Abstract
Four experiments revealed arousal-enhanced location memory for pictures. After an incidental encoding task, participants were more likely to remember the locations of positive and negative arousing pictures than the locations of non-arousing pictures, indicating better binding of location to picture. This arousal-enhanced binding effect did not have a cost for the binding of nearby pictures to their locations. Thus, arousal can enhance binding of an arousing picture’s content to its location without interfering with picture-location binding for nearby pictures. In addition, arousal-enhanced picture-location memory binding is not just a side effect of enhanced memory for the picture itself, as it occurs both when recognition memory is good and when it is poor.
Keywords: memory binding, arousal, emotion, location, interference
Emotionally arousing events are more likely to be recalled later than non-arousing events. This emotional memory enhancement has been demonstrated in many lab studies using stimuli such as pictures and words (Ochsner, 2000; Denburg, Buchanan, Tranel, & Adolphs, 2003; Mather & Knight, 2005; Kensinger & Corkin, 2003; Kensinger et al., 2002; Charles, Mather, & Carstensen, 2003; Canli et al., 1999; LaBar & Phelps, 1998; Hamann, Ely, Grafton, & Kilts, 1999). These studies demonstrate that recall and recognition is more likely for emotional than for neutral items. However, memory for individual items is only one aspect of episodic memory. Remembering how various elements of an event were associated is another important aspect; one that is necessary in order to remember the peripheral and contextual details that specify the source of information (Chalfonte & Johnson, 1996; Henkel, Franklin, & Johnson, 2000; Mitchell et al., 2000).
Memory for items and for their associations with other information can be influenced in different ways by the same factor (Jurica & Shimamura, 1999; Mather, Johnson, & De Leonardis, 1999; Johnson, Nolde, & De Leonardis, 1996). For example, participants asked to focus on their own feelings while two speakers make statements are later better able to recognize the statements, but are worse at identifying who said them than participants asked to focus on the speakers’ feelings (Mather et al., 1999; Johnson et al., 1996). Given these findings of item-source tradeoffs, it is possible that the enhanced attention that emotionally arousing items attract (Schimmack, 2005; Phan, Wager, Taylor, & Liberzon, 2002; Easterbrook, 1959) may not enhance memory for contextual information even though it enhances item memory.
However, initial data from laboratory studies reveal that people are better at remembering the color or location of emotional or taboo words than of neutral words (Kensinger & Corkin, 2003; D’Argembeau & Van der Linden, 2004; Doerksen & Shimamura, 2001; MacKay et al., 2004; MacKay & Ahmetzanov, 2005). The presentation rates and encoding tasks varied across these studies, but in each one, participants saw an intermixed list of emotional and neutral words, with words presented one at a time. In addition, compared with neutral items, participants are less likely to incorrectly remember imagined emotional items as previously seen, also indicating enhanced memory for contextual detail of emotional stimuli (Kensinger, Garoff-Eaton, & Schacter, 2006; Kensinger & Schacter, 2005).
One potential mechanism to explain the enhanced memory for features of emotional items is that it is an interference effect that occurs during presentation of mixed lists of emotional and neutral items (none of the studies cited above compared context memory for emotional and neutral stimuli in a between-subjects design or a blocked format). A number of studies have shown that arousing stimuli can interfere with memory for spatially or temporally nearby neutral items (Bornstein, Liebel, & Scarberry, 1998; Detterman & Ellis, 1972; Ellis et al., 1971; Erdelyi & Blumenthal, 1973; Hadley & MacKay, 2006; Hurlemann et al., 2005; Johnson et al., 2005; MacKay et al., 2004; Miu, Heilman, Opre, & Miclea, 2005; Runcie & O’Bannon, 1977; Schmidt, 2002; Strange, Hurlemann, & Dolan, 2003). Although some of these studies used very rapid presentation, item memory impairment for temporally adjacent items also has been found with up to three or four seconds between the arousing and neutral items (Detterman & Ellis, 1972; Hurlemann et al., 2005; Runcie & O’Bannon, 1977) and sometimes even for items appearing six seconds after an arousing item (Schmidt, 2002). However, memory for contextual information was not tested in any of these studies, so it is not clear whether interference from adjacent emotionally arousing items also impairs binding of contextual features to neutral items.
In the current series of experiments, we examined whether interference from arousing pictures impairs binding of non-arousing pictures to location or whether arousal leads to enhancements in binding without a cost to nearby items. Any random set of arousing and non-arousing pictures may vary on dimensions other than just arousal, such as visual complexity or layout. Thus, we assembled a set of matched pictures in which each arousing picture was yoked with a visually similar but less arousing picture (see Figure 1 for examples). Some of these were International Affective Picture System (IAPS: Lang, 1995) pictures, some were IAPS pictures that we modified and some were from other sources.
Figure 1.
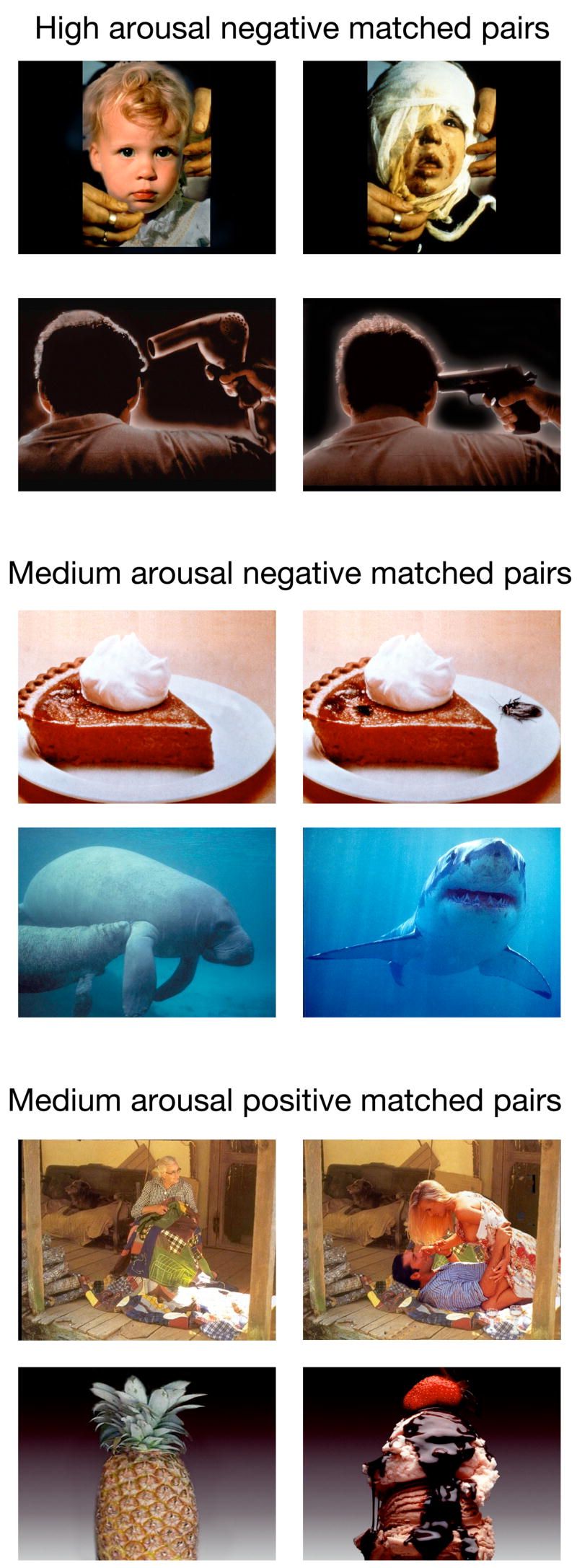
Examples of matched pairs of similar non-arousing (left column) and arousing (right column) pictures from the negative high arousal set, negative medium arousal set and positive medium arousal set.
A couple of previous studies have included both positive and negative words but did not report whether they yielded the same enhancement in binding (D’Argembeau & Van der Linden, 2004; Doerksen & Shimamura, 2001). Thus, in order to see whether arousal induced by positively and negatively valenced pictures would have the same effect on memory binding, we included a set of moderately arousing positive as well as a set of moderately arousing negative pictures and a set of highly arousing negative pictures (we could not find enough positive pictures with very high arousal ratings to create a highly arousing positive set). Each picture in these three emotional sets had a less arousing but visually similar matched picture (we will refer to these as non arousing).
In addition to questions about the role of competition between items and the impact of valence versus arousal in emotional memory binding effects, we were also interested in whether the emotion-enhanced memory-binding effect seen for words would replicate for pictures. At least under some circumstances, location memory for pictures decreases as arousal increases (Mather et al., 2006; Mitchell et al., 2006). In Mather et al.’s Experiment 1, participants viewed a sequence of four pictures, each presented alone on the screen for 750 ms in one of eight locations. All four pictures in each set were from the same arousal category (low, medium or high). This study phase was followed by a 7-second delay and then a test, consisting of one of the four pictures in one of the four locations. Participants indicated whether or not the picture-location conjunction was the same as before. As arousal for the set of four pictures increased, picture-location conjunction accuracy declined. This arousal-based impairment was seen for both positive and negative pictures. Two subsequent functional magnetic resonance imaging experiments revealed that both medium and high arousal trials resulted in greater activity in visual processing brain regions, but less activity in superior precentral gyrus and the precentral-superior temporal intersect, regions which play a role in feature integration (Mather et al., 2006).
The Mather et al. findings suggest that maintaining multiple highly arousing representations in working memory leads to disruptions in binding, whereas previous findings suggest that arousal enhances source memory when items are seen one at a time and there is no requirement to keep them active in working memory (MacKay & Ahmetzanov, 2005; MacKay et al., 2004; Doerksen & Shimamura, 2001; D’Argembeau & Van der Linden, 2004). However, it is possible that the contrast between the two patterns of results could be the result of differences in the impact of pictures versus words rather than the difference between maintaining competing representations in working memory and just processing stimuli while they are seen on the screen. By using pictures and a paradigm more similar to the previous word experiments, this study allowed us to see whether the materials or the task format is the critical factor in the contrasting effects of emotional arousal on source memory across these two types of paradigms.
In Experiment 1, we presented pictures one at a time in a mixed list format. Encoding of the pictures was incidental and participants did not have to make any response to them. Instead, to keep their attention engaged, presentations of pictures in different locations alternated with presentations of dots that participants had to make a color judgment about. In this experiment, we tested the role of interference by manipulating whether there was an additional intertrial interval between each picture or not, as increasing the interval between pictures should decrease the degree of interference. In Experiment 2, we presented the pictures blocked by arousal rather than in a mixed list, to see if performance for arousing and non-arousing items could be equated by having them appear separately rather than in intermixed lists in which they might compete for resources. In Experiment 3, we presented two pictures on the screen at the same time, to see if arousing pictures interfered with binding of pictures shown at the same time. In Experiment 4, we examined the relationship between item memory and item-location conjunction memory. In all experiments, participants completed forced-choice memory tests for the picture-location conjunctions after the incidental encoding session ended. As we will report, all four studies showed robust arousal-based enhancement of location memory. In addition, there was no evidence that seeing an arousing picture interfered with feature binding for temporally or spatially adjacent items.
Experiment 1
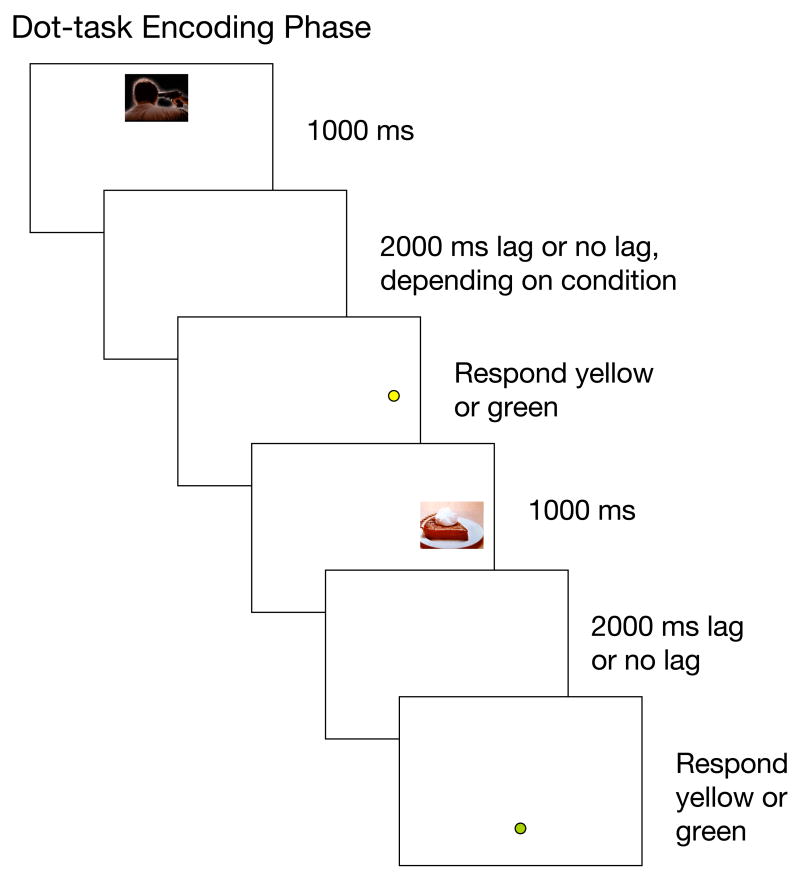
This experiment tested whether there would be an effect of arousal on memory for the locations of individual pictures. In an incidental encoding task, participants each viewed some arousing and some non-arousing pictures that appeared one at a time in a particular location and alternated with dots. The task was to indicate the color of the dots. Later, participants were tested on their memory for the picture-location conjunctions. In addition, across participants we manipulated whether there was a 2000 ms presentation lag between each picture and subsequent dot or not (see Figure 2A). According to the interference hypothesis, arousing items should interfere more with binding of non-arousing items as the lag between items decreases.
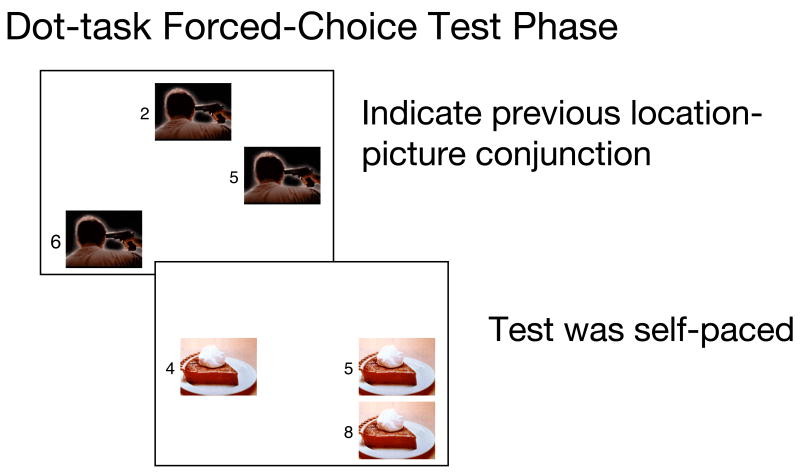
Figures 2A–B.
During the dot-task encoding phase in Experiment 1 (A), dots were shown in yellow or green and stayed on the screen until a response was made, at which point the next picture appeared. During the test phase (B), each picture was shown in three locations with numerical labels and participants were asked to indicate which was the picture-location conjunction shown previously. Pictures are not shown to scale.
Method
Participants
Forty undergraduates participated for course credit (M age = 19.65, SD = 2.0, 14 male and 26 female) and were randomly assigned to each of the lag conditions (20 to each condition).
Materials
We matched each arousing picture to a non-arousing picture that was similar in appearance, complexity, content, and focus of interest. We administered a series of pretest rating sessions with different raters to help us develop and revise the picture pairs (none of the raters were participants in the studies). Based on the final round of pretest ratings from a group of four undergraduates, we selected pairs that: 1) differed by at least 1.5 points in arousal on a 1–9 scale where 9 was high arousal and 1 was low arousal; and 2) were rated as being similar to each other with at least a 5.3 rating on a scale of 1 to 9, where 9 was “extremely similar” and 1 was “not at all similar.” The 72 picture pairs (144 pictures) were grouped into three sets of 24 pairs based on the type of arousing picture in the pair: medium arousal negative, medium arousal positive and high arousal negative. Example picture pairs from each set are shown in Figure 1.
Each participant was shown just one of the pictures from a matched pair; whether it was the arousing or the non-arousing version was counterbalanced across participants. Thus, 72 pictures were shown to each participant, in a mixed list with equal representation of the arousing and non-arousing versions for each of the three subsets of pictures. In order to equate conditions as much as possible across the arousing and non-arousing pictures, the members of each matched pair of pictures shared a display location on the dot task and two lure locations on the test. Each matched pair of pictures also appeared in the same place within a random list order during the dot task. Thus, across participants, each arousing picture had a yoked non-arousing control picture that was seen in the same configuration.
We presented stimuli using Psyscope (Cohen, MacWhinney, Flatt, & Provost, 1993). The screen (on a 17” monitor) was divided into a three by three grid (without any visible lines) and the outer eight cells were locations for the pictures. Pictures were presented as large as possible within the boundaries of the cells (each one 7.6 mm wide and 6.5 mm high, with 2 mm separation between cells) without distorting the picture dimensions.
Procedure
For the dot-task phase of the experiment, the instructions informed participants that their task was to indicate whether each dot they saw was yellow or green; in addition, they would see pictures, but no response was needed when they saw the pictures and they should just view them as though they were pictures in a slide show. During the dot-task phase, 72 pictures appeared once alone on the screen for 1000 ms in a random sequence. In the no-lag condition, the picture was immediately followed by the yellow or green dot in a random location on the screen (see Figure 2A for trial sequence). The dot remained on the screen until the participant indicated which color it was, at which point the next picture appeared. In the lag condition, the picture was followed by a 2000 ms interval with a blank screen, then the dot. As in the no-lag condition, the participants’ response led the dot to be replaced by the next picture.
Immediately after the dot-task phase, participants completed a forced-choice memory test (see Figure 2B). Each test trial consisted of one of the pictures shown in the dot-task phase, displayed simultaneously in three different locations on the screen. One of the locations was the same one the picture had appeared in during the dot-task phase. Numbers were printed next to each of the three copies of the picture. Participants typed in the number they thought corresponded with the picture-location conjunction they had seen before.
After the memory test, participants completed arousal ratings for each of the 144 pictures, followed by valence ratings for each picture. For the arousal ratings, participant were asked to rate each picture’s “emotional intensity” on a scale of 1 to 9 with 9 equaling “very emotionally intense.” A picture rated high in emotional intensity was defined for participants as evoking strong emotions such as being excited, disgusted, amazed, or fearful, whereas a picture low in emotional intensity would evoke calm or bored feelings. The valence ratings were also made on a scale of 1 to 9, with 1 equal to ‘very negative,’ 5 equal to ‘neutral’ and 9 equal to ‘very positive.’
Results
Examination of the median reaction times in the dot task revealed no significant difference in response speed between the lag (M = 780.4 ms, SE = 89.1) and the no-lag (M = 794.1 ms, SE = 96.5) conditions, t(38) = .10, p>.9, indicating that other than our manipulation of intertrial interval, the timing across the two conditions was similar.1
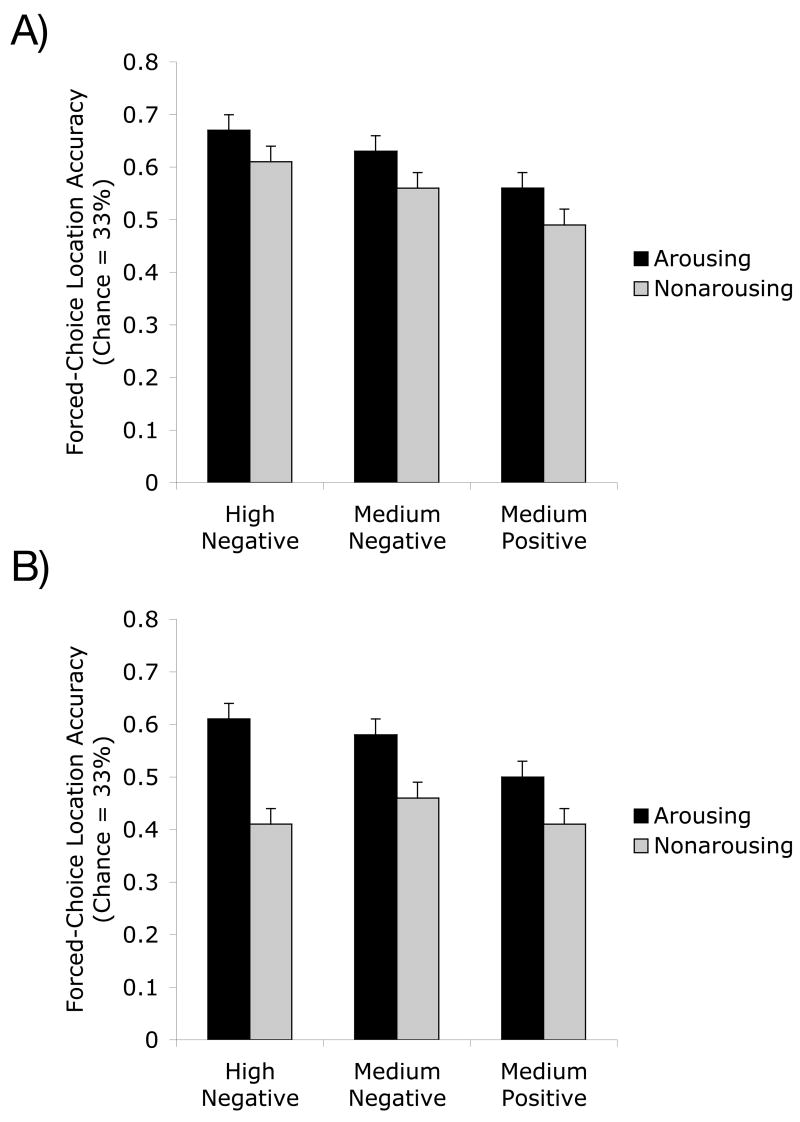
To examine whether arousing content enhances or impairs memory for picture location, we computed the proportion of correct picture-location conjunction identifications for the arousing and non-arousing pictures from each set, and submitted these scores to a 2 (picture version: arousing, non-arousing) X 3 (pair type: high negative, medium negative, medium positive) X 2 (condition: lag, no lag) ANOVA. As can be seen in Figures 3A and 3B, across the two lag conditions, participants remembered the picture-location conjunctions better for the arousing than the non-arousing versions of matched pictures for each of the three emotion types represented, F(1,38) = 21.06, p<.001, ηp2=.36.
Figures 3A–B.
The percentage of correct identifications of old picture-location conjunctions was higher for arousing than non-arousing pictures within each emotion-type set for the no-lag condition (A) and lag condition (B) of Experiment 1.
There also was a main effect of emotion type, F(2,76)=8.39, p<.01, ηp2=18; on average, the locations of pictures from the positive pairs (averaged across the arousing and non-arousing versions) were remembered less well (M=.49, SE=.02) than the locations of pictures from medium (M=.56, SE=.03) or high (M=.57, SE=.03) arousal negative picture pairs. However, arousal did not interact with emotion type, F(2,76)=.92, p>.4, ηp2=.02, indicating that the effect of arousal was equivalent across the different types of pairs. In addition, running separate analyses for each emotion category yielded significant effects of arousal in all three cases. This pattern reveals the benefits of having separate matched control pictures for positive and negative emotional pictures; without them, it might appear as though the positive and neutral conditions were equivalent and only negative arousal enhanced location memory (for instance, see Figure 3A).
There was also a main effect of condition, with conjunction memory better in the no-lag condition (M=.59, SE=.03) than in the lag condition (M=.49, SE=.03), F(1,38) = 5.32, p<.05, ηp2=.12, but condition did not interact significantly with any factors. The better picture-location binding in the no-lag condition may result from the dot-task session taking less overall time in the no-lag condition, leading a shorter delay on average for testing of items. In any case, having an unfilled interval in between items did not decrease the impact of picture arousal on memory for location, F(1,38) = 2.23, p=.14, ηp2=.06. If anything, the effect was in the opposite direction, as in the no-lag condition the difference between location memory for arousing (M = .62, SE = .03) and non-arousing (M = .55, SE = .03) was not as large as it was in the lag condition (M arousing = .56, SE = .03, M non-arousing = .43, SE = .03). Thus, the arousal-enhanced location memory found in this experiment does not appear to be the result of interference.
Repeating the overall ANOVA with gender as a factor revealed no significant effects of gender. We review the arousal and valence ratings from the first three experiments in the Appendix.
Discussion
In this experiment, participants remembered the location of arousing pictures better than the location of non-arousing pictures and this arousal enhancement effect was consistent for both positive and negative pictures. Having an additional unfilled interval after seeing each picture did not diminish the arousal effect, suggesting that this arousal-enhanced location memory cannot be explained by an interference account.
Experiment 2
In this experiment, we examined whether the arousal advantage would occur even if the pictures were grouped such that all the arousing pictures occurred in a row and all the non-arousing pictures occurred in a row. In addition, in one condition, participants saw the pictures and the dots in separate mini-blocks, with four pictures appearing one after the other without any intervening stimuli followed by four dots in a row. We compared this blocked-dot presentation condition to an alternating-picture-and-dot condition (as in Experiment 1). In both conditions, the arousing and non-arousing items were presented in separate halves of the list. If Experiment 1’s arousal-enhanced location binding was due to the arousing items delaying the binding of preceding or following non-arousing items, the effect should disappear in this experiment.
Method
Participants
Sixteen undergraduates participated for course credit (M age = 19.00, SD = .79, 8 male and 8 female) and eight were randomly assigned to each condition.2
Materials
We used the same pictures as in Experiment 1. For half the participants, 36 arousing pictures appeared first in a block, followed by 36 non-arousing pictures. Each picture was from a different matched pair. The matched picture pair versions were swapped for the other half of the participants, so that non-arousing pictures appeared first. In addition, pictures from each matched pair appeared in the first block for half the participants and in the second block for the other half. Since in Experiment 1 the effect of arousal was equivalent across the different picture pair types (e.g., positive and negative), we simplified the experimental design by not counterbalancing the order of the different emotion types. Instead, within each of the two blocks, the medium arousal negative pictures (or their non-arousing counterparts) appeared first, followed by pictures from the medium arousal positive pairs and then by pictures from the high arousal negative pairs. Thus, in the subsequent analyses, we examine the effects of arousal (which was fully counterbalanced) and not the effects of emotion type.
Procedure
There were two between-subjects conditions for the dot-task phase. In the alternating-picture-and-dot condition, participants saw each picture for 2000 ms followed by a 500 ms interval. Next, the yellow or green dot appeared and remained on the screen until a response was made and was then followed by the next picture. In the blocked-dot condition, four pictures appeared in a row for 2000 ms each, followed by four trials consisting of a 500 ms interval and a dot. Thus, the overall time of the two types of session was equivalent, but in the alternating-picture-and-dot condition the pictures were always separated by dots, whereas in the blocked-dot condition, every four pictures appeared in immediate succession. In both conditions, however, the item order itself was grouped such that the arousing pictures all appeared together in one half of the list and the non-arousing pictures appeared in the other half.
After the dot-task encoding phase, participants completed a forced-choice recognition test for the 72 picture-location conjunctions. Each picture appeared in two locations on the screen with number labels (rather than three location options as in Experiment 1). Participants selected which picture-location conjunction had been shown earlier. The subsequent ratings phase was the same as in Experiment 1, except that participants only gave arousal and valence ratings for the 72 pictures they saw during the encoding and memory test phase, instead of rating all 144 pictures.
Results
Whether picture and dot presentation was blocked (M = 620.9 ms, SE = 18.1) or alternated (M = 650.0 ms, SE = 46.2) did not significantly influence the speed of dot-color median response times, t(14) = .59, p>.5.
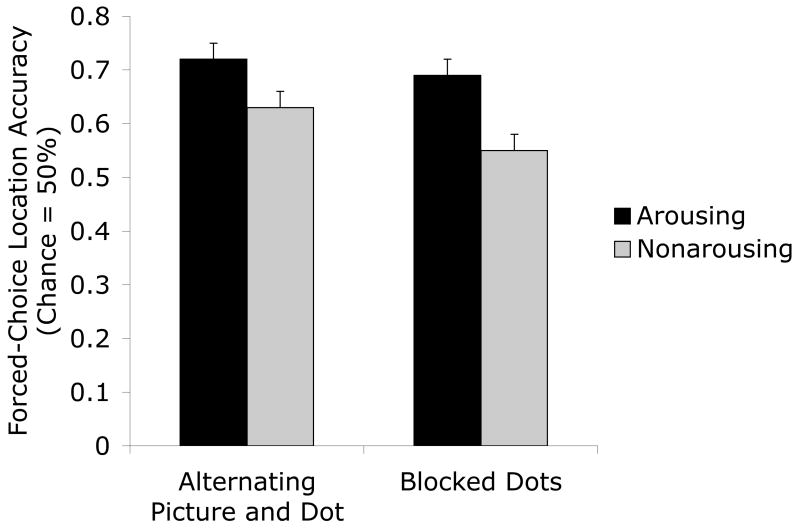
We analyzed the percentage of accurate responses on the location memory test in a 2 (picture version: arousing, non arousing) X 2 (list block order: arousing first, non arousing first) X 2 (picture and dot presentation: blocked, alternating) ANOVA. As shown in Figure 4, picture-location conjunctions were remembered better for the arousing versions than for the non-arousing versions of pictures in both conditions, leading to a main effect of picture version, F(1,12) = 16.96, p=.001, ηp2=.59. Thus, the arousal advantage held up when pictures were grouped together by arousal level. No other effects were significant.
Figure 4.
Percentage of correct identifications of old arousing and non-arousing picture-location conjunctions in the alternating-picture-and-dot and the blocked-dots conditions in Experiment 2. Both of these conditions showed pictures blocked by arousal type and both yielded arousal-enhanced location memory.
In particular, there was no interaction of picture version and whether the picture and dot presentation was blocked (F<1), suggesting that the arousal advantage occurred in both conditions. To test whether this was the case, we conducted separate ANOVAs for each condition. In both the alternating-picture-and-dot and the blocked-dot conditions, the main effect of arousal was significant (alternating F(1,6) = 7.14, p<.05, ηp2=.54; blocked F(1,6) = 9.91, p<.05, ηp2=.62) and no other effects were significant. Thus, the arousal advantage is quite reliable even when the arousing and non-arousing pictures are not intermixed in the presentation list.
In addition, including gender as a factor in the overall ANOVA did not yield any significant effects of gender.
Discussion
This experiment reveals that arousal-based differences in binding occur even when arousing and non-arousing stimuli are not presented within seconds of each other. Thus, these arousal effects cannot be explained by an interference account in which arousing items interfere with binding of non-arousing stimuli that might otherwise be occurring at the same time. Instead, together with the findings from Experiment 1, these results suggest that binding location information to arousing stimuli does not interfere with feature binding of temporally adjacent stimuli. In Experiment 3, we directly examined the effects of arousing stimuli on binding of nearby stimuli.
Experiment 3
In Experiment 3, we examined whether memory for a picture’s location is impaired or enhanced when an arousing picture is shown at the same time in another location on the screen. As in Experiment 1, pictures alternated with dots. However, in each trial two pictures were shown at the same time on the screen.
Method
Participants
Forty-eight undergraduates participated for course credit (M age = 18.81, SD = 1.45, 15 male and 33 female).
Materials
We used the same pictures as in Experiment 1. In this experiment, however, participants saw the pictures two at time, leading to 36 trials (with 72 pictures seen). Pictures seen together were from different matched pairs. Across participants, each picture was seen with the arousing and a non-arousing version of its presentation companion picture, yielding four counterbalanced lists. Thus, for example, the matched pair with the neutral child/bandaged child and the matched pair of the man with a hairdryer/gun (see pictures in Figure 1) might comprise one presentation pair for which the four different lists would have the following pictures presented together: 1) neutral child/hairdryer; 2) neutral child/gun; 3) bandaged child/hairdryer; 4) bandaged child/gun. As in that example, pictures were always presented with pictures from the same emotion set, so if two arousing pictures were presented together, they were always of the same valence. The presentation pairs appeared in the same two locations across all four lists, so that the arousing and non-arousing version of each picture was shown in the same place. The sequence of the presentation pairs was random. In addition, as in the previous two experiments, each participant only saw one picture version from each matched pair (i.e., if they saw the gun picture, they did not see the hairdryer picture, and vice versa).
Procedure
Each trial consisted of two pictures shown on the screen simultaneously for 2000 ms, followed by a sequence of five dots requiring a yellow/green judgment. Each of the first four dots had a 1500 ms time window in which a response could be made. If a response was made before that time was up, the dot disappeared, but the next dot did not appear until 1500 ms was up. The final dot remained on the screen until a response was made, at which point the next picture pair appeared for 2000 ms.
Immediately after the dot-task encoding phase, participants completed a forced-choice memory test with separate trials for each of the 72 pictures. Each trial consisted of one of the previously presented pictures presented in three locations on the screen simultaneously, with number options printed next to them. One location was the correct one, one was the location of the picture previously shown with the target picture and one was a different location. The pictures were presented in a random order and the three copies of each one remained on the screen until participants made their response. Once participants had completed the memory test, they did the ratings task, as in Experiment 1, again making valence and arousal ratings for all 144 pictures.
Results
Averaging each participant’s median response time in the dot task yielded an average response time of 621.5 ms (SE = 10.8). Dot-color accuracy was 93% (SE = 1%).
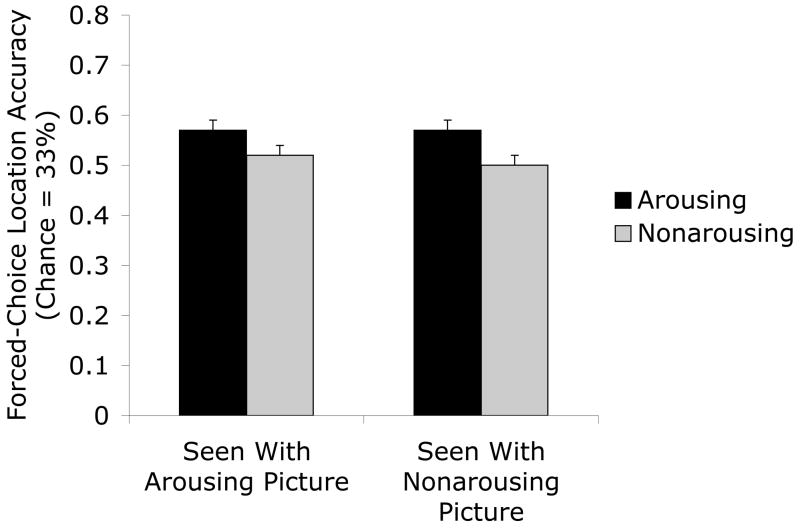
An ANOVA comparing the percent picture-location conjunction accuracy for arousing versus non-arousing pictures replicated the previous experiments, with the locations of arousing pictures more likely to be identified (M = .57, SE = .02) than the locations of non-arousing pictures (M = .51, SE = .02), F(1,47) = 10.51, p<.01, ηp2=.18. A follow-up ANOVA comparing the two possible types of errors (misbinding with presentation pair’s location or an unrelated location) revealed no significant effects of error type. Of particular interest, participants showed no bias to select the presentation pair’s location over the unrelated location for either arousing (M pair = .22, SE = .01; M unrelated = .22, SE = .01) or non-arousing (M pair = .24, SE = .01; M unrelated = .25, SE = .01) pictures, F(1,47) = .28, p>.5, ηp2=.01. Thus, participants were not likely to confuse the two pictures’ locations with each other regardless of whether the pictures that had been shown together were arousing or not.
The other question of interest in this study was how the presence of an arousing picture might influence binding of another picture’s location. To test this, we conducted a 2 (picture version: arousing, non arousing) X 2 (picture shown at same time: arousing, non arousing) ANOVA on the percentage of correct responses. There were no significant effects of the type of picture shown at the same time as the tested picture during the dot-color task. The presence of an arousing picture did not impair location accuracy (M = .55, SE = .02) compared with the presence of a non-arousing picture (M = .53, SE = .01), F(1,47) = 1.25, p>.2, ηp2=.03. There also was no interaction of picture version and pair type, F(1,47) = .11, p>.7, ηp2=.00 (see Figure 5). Repeating the above analyses with gender as a factor did not lead to any significant effects of gender.
Figure 5.
Percentage of correct identifications of old arousing and non-arousing picture-location conjunctions displayed with an arousing picture versus those displayed with a non-arousing picture in Experiment 3. The type of picture shown at the same time did not significantly affect later location memory.
Discussion
These results indicate that the enhanced location memory for arousing pictures does not spillover to other stimuli presented at the same time, as they show no enhancement in binding to their locations. In addition, there was no decrement in location accuracy for pictures seen at the same time as arousing pictures, indicating that the arousal-enhanced-binding effect cannot be explained by interference for the neutral stimuli. Instead, the arousal-enhanced-binding effect was specific to the item that induced the arousal, with no effect on picture-location binding for nearby items. Also of interest was the finding that misbinding, in which the locations of the two pictures were confused with each other, was no more likely to occur for non-arousing than arousing pictures. Thus, the worse location memory for the non-arousing pictures did not appear to be the result of confusion with locations of arousing pictures shown at the same time.
Experiment 4
Experiments 1–3 reveal a consistent pattern of better location memory for arousing than for non-arousing pictures. An important question is whether this enhanced location memory is the result of the same mechanisms that enhance memory for arousing pictures. Although we did not test item memory, results of previous studies indicate that recognition or recall of the pictures themselves should also be better when they are emotionally arousing than non-arousing. Enhanced location memory may be a side effect of enhanced item memory, or it may be an independent effect. In Experiment 4, we investigated the relationship between arousal effects on recognition memory and location memory for pictures. We used a shortened version of our previous experiments run over the Internet that included both a recognition test and a location memory test.
Method
Participants
Postings on lists of online psychology experiments recruited participants for a study of attention and cognition. One hundred and eighty-six participants completed the experiment (M age = 23.6, SD = 8.72, range 15–56; 62 males, 123 females, 1 no gender indicated). Five participants were excluded from further analyses for failures to follow instructions. No compensation was offered.
Materials
The experiment was programmed using WebExp2 (Mayo, Corley, Keller, & Jaeger, 2006). This Java-based program displayed instructions and picture stimuli in participants’ web browser window. We used 24 of the matched-picture pairs used in the previous experiments (48 pictures), with eight pairs from each of the three categories (high arousal negative, medium arousal negative and medium arousal positive). There were four versions of the encoding lists, to counterbalance whether the arousing or non-arousing picture from each pair was seen and whether pictures were seen during encoding and thus were old items on the test or served as new items. Each participant saw 12 pictures from the matched-picture set—six arousing and six neutral—and was tested on 24 pictures (one from each matched-picture pair, with 12 old items). To avoid ceiling levels on the recognition test, we also included a set of 12 filler IAPS pictures in the encoding task, selected to match the composition of the target picture set in terms of arousal and valence. Memory for these pictures was not tested.
Procedure
A consent page invited participation in a study on attention that involved indicating the locations of pictures as quickly as possible (no mention was made of the memory tests). When participants clicked on the study link, they were randomly assigned to one of the four counterbalancing conditions of the study and asked to answer several demographic questions. Next, they were given instructions for the encoding phase, in which they were asked to respond as quickly as they could to indicate whether each picture that appeared on the screen was on the top or bottom half of the window. The 12 target and 12 filler pictures were each presented (in a random order for each participant) in one of four quadrants within the display frame. The size of the pictures depended upon the screen resolution of the viewer. Each picture remained on the screen until the participant entered either the “t” (top) or “b” (bottom) key. After completing this phase, participants were told we were interested in their memory of the pictures and were asked to indicate whether they had seen pictures in the top/bottom task (“s” key) or not (“n” key). The 12 target old pictures and 12 new pictures were each presented in the center of the window in a random order. After completing this recognition memory test, participants were given the location memory test, in which they were shown each of the 12 target old pictures in two of the quadrants of the screen and asked which of the two locations the picture had been seen in during the location task. Finally, participants were asked if they had expected a memory test (a response was required) and if they had any other comments about the study (a response was optional) and were debriefed.
Results
Encoding task
Accuracy on the initial top/bottom task was quite high and did not differ for arousing and non-arousing target pictures (M = .97, SE = .01 for both), F(1,180) = .25, p>.6, ηp2=.00. The program recorded the time from one response to the next, which included picture loading time as well as display time. For this and subsequent response time analyses, we excluded responses more than 2 SD slower than the overall average response time (1.5% of all responses). Response times were slower for arousing (M = 822.9 ms, SE = 27.3) than for non-arousing pictures (M = 797.7 ms, SE = 22.1), but the difference was not significant, F(1,179) = 2.45, p>.1, ηp2=.01.
Item and location memory
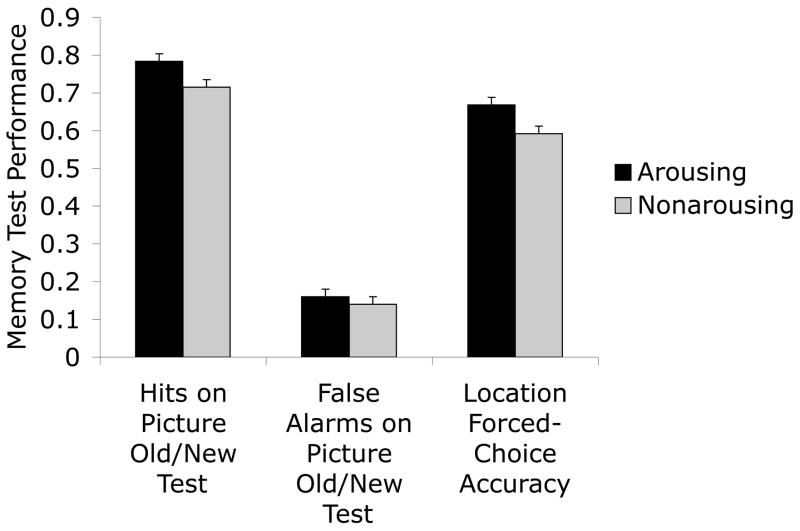
On the recognition test, participants made more correct identifications of previously-seen arousing pictures than of non-arousing pictures, F(1,180) = 13.42, p<.001, ηp2=.07 (see Figure 6 for means). False alarms did not differ significantly for new arousing pictures and non-arousing pictures, F(1,180) = 1.68, p>.19, ηp2=.01 (see Figure 6). However, corrected recognition (hits - false alarms) was not significantly greater for arousing pictures (M = .62, SE = .02) than for non-arousing pictures (M = .57, SE = .02), F(1,180)= 3.29, p=.07, ηp2=.02 (the same p value was obtained when comparing d’ for arousing and non-arousing pictures).
Figure 6.
Recognition (hits and false alarms) and picture-location conjunction test performance in Experiment 4 for arousing and non-arousing pictures.
In addition, as expected, the arousal-enhanced location memory seen in the previous experiments was replicated in this experiment, F(1,180) = 14.90, p<.001, ηp2=.08 (see right side of Figure 6).3
Relationship between item and location memory arousal effects
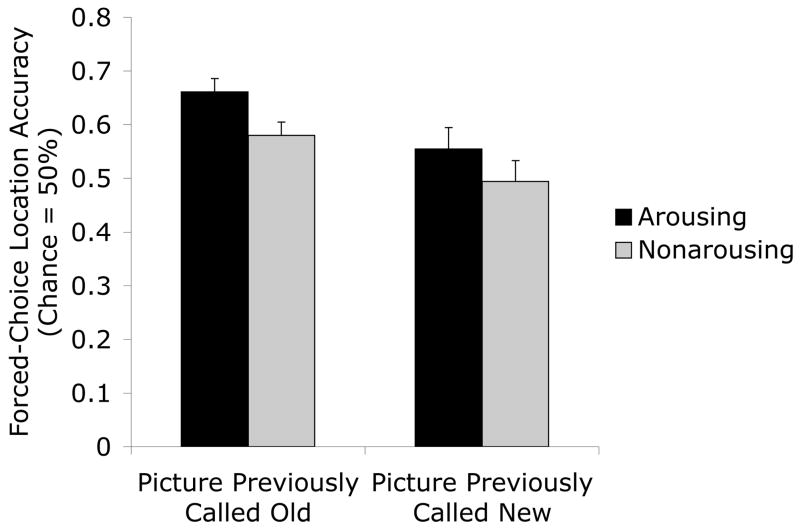
We categorized each picture on the location memory test by whether it had been correctly identified as old on the preceding recognition memory test (a hit) or whether it had been incorrectly called new (a miss). Then we examined results from the 101 participants who had both hits and misses using a 2 (version: arousing, non-arousing) X 2 (item memory: hit, miss) repeated-measures ANOVA. As in the overall analysis, there was a main effect of arousal, F(1,100) = 4.25, p<.05, ηp2=.04. In addition, not surprisingly, there was a main effect of item memory, with better location memory for old pictures correctly identified as old than for those misidentified as new, F(1,100) = 8.76, p<.01, ηp2=.08. However, there was no significant interaction of the two factors, F(1,100) = .10, p>.7, ηp2=.00. As can be seen in Figure 7, arousal-enhanced location memory occurred both when item memory for that picture was good (hits) and when it was poor (misses). Thus, the arousal-enhanced location memory effect was not simply a by-product of good item memory.
Figure 7.
Picture-location conjunction memory performance was significantly better for arousing than for non-arousing pictures, across previously recognized (called old) and previously forgotten (called new) pictures.
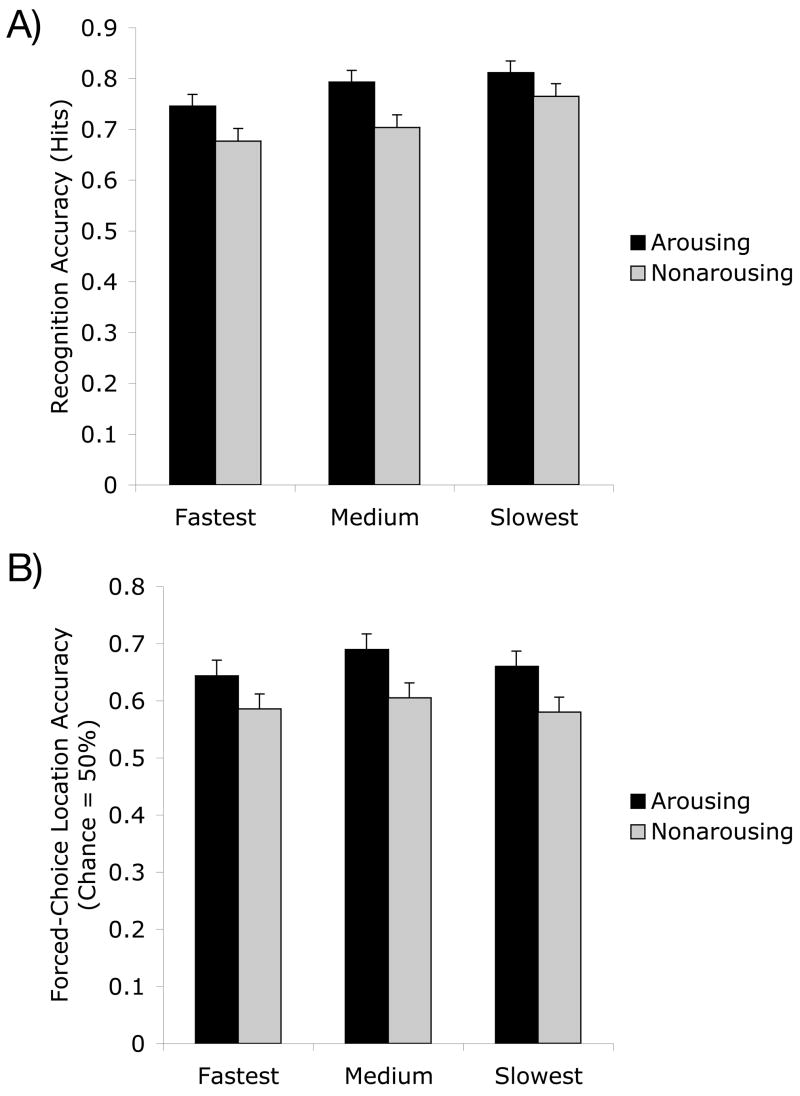
Further evidence that location and item memory were influenced by different factors is that participants’ response times to pictures on the top/bottom task predicted later recognition accuracy for those pictures, but not location memory accuracy. These analyses were done by rank ordering top/bottom response times separately for arousing and neutral pictures for each participant and dividing these items into categories based on these individual rankings: the fastest, middle and slowest third of items. As shown in Figure 8A, a 2 (version: arousing, non-arousing) X 3 (response time: fastest, medium, slowest) repeated-measures ANOVA with recognition accuracy (hits) as the dependent measure revealed a significant linear effect of response time, F(1,180) = 14.80, p<.001, ηp2=.08 and no interaction of version and response time (F<1). In contrast, as shown in Figure 8B, the same analysis for location accuracy revealed no significant effects of response time (F<1). Thus, in this task, lingering longer over the picture in the initial task improved later recognition memory but not location memory.
Figures 8A–B.
Participants were more likely to correctly recognize pictures they had responded to more slowly than those they had responded to more quickly on the top/bottom task (A) but longer response times on the top/bottom encoding task did not predict location memory accuracy (B).
Other factors
There were no significant effects of gender when it was added as a factor in the analyses of item and location memory. In addition, age was not significantly correlated with the degree to which arousal enhanced item memory, r(179) = .04, p>.6, or location memory, r(179) = .01, p>.8. However, response times were significantly slower on the top/bottom task as age increased, for both arousing, r(179) = .21, p<.01, and non-arousing target pictures, r(179) = .19, p<.05, consistent with previous findings of decreases in speed of processing with aging (Salthouse, 2000).
Looking at memory performance separately for the 27 participants who said they suspected there would be a memory test revealed no significant effects of arousal on location memory accuracy (M arousing = .67, SE = .04; M non-arousing = .65, SE = .03), F(1,26) = .16, p>.6, ηp2=.01, or corrected recognition (M arousing = .67, SE = .06; M non-arousing = .67, SE = .06), F(1,26) = .00, p>.9, ηp2=.00. However, including memory test expectations as a factor in the analyses for the overall group did not reveal significant interactions, thus it is not clear whether we lacked power to detect arousal-based effects in this subgroup of participants or whether intentionally trying to memorize the pictures eliminated arousal-based effects in this task.4 In addition, these participants may have differed in other ways from those who did not suspect a memory test. However, in any case, it is clear that any intentional encoding strategies employed by this subgroup did not drive the arousal enhancement effects we found among the overall group of participants.
Discussion
As expected, this study revealed arousal-based enhancement for location memory for pictures, extending the findings from our previous experiments to a different encoding task (in this experiment, participants indicated the location of the pictures rather than the color of intervening dots). Most interesting, however, is the finding that arousal-enhanced location memory occurred to the same extent for pictures that were recognized as old as for pictures that were not recognized. This suggests that enhanced location memory for arousing pictures is not simply a side effect of enhanced memory for the pictures themselves. It would be interesting in further studies to examine whether this lack of relationship between arousal-enhanced location memory and item memory strength holds up across more fine-grained levels of item memory strength, for instance using confidence ratings. In addition, a qualification of this finding is that we used separate memory tests for recognition and location memory; it is possible that a stronger relationship would be seen if participants were queried about both item and location information on the same trial for each picture.
Another interesting finding was that the time spent looking at a picture predicted recognition accuracy but not location accuracy for that picture, a finding that is consistent with the “one-shot” hypothesis for context storage—that beyond an initial context encoding, increases in study time do not increase encoding of contextual information (Malmberg & Shiffrin, 2005).
General Discussion
In each of the four experiments, we found that people are better at remembering the location of arousing pictures than non-arousing pictures seen during an incidental encoding task. This arousal-enhanced location binding for pictures appears to be a highly reliable effect and is consistent with previous studies showing better memory for the color or location of emotional than neutral words (Kensinger & Corkin, 2003; D’Argembeau & Van der Linden, 2004; Doerksen & Shimamura, 2001; MacKay et al., 2004; MacKay & Ahmetzanov, 2005). In addition, because the enhancement in location memory occurred for both positive and negative arousing pictures, this study reveals that arousal is the critical factor, rather than valence.
The results from these experiments also indicate that this enhanced binding has no benefit or cost for binding of other picture-location conjunctions that appear within a few seconds of the arousing pictures or on the screen at the same time. These findings rule out two possible accounts of the arousal-enhanced binding seen here. First, the effect cannot be explained by a global enhancement in memory binding during the trial in which an arousing picture appears, because the enhanced binding is specific to the picture that induced the arousal and no carry-over is seen for nearby pictures. Second, the arousal-enhanced binding seen in this study cannot be explained by an interference mechanism in which arousal-enhanced binding on mixed lists is due to disrupted binding of non-arousing items resulting from interference from nearby arousing items. Given that neither the global-enhancement nor the interference mechanisms can account for our findings, what can? Below we discuss findings from previous attention and cognitive neuroscience research that may help explain our findings.
Mechanisms of arousal-enhanced binding
Binding features to create coherent representations starts with initial perception. Treisman’s feature integration theory proposes that features such as color and shape are registered automatically and in parallel in the visual field, but that to bind features within an object together, attention must be focused on that object (Treisman, 1998). Furthermore, because of capacity limits, attention can only be focused on one spatial region at a time. Thus, when searching for two features that co-occur within the same visual object, people need to look at each object on the screen one at a time. In contrast, when the task is to find any target that contains one of the two features, increasing the number of objects in the display does not slow people’s search down much, indicating that they do not have to look at each object to detect their individual features (Treisman & Gelade, 1980).
These findings about the role of attention in binding suggest that emotionally arousing stimuli might be more effectively bound with their features in initial perception, insofar as they elicit more intense focused attention (for a review see Mather, 2007). Previous studies have shown enhanced initial perceptual processing for emotional stimuli, as they are more likely than nonemotional stimuli to be correctly identified when shown very briefly (Zeelenberg, Wagenmakers, & Rotteveel, 2006) and less likely to be missed when presented in a rapid serial visual presentation with other stimuli (Anderson, 2005; Keil & Ihssen, 2004). Brain imaging studies reveal that when shown a series of pictures, people show more activation in visual processing regions for emotionally intense pictures than for emotionally neutral pictures (Bradley et al., 2003; Phan et al., 2002; Mather et al., 2006). The amygdala plays a key role in supporting these attentional advantages for emotional stimuli (e.g., Anderson & Phelps, 2001).
According to feature integration theory, in order to bind an item and its location together, an “attention window” targets a particular location and selects whatever features are currently linked to that location, “temporarily excluding the features of all other objects from the object perception level” (Treisman, 1998, p. 1296). Arousal may enhance this binding process by increasing both the selectivity of attention and the activation level of the features associated with the arousing object. If not disrupted during later stages of encoding or rehearsal, this enhanced perceptual binding should provide the basis for enhanced memorial binding, such as that seen in the current experiments and in previous work.
Furthermore, our findings suggest that arousal-enhanced location-picture binding is not due to arousing stimuli drawing attention for longer than non-arousing stimuli. In Experiment 4, lingering longer over pictures at encoding enhanced picture recognition but not picture-location conjunction memory. In addition, being shown on the screen at the same time as an arousing picture did not influence picture-location binding, despite the fact that previous research demonstrates that when a non-arousing and an arousing picture are shown together on a screen, people look longer at the arousing picture (e.g., Knight et al., under review; LaBar, Mesulam, Gitelman, & Weintraub, 2000; Rosler et al., 2005). Thus, looking time does not seem to influence picture-location binding, at least not in an incidental encoding task in which there is sufficient time (i.e., 2000 ms in Experiment 3) to look at both of the pictures on the screen. Instead, picture-location binding seems to occur automatically at the moment that the picture is first looked at. Likewise, the arousal-based enhancement appears to be limited to the initial moment of perception and does not consume resources needed to subsequently bind location to other nearby pictures.
How these findings contrast with other effects of emotion
It would be interesting in future research to compare item and location memory in the type of experimental designs that in past studies has led to item-memory impairments for items seen before or after arousing items. These typically either involve sequences with just one arousing item (Hurlemann et al., 2005; Strange et al., 2003; Schmidt, 2002; Erdelyi & Blumenthal, 1973; Detterman & Ellis, 1972; Ellis et al., 1971; Runcie & O’Bannon, 1977; MacKay et al., 2004) or rapid serial visual presentation without responses required from the participants until the end of the list (Hadley & MacKay, 2006). The current findings suggest that this type of arousal-based interference seen for item memory will not extend to item-location conjunction memory.5
In the present experiments, participants did encoding tasks that made it unlikely that they would rehearse the pictures during the encoding phase. In contrast, in working memory studies requiring rehearsal of multiple picture-location conjunctions immediately after seeing them in a sequence, participants were worse at remembering the locations if all four pictures were arousing than if they were non-arousing (Mather et al., 2006; Mitchell et al., 2006). Rehearsing two items at the same time in working memory (Reinitz & Hannigan, 2004; Hannigan & Reinitz, 2000) or alternating attention between two different items (Reinitz & Hannigan, 2001) can dramatically increase conjunction errors over conditions in which the two items are seen in the same study list, but are not simultaneously rehearsed. The findings of impaired source memory for arousing items (Mather et al., 2006; Mitchell et al., 2006) indicate that having multiple emotionally arousing elements in working memory simultaneously makes it even more difficult to maintain multiple bound representations of objects and their features, perhaps because the emotionally arousing elements demand focused attention during rehearsal. Focusing on one picture at a time during rehearsal makes it less likely that all of the bound representations can be maintained simultaneously than if attention is more distributed across the picture-location representations in working memory. In contrast, the current study suggests that the emotional arousal associated with an object enhances initial perceptual binding and that this enhancement is maintained in memory as long as there is no rehearsal task in which that representation must compete with bound representations for other arousing objects.
Conclusion
In order to accurately remember an event, one must remember not only the various elements, but also the way that they were related to each other. This study provides evidence that the emotional arousal associated with one element of an event enhances memory for the location of that element but does not have any impact on how well the location of other elements will be recalled. These findings provide new information about how emotional arousal shapes memory for the relationships among different components of an event.
Acknowledgments
This work was supported by a grant from the National Institute on Aging (AG025340).
Appendix
Experiments 1–3 Ratings of Pictures
Participants in the first three experiments completed the same ratings task (the only difference was that in Experiment 2, participants only rated the 72 pictures they had seen in the dot-color encoding session, rather than all 144 pictures). Separate analyses for these three experiments yielded the same pattern of results and significant findings, thus to simplify reporting we combined the data. We conducted 2 (version: arousing, non arousing) X 3 (emotion type: high negative, medium negative, medium positive) ANOVAs for both the arousal and valence ratings (see Table A1 for means). For the arousal ratings, as expected, there was a main effect of version, with the arousing versions of pictures rated as more arousing (M = 6.01, SE = .11) than the non-arousing versions (M = 2.57, SE = .09), F(1,102) = 927.27, p<.001, ηp2=.90. In addition, there was a main effect of emotion type, F(2,204) = 281.93, p<.001, ηp2=.73, and an interaction of version and emotion type, F(2,204) = 258.34, p<.001, ηp2=.72. As can be seen in Table A1, the arousal levels of the non-arousing matched pictures did not vary much across the different picture types, but differed more for the arousing versions from the three emotion types, with high arousal negative pictures having the highest arousal, followed by medium arousal negative pictures and then by medium arousal positive pictures.
As expected, the valence rating analyses yielded a main effect of emotion type, F(2,204) = 831.77, p<.001, ηp2=.89, and an interaction of emotion type and version, F(2, 204) = 986.65, p<.001, ηp2=.91. As can be seen in Table A1, the non-arousing matched control pictures were all about the same valence, whereas the positive pictures were rated more positively and the negative pictures were rated more negatively than the matched control pictures.
Table A1.
Mean Arousal and Valence Ratings in Experiments 1–3.
| Picture Type | Arousal | Valence |
|---|---|---|
| Negative High Arousal | 7.64 (.11) | 1.71 (.06) |
| Negative Medium Arousal | 5.88 (.14) | 2.71 (.09) |
| Positive Medium Arousal | 4.50 (.14) | 6.62 (.08) |
| Non-arousing (Neg. High Arousal Match) | 2.63 (.09) | 5.32 (.06) |
| Non-arousing (Neg. Med. Arousal Match) | 2.66 (.10) | 5.71 (.06) |
| Non-arousing (Pos. Med. Arousal Match) | 2.43 (.10) | 5.49 (.06) |
Notes. Ratings were made using 1–9 scales. Standard errors are listed in parentheses.
Neg. = Negative, Pos. = Positive, Med. = Medium.
Footnotes
Whether dots were yellow or green was randomly determined by the program and not recorded in Experiments 1 and 2. Thus, we cannot determine dot-color accuracy for these experiments.
Fewer participants were run in this experiment than in the others because our main objective was to show that arousal-enhanced location memory occurred even when items were not intermixed, and given the large effect size, we did not need a large N for this purpose.
Although the main focus of Experiments 2–4 was on arousal effects rather than valence effects, we did follow-up analyses to check if the arousal effect differed across valence types. Including picture pair type (i.e., high arousal negative, low arousal negative and low arousal positive) as a factor did not lead to any significant interactions of pair type and arousal level for location memory (all p>.4 across Experiments 2–4), indicating that, as in Experiment 1, the arousal-enhanced location memory effect was similar for the negative and positive pictures.
We believe that the likely explanation is a lack of power, as we ran a follow-up to Experiment 3 in which all the methods were identical as before except that participants were given intentional encoding instructions for the pictures. Because of the dot task, they were not able to do much rehearsal between picture viewings (unlike in Mather et al., 2006). The findings from this experiment were the same as in Experiment 3 (a large arousal-enhanced location memory effect and no significant effects of which pictures were shown together).
Exploratory analyses of sequence memory effects in Experiment 4 (in which order was randomized for each participant) revealed no item memory or item-location conjunction memory effects of being seen before (or after) an arousing versus a non-arousing picture. The lack of interference effects for location memory is consistent with the previous three experiments and further supports our conclusion that the arousal-enhanced picture-location binding seen in these experiments was not due to interference from nearby pictures. However, future studies are needed to contrast item memory and item-location binding under conditions in which arousal-based interference effects are seen for item memory.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AK. Affective influences on the attentional dynamics supporting awareness. Journal of Experimental Psychology: General. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Bornstein BH, Liebel LM, Scarberry NC. Repeated testing in eyewitness memory: A means to improve recall of a negative emotional event. Applied Cognitive Psychology. 1998;12:119–131. [Google Scholar]
- Bradley MA, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE. fMRI identifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology. 1999;27:441–452. [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt RR, Provost J. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- D’Argembeau A, Van der Linden M. Influence of affective meaning on memory for contextual information. Emotion. 2004;4:173–188. doi: 10.1037/1528-3542.4.2.173. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan D, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal elderly persons. Emotion. 2003;3:239–254. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Detterman DK, Ellis NR. Determinants of induced amnesia in short-term memory. Journal of Experimental Psychology. 1972;95:308–316. doi: 10.1037/h0033629. [DOI] [PubMed] [Google Scholar]
- Doerksen S, Shimamura AP. Source memory enhancement for emotional words. Emotion. 2001;1:5–11. doi: 10.1037/1528-3542.1.1.5. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Ellis NR, Detterman DK, Runcie D, McCarver RB, Craig EM. Amnesic effects in short-term memory. Journal of Experimental Psychology. 1971;89:357–361. doi: 10.1037/h0031192. [DOI] [PubMed] [Google Scholar]
- Erdelyi MH, Blumenthal DG. Cognitive masking in rapid sequential processing: The effect of an emotional picture on preceding and succeeding pictures. Memory & Cognition. 1973;1:201–204. doi: 10.3758/BF03198095. [DOI] [PubMed] [Google Scholar]
- Hadley CB, MacKay DG. Does emotion help or hinder immediate memory? Arousal versus priority-binding mechanisms. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:79–88. doi: 10.1037/0278-7393.32.1.79. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hannigan SL, Reinitz MT. Influences of temporal factors on memory conjunction errors. Applied Cognitive Psychology. 2000;14:309–321. [Google Scholar]
- Henkel LA, Franklin N, Johnson MK. Cross-modal source monitoring confusions between perceived and imagined events. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2000;26:321–335. doi: 10.1037//0278-7393.26.2.321. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, Vogeley K, Maier W, Dolan RJ. Noradrenergic modulation of emotion-induced forgetting and remembering. Journal of Neuroscience. 2005;25:6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Nolde SF, De Leonardis DM. Emotional focus and source monitoring. Journal of Memory and Language. 1996;35:135–156. [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: Prefrontal correlates of refreshing a just-activated representation. Cognitive Affective & Behavioral Neuroscience. 2005;5:339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Shimamura AP. Monitoring item and source information: Evidence for a negative generation effect in source memory. Memory & Cognition. 1999;27:648–656. doi: 10.3758/bf03211558. [DOI] [PubMed] [Google Scholar]
- Keil A, Ihssen N. Identification facilitation for emotionally arousing verbs during the attentional blink. Emotion. 2004;4:23–35. doi: 10.1037/1528-3542.4.1.23. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory & Cognition. 2003;31:1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54:99–112. [Google Scholar]
- Kensinger EA, Schacter DL. Emotional content and reality-monitoring ability: fMRI evidence for the influences of encoding processes. Neuropsychologia. 2005;43:1429–1443. doi: 10.1016/j.neuropsychologia.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt J, Baker C, Nesmith K, Mather M. Aging and goal directed emotional attention: Distraction reverses emotional biases. doi: 10.1037/1528-3542.7.4.705. under review. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Mesulam MM, Gitelman DR, Weintraub S. Emotional curiousity: Modulation of visuospatial attention by arousal is preserved in aging and early-stage Alzheimer’s disease. Neuropsychologia. 2000;38:1734–1740. doi: 10.1016/s0028-3932(00)00077-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9:490–493. [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Ahmetzanov MV. Emotion, memory, and attention in the taboo Stroop paradigm: An experimental analogue of flashbulb memories. Psychological Science. 2005;16:25–32. doi: 10.1111/j.0956-7976.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Shafto M, Taylor JK, Marian DE, Abrams L, Dyer JR. Relations between emotion, memory, and attention: Evidence from taboo Stroop, lexical decision, and immediate memory tasks. Memory & Cognition. 2004;32:474–488. doi: 10.3758/bf03195840. [DOI] [PubMed] [Google Scholar]
- Malmberg KJ, Shiffrin RM. The “one-shot” hypothesis for context storage. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:322–336. doi: 10.1037/0278-7393.31.2.322. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Johnson MK, De Leonardis DM. Stereotype reliance in source monitoring: Age differences and neuropsychological test correlates. Cognitive Neuropsychology. 1999;16:437–458. [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Mitchell KJ, Raye CL, Novak DL, Greene EJ, Johnson MK. Emotional arousal can impair feature binding in working memory. Journal of Cognitive Neuroscience. 2006;18:614–625. doi: 10.1162/jocn.2006.18.4.614. [DOI] [PubMed] [Google Scholar]
- Mayo N, Corley M, Keller F, Jaeger F. WebExp2 Experimental Software. 2006 Retrieved January 2, 2006, from http://www.hcrc.ed.ac.uk/web_exp/
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D’Esposito M. Aging and reflective processes of working memory: Binding and test load deficits. Psychology and Aging. 2000;15:527–541. doi: 10.1037//0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Mather M, Johnson MK, Raye CL, Greene EJ. A functional magnetic resonance imaging investigation of short-term source and item memory for negative pictures. NeuroReport. 2006;17:1543–1547. doi: 10.1097/01.wnr.0000234743.50442.e5. [DOI] [PubMed] [Google Scholar]
- Miu AC, Heilman RM, Opre A, Miclea M. Emotion-induced retrograde amnesia and trait anxiety. Journal of Experimental Psychology: Learning Memory and Cognition. 2005;31:1250–1257. doi: 10.1037/0278-7393.31.6.1250. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology: General. 2000;129:242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Reinitz MT, Hannigan SL. Effects of simultaneous stimulus presentation and attention switching on memory conjunction errors. Journal of Memory and Language. 2001;44:206–219. [Google Scholar]
- Reinitz MT, Hannigan SL. False memories for compound words: Role of working memory. Memory & Cognition. 2004;32:463–473. doi: 10.3758/bf03195839. [DOI] [PubMed] [Google Scholar]
- Rosler A, Ulrich C, Billino J, Sterzer P, Weidauer S, Bernhardt T, Steinmetz H, Frohich L, Kleinschmidt A. Effects of arousing emotional scenes on the distribution of visuospatial attention: changes with aging and early subcortical vascular dementia. Journal of the Neurological Sciences. 2005;229–30:109–116. doi: 10.1016/j.jns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Runcie D, O’Bannon RM. An independence of induced amnesia and emotional response. American Journal of Psychology. 1977;90:55–61. [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biological Psychology. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Schimmack U. Attentional interference effects of emotional pictures: Threat, negativity, or arousal? Emotion. 2005;5:55–66. doi: 10.1037/1528-3542.5.1.55. [DOI] [PubMed] [Google Scholar]
- Schmidt SR. Outstanding memories: The positive and negative effects of nudes on memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:353–361. doi: 10.1037//0278-7393.28.2.353. [DOI] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. Feature binding, attention and object perception. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1998;353:1295–1306. doi: 10.1098/rstb.1998.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A, Gelade G. A feature integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Zeelenberg R, Wagenmakers EJ, Rotteveel M. The impact of emotion on perception. Psychological Science. 2006;17:287–291. doi: 10.1111/j.1467-9280.2006.01700.x. [DOI] [PubMed] [Google Scholar]