Abstract
The lin-12/Notch signaling pathway is conserved from worms to humans and is a master regulator of metazoan development. Here, we demonstrate that lin-12/Notch gain-of-function (gf) animals display precocious alae at the L4 larval stage with a significant increase in let-7 expression levels. Furthermore, lin-12(gf) animals display a precocious and higher level of let-7 gfp transgene expression in seam cells at L3 stage. Interestingly, lin-12(gf) mutant rescued the lethal phenotype of let-7 mutants similar to other known heterochronic mutants. We propose that lin-12/Notch signaling pathway functions in late developmental timing, upstream of or in parallel to the let-7 heterochronic pathway. Importantly, the human microRNA let-7a was also up-regulated in various human cell lines in response to Notch1 activation, suggesting an evolutionarily-conserved cross-talk between let-7 and the canonical lin-12/Notch signaling pathway.
Keywords: Heterochronic genes, developmental timing, lin-12/Notch, let-7, lin-41, microRNA molting, cell fate, seam cells
Introduction
MicroRNAs are single strand non-coding RNAs of ~22 nucleotides that function as regulators of gene expression and are found in viruses, fungi, plants and animals [1–3]. In animals, microRNAs bind to complementary sequences within the 3′ untranslated regions (UTRs) of their target mRNAs leading to translational repression and mRNA destabilization [4]. Importantly, these new class of regulators have been implicated in stem cell differentiation, organ development and oncogenesis [5]. The molecular mechanisms regulating microRNA expression and function are therefore of great interest in understanding both normal and abnormal biological pathways.
The evolutionarily-conserved microRNA genes lin-4 and let-7 play key roles in the temporal control of larval developmental in C. elegans [6]. lin-4 functions in early (L1 to L2) larval developmental decisions, while let-7 functions as a core regulator of the late (L4-to-Adult) larval developmental pathway [7, 8]. In wild-type animals, let-7 is temporarily expressed in seam cells (hypodermal skin cells) at the L4 larval stage (L3 molt) and negatively regulates lin-41, which encodes a RING-finger B-box Coiled-coil-containing protein [9, 10]. The down-regulation of the LIN-41 protein levels releases the repression on the adult-specific transcription factor LIN-29. Upon LIN-29 activation, seam cells exit the cell cycle, fuse and secrete a cuticular structure known as alae [11]. Furthermore, LIN-29 promotes the synthesis of adult cuticle and cessation of molting [12, 13]. Loss-of-function mutations in lin-41 result in a precocious alae phenotype. In this situation, seam cells prematurely adopt the adult fate and secrete alae during the L3 molt. Conversely, mutations in let-7 or lin-29 [9, 13] cause a retarded phenotype where seam cells continue to proliferate and fail to adopt the adult cell fate at the adult stage (L4 molt). Although the role of let-7 has been well established in C. elegans, its regulation in both C. elegans and mammals is poorly understood.
The canonical lin-12/Notch signaling pathway controls a broad spectrum of cell fate decisions and developmental processes in diverse metazoans [14]. In C. elegans, LIN-12 is involved in cell fate specification of the vulval precursor cells, sex myoblasts and Π cells during the hermaphrodite gonad development [15]. The LIN-12/Notch receptor is activated upon binding to the DSL (Delta/Seratte/Lag-2) family of ligands on the surface of adjacent signal-sending cells. Ligand binding leads to cleavage of the extra-cellular domain of the receptor by the ADAM family proteases generating the membrane tethered intracellular domain (ICN). The ICN is subsequently cleaved from the membrane by the γ-secretase complex and translocated to the nucleus to interact with CSL (CBF1, Human/Su(H) Drosophila/Lag-1 C. elegans), the major downstream effector of Notch signaling, to regulate transcription of specific target genes [14]. In this report, we demonstrate that lin-12/Notch gain-of-function (gf) animals display precocious alae at the L4 larval stage with a significant increase in let-7 expression levels. Our genetic and molecular data suggests that lin-12/Notch can positively regulate the let-7 in the heterochronic pathway in C. elegans. Furthermore, we provide evidence that activated human Notch1 also increases the levels of let-7a microRNA in human cell lines, suggesting an evolutionarily conserved cross-talk between let-7 and Notch receptors in metazoans.
Materials and Methods
C. elegans culture and strains
C. elegans strains were maintained on NGM plates with OP50 as described [30]. The following strains were used in this study: N2 (wild-type), lin-12(n137gf), lin-12(n137n460gf), lin-12(n472gf), lin-12(n302gf), lin-12(n676n930lf), lin-12(n676gf), let-7(n2853lf), let-7(mn112); unc-3(e151), lin-41(ma104lf), lin-29(n333lf), lag-1(om13lf), wls78 and ZaEx5. The double mutants: let-7(n2853lf); lin-12(n137n460gf), let-7(mn112null); lin-12(n137n460gf), lin-41(ma104lf); lin-12(n137n460gf), lin-29(n333lf); lin-12(n137n460gf) and lin-12(427gf); lag-1(om13lf). Details on double mutant crosses are upon request.
