of H-8, and a general TLC Rf value. Our generalized NMR data should provide reliable information for identification of future salvinorin analogs including C-8 epimers.
Table 1.
1H NMR Data (300 MHz) at H-8 and -12 for 1a, 1b and 2b in CDCl3
Table 2.
13C NMR Data (75 MHz) for 1a, 1b and 2b in CDCl3
| Carbon # | 1a | 1b | Δδ1b-1aa | 2b | Carbon # | 1a | 1b | Δδ1b-1aa | 2b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 202.0 | 202.3 | 145.1 | 13 | 125.2 | 123.4 | − 1.8 | 124.4 | |
| 2 | 75.0 | 75.2 | 180.7 | 14 | 108.4 | 108.5 | 108.4 | ||
| 3 | 30.7 | 30.7 | 128.2 | 15 | 143.7 | 143.5 | 143.7 | ||
| 4 | 53.6 | 52.9 | 157.5 | 16 | 139.4 | 139.7 | 139.6 | ||
| 5 | 42.1 | 42.2 | 42.3 | 17 | 171.1 | 173.4 | + 2.3 | 173.2 | |
| 6 | 38.1 | 34.0 | − 4.1 | 28.3 | 18 | 171.5 | 171.8 | 165.4 | |
| 7 | 18.1 | 17.6 | 21.9 | 19 | 16.4 | 15.2 | − 1.2 | 30.3 | |
| 8 | 51.4 | 45.2 | − 6.2 | 44.8 | 20 | 15.2 | 24.6 | + 9.4 | 24.4 |
| 9 | 35.4 | 34.7 | 37.6 | -COOMe | 52.0 | 51.7 | 52.6 | ||
| 10 | 64.1 | 64.0 | 140.0 | -COCH3 | 170.0 | 169.7 | - | ||
| 11 | 43.4 | 48.0 | + 4.6 | 36.8 | -COCH3 | 20.6 | 20.5 | - | |
| 12 | 72.0 | 70.0 | − 2.0 | 70.8 |
Data is given when Δδ1b-1a is more than 1.0 ppm.
We reported previously that treatment of 1a with Ba(OH)2 in MeOH gave an unexpected oxidative-elimination product that was assigned as structure 3.8 In a very short time span, the structure of 3 was revised to deacetyl-1,10-didehydrosalvinorin G (2a), which was synthesized in 1 M KOH methanol solution.13 The structural revision was based on extensive NMR experiments including 1H NMR, 13C NMR, DEPT, COSY, HMQC, HMBC and NOE, and HR-ESI-MS. Following the published procedure (Scheme 1),13 indeed, we isolated the same product.24 The NMR data were in full agreement with previous report13 except that the assignments of H-7α (δ1.98) and H-7β (δ2.24) should be reversed as H-7α (δ 2.24) and H-7β (δ1.98). After careful comparison of our 1H and 13C NMR data with those of 1a and 1b (Tables 1 and 2), we found that structure 2a assigned to the product was incorrect,13 and the correct structure should be 8-epi-deacetyl-1,10-didehydrosalvinorin G (2b, Figure 1). In the 1H NMR spectrum, the C-8-H of 2b shifted much lower field to δ 2.99 (dd, J = 5.1 and 9.6 Hz), while the C-12-H shifted upfield slightly compared with that of 1a (Table 1). The H-12 coupling constants (dd, J = 2.4 and 12.3 Hz) of 2b were closer to those of 1b (dd, J = 1.8 and 12.0 Hz) than of 1a (dd, J = 4.8 and 11.7 Hz), but the H-8 of 2b showed the J values (5.1 and 9.6 Hz) for axial orientation.13 However, this perplexing configuration at C-8-H was soon resolved by comparing the C-ring 13C NMR data of 2b with those of 1a and 1b (Table 2). The 13C NMR chemical shifts at C-8, C-12, C-17 and C-20 are very similar to those of 1b, indicating the orientation of H-8 is α. The revised structure 2b was further confirmed by the NOE interactions shown in Figure 2. In the NOESY spectrum (see Supplementary Data), H-8 (δ2.99) showed cross peaks to H-7α (δ2.24, strong), H-7β (δ1.98, weak), H-19 (δ1.72) and H-20 (δ1.67), while H-12 (δ5.45) related to H-11α (δ3.11, strong), H-11β (δ2.02, weak) and H-20 (δ1.67). It should be noted that the crossed peak between H-8 and H-12 was very weak. Furthermore, when 1b, the 8-epimer of 1a, was treated with 1 M KOH in MeOH, it afforded the endione 2b in a 67% isolated yield (Scheme 1).24 Valdes et al.2 reduced 1a with NaBH4 in i-PrOH and afforded equal amounts of 8-epimeric mixtures (diol 4a and 8-epi-diol 4b), while Munro et al.13 reduced 2a with NaBH4 in EtOH/CH2Cl2 and obtained sole product - 8-epi-diol 4b. All of this evidence supports the structure 2b.
Scheme 1.
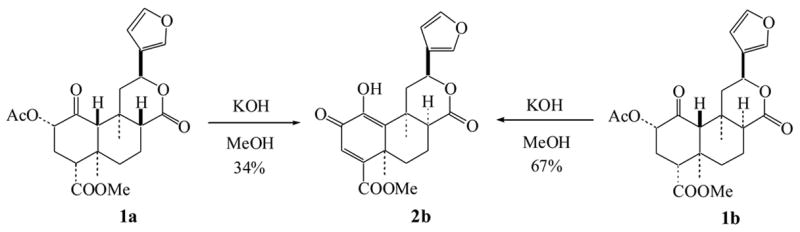
Figure 2.
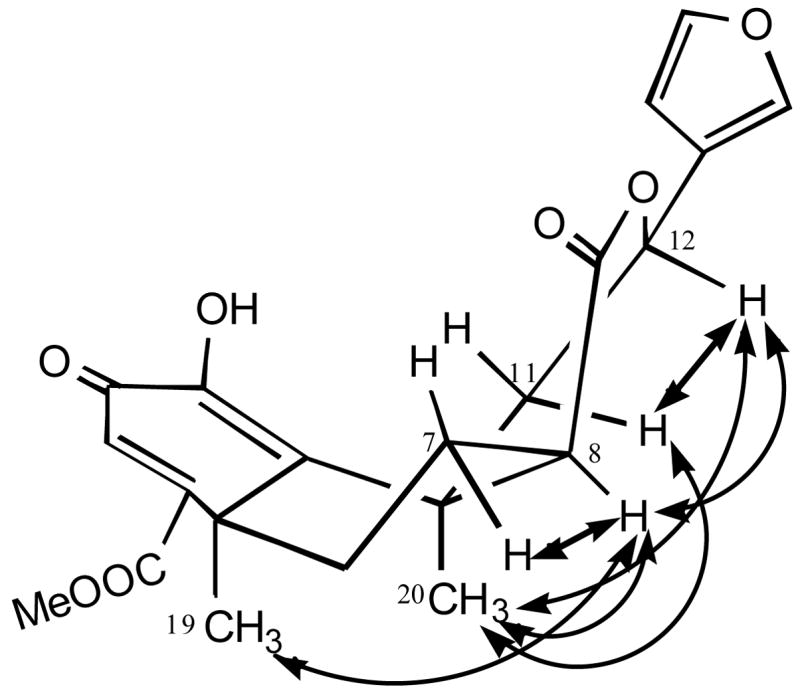
Key NOE interactions of 2b
The coupling constants of H-8 in 2b were misleading in comparison with those of other 8-epi-salvinorins. This is the main reason that Munro et al.13 determined H-8 as β configuration. Because of the double bond between C-1 and C-10, the conformation of 2b is distorted. It is known that A/B/C rings in 1a are in chair/chair/chair conformation1,2 and A/B/C rings in 1b are in chair/chair/boat conformation.21 Based on NOESY and molecular modeling analyses, A/B/C rings in 2b should be face-down boat /twist-chair/twist-chair conformation (Figure 1). Under this circumstance, both H-7β and H-8 resemble axial orientations and showed a closer diaxial coupling constant (9.6 Hz). Without sufficient NOE data,13 the incorrect assignments of H-7α and H-7β in 2b might depend on those of the known salvinorins C (5a), D (5b), E (5c) and F (5d), isolated from S. divinorum by Munro and Rizzacasa.25 Because the chemical shifts of H-7 and H-8 in 5a, 5b, 5c and 5d overlapped,19, 25 the unambiguous assignments of H-7α and H-7β with NOESY spectra became very difficult. Unexpectedly, one year latter, Munro gave the revised assignments of H-7α and H-7β of 2b without any scientific explanation.20 Based on our NOE data, all protons in 2b were fully assigned.24
In conclusion, a concise and informative NMR method for the determination of the C-8-H configuration of salvinorins was established. Based on this method, the correct product obtained by the treatment of 1a with hydroxide in MeOH has been identified. The C-8 epimeric structure (2b) was confirmed by NOESY spectrum and chemical conversion.
Supplementary Material
Supplementary data
Copies of 1H and 13C NMR spectra of 1a, 1b and 2b, and 2D NOESY spectrum of 2b.
Acknowledgments
The authors wish to thank Ms. Jane F. McNeil for EI-MS determination. This work was supported by research grant (R01-DA023025) from NIDA to D.Y.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ortega A, Blount JF, Manchand PS. J Chem Soc, Perkin Trans. 1982;1:2505–2508. [Google Scholar]
- 2.Valdes LJ, III, Butler WM, Hatfield GM, Paul AG, Koreeda M. J Org Chem. 1984;49:4716–4720. [Google Scholar]
- 3.Roth BL, Baner K, Westkaemper R, Siebert DJ, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Proc Natl Acad Sci. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheffler DJ, Roth BL. Trends Pharmacol Sci. 2003;24:107–109. doi: 10.1016/S0165-6147(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 5.Siebert DJ. J Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 6.Chavkin C, Sub S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- 7.Beguin C, Richards MR, Wang Y, Chen Y, Liu-Chen LY, Ma Z, Lee DYW, Carlezon WA, Cohen BM. Bioorg Med Chem Lett. 2005;15:2761–2765. doi: 10.1016/j.bmcl.2005.03.113. [DOI] [PubMed] [Google Scholar]
- 8.Lee DYW, Karnati VVR, He M, Liu-Chen LY, Kondaveti L, Ma Z, Wang Y, Chen Y, Beguin C, Carlezon WA, Cohen B. Bioorg Med Chem Lett. 2005;15:3744–3747. doi: 10.1016/j.bmcl.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Lee DYW, Ma Z, Liu-Chen LY, Wang Y, Chen Y, Carlezon WA, Cohen B. Bioorg Med Chem. 2005;13:5635–5639. doi: 10.1016/j.bmc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Munro TA, Rizzacasa MA, Roth BL, Toth BA, Yan F. J Med Chem. 2005;48:345–348. doi: 10.1021/jm049438q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DYW, He M, Kondaveti L, Liu-Chen LY, Ma Z, Wang Y, Chen Y, Li JG, Beguin C, Carlezon WA, Cohen B. Bioorg Med Chem Lett. 2005;15:4169–4173. doi: 10.1016/j.bmcl.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 12.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. J Med Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 13.Munro TA, Goetchius GW, Roth BL, Vortherms TA, Rizzacasa MA. J Org Chem. 2005;70:10057–10061. doi: 10.1021/jo051813e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding WW, Schmidt M, Tidgewell K, Kannan P, Holden KG, Dersch CM, Rothman RB, Prisinzano TE. Bioorg Med Chem Lett. 2006;16:3170–3174. doi: 10.1016/j.bmcl.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Beguin C, Richards MR, Li JG, Wang Y, Xu W, Liu-Chen LY, Carlezon WA, Cohen BM. Bioorg Med Chem Lett. 2006;16:4679–4685. doi: 10.1016/j.bmcl.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 16.Lee DYW, He M, Liu-Chen LY, Wang Y, Li JG, Xu W, Ma Z, Carlezon WA, Cohen B. Bioorg Med Chem Lett. 2006;16:5498–5502. doi: 10.1016/j.bmcl.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 17.Tidgewell K, Harding WW, Lozama A, Cobb H, Shah K, Kannan P, Dersch CM, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. J Nat Prod. 2006;69:914–918. doi: 10.1021/np060094b. [DOI] [PubMed] [Google Scholar]
- 18.Bikbulatov RV, Yan F, Roth BL, Zjawiony JK. Bioorg Med Chem Lett. 2007;17:2229–2232. doi: 10.1016/j.bmcl.2007.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdes LJ, III, Chang H-M, Visger DC, Koreeda M. Org Lett. 2001;3:3935–3937. doi: 10.1021/ol016820d. [DOI] [PubMed] [Google Scholar]
- 20.Munro TA. PhD Thesis. University of Melbourne; Victoria, Australia: 2006. The Chemistry of Salvia divinorum; p. 92.p. 121.p. 188.p. 224. http://eprints.infodiv.unimelb.edu.au/archive/00002327. [Google Scholar]
- 21.Munro TA, Duncan KK, Staples RJ, Xu W, Liu-Chen LY, Beguin C, Carlezon WA, Cohen BM. Beilstein J Org Chem. 2007;3 doi: 10.1186/1860-5397-3-1. No PP. given. [ http://bjoc.beilstein-journals.org/content/pdf/1860-5397-3-1.pdf] [DOI] [PMC free article] [PubMed]
- 22.Ma ZZ, Hano Y, Qiu F, Chen YJ, Nomura T. Tetrhedron Lett. 2004;45:3261–3263. [Google Scholar]
- 23.Giner JL, Kiemle DJ, Kutrzeba L, Zjawiony J. Magn Reson Chem. 2007;45:351–354. doi: 10.1002/mrc.1972. [DOI] [PubMed] [Google Scholar]
- 24.Synthesis of 2b. 1a (40 mg, 92.6 μmol) was added to a solution of KOH in MeOH (1 M, 3 mL) and stirred at room temperature for 1 h. Following the previous procedure,13 crude 2b was obtained. Further purification by silica gel column [hexane:AcOEt (2:1)] gave pure 2b (12 mg, yield 34%). In the same manner, 2b was also obtained from 1b in 67% isolated yield. The 1H and 13C NMR chemical shifts of 2b were in full agreement with those of published data.12,17 The H-6α and H-6β were assigned as δ 2.53 and δ 1.67–1.77, respectively. The H-7α and H-7β were revised to δ 2.24 and δ 1.98, respectively. EI-MS m/z: 386 (M+).
- 25.Munro TA, Rizzacasa MA. J Nat Prod. 2003;66:703–705. doi: 10.1021/np0205699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Copies of 1H and 13C NMR spectra of 1a, 1b and 2b, and 2D NOESY spectrum of 2b.