Phenotype analysis
The precocious and retarded alae phenotypes were observed in L4 stages using Nomarski optics. The different developmental stages were determined by stage-specific gonad morphology [11] and by specific stage developmental markers: col-19, col-17 and col-3 [31]. To visualize seam cell junctions and nuclei, we used an integrated array (wIs78) containing both ajm-1::gfp and scm-1::gfp.
Northern analysis
Total RNA was extracted from synchronized cultures of worms or sub-confluent cultures of human cell lines using the Trizol reagent (Invitrogen). The small RNAs were resolved on pre-cast 15% Urea-TBE gels (Bio-Rad) for 1h and transferred to Zeta-probe blotting membrane (Bio-Rad) overnight using a Bio-Rad Criterion blotter. After transfer, the membranes were air-dried, UV cross-linked (1200J) and baked for 1h at 80°C. Blots were pre-hybridized in 50 ml hybridization solution (5X SCC, 20mM NaH2PO4, 7% SDS, 1X Denhardt’s Solution) for 1 hour and subsequently probed overnight with ∝-32P labeled let-7 probe (enhanced with LNA, Exiqon) with a probe concentration of 106 cpm/ml. The C. elegans let-7 and human let-7a detection utilized the same probe sequence 5′-aactatacaacctactacctca-3′ and for loading control we used the U6 probe 5′ cacgaatttgcgtgtcatcctt-3′. Prior to film exposure, the blots were washed twice for 30 min each in 6X SCC, 20X Denhardt’s Solution, 10% SDS, 50 mM NaH2PO4 and twice for 10 minutes each in 1X SCC, 1% SDS.
qRT-PCR and RT-PCR
The quantitative real-time PCR (qRT-PCR) experiments were performed by the TaqMan method and the Stratagene MX3000P QPCR detection system. The fold-change in lin-41 mRNA levels was calculated using the ΔΔCt method and normalized to eft-2 loading control as described [4]. Standard RT-PCR experiments were performed using the MMLV Reverse Transcriptase kit (Stratagene) followed by PCR with primers that span the 3′-UTR of the target gene as described [4].
Cell Culture
The human embryonic kidney cell Hek293T and the mammary epithelial cell (MEC) lines 16A5 and 76NTert were used in our study. 16A5 and 76NTert cell lines are HPV-E6/E7 and hTert-immortalized, respectively, derivatives of 76N normal MEC line established from a reduction mammoplasty [32]. Mammary cells were cultured in the DFCI-1 medium supplemented with 12.5 ng/ml EGF and 293T cells were cultured in Dulbecco’s Modified Essential Medium supplemented with 5 % fetal bovine serum.
Ectopic Notch expression
Intracellular region of human Notch-1 (ICN1) cloned in a retroviral vector (MIGR1) was kindly provided by Dr. Warren Pear (University of Pennsylvania). Retroviral supernatants were generated by transfecting 10ug of the MIGR1-ICN1 or control vector along with the pIK packaging vector into tsa54 packaging cells (CellGenesys) [33] using the calcium phosphate method. Viral supernatants were collected, cleared through a 45um cellulose filter and freshly applied to proliferating target cells during two serial incubations as previously described [34]. Aliquots of cells were processed for RNA extraction (see Northern blotting) or for protein extraction and Western blot.
Western blot analysis
Cells were lysed in 3% SDS, 50mmol/L Tris-HCl, pH 7.4. Aliquots of 100 ug protein were measured using the bicinchonic acid protein assay kit (Pierce), separated on SDS-PAGE gels, transferred to a PVDF membrane (PerkinElmer) and blotted with a rabbit anti-human Notch 1 cleaved N terminal polyclonal antibody (100-401-407; Rockland) or anti-beta-actin mouse monoclonal antibody (Sigma- Aldrich) as loading control. Blots were incubated with the primary antibody for 1h at room temperature and washed 3 times in Tween20-TBS buffer. Appropriate HRP-labeled secondary antibodies were applied for 1h and washed again in Tween20-TBS buffer. Signals were developed using the Western Lightning Chemiluminescence Reagent Plus Kit (PerkinElmer). The specificity of anti-Notch antibody was verified by competition with the specific control peptide (Rockland, data not shown).
Results and discussion
lin-12/Notch genetically interacts with the heterochronic gene lin-41
During an RNAi screen for potential regulators of lin-12/Notch in C. elegans (Liu, Solomon & Band, unpublished), we observed a molting defect (Mlt) phenotype associated with increased lin-12/Notch activity (Fig 1A). We found that the gain-of-function (gf) temperature sensitive lin-12(n137n460gf) animals display an intriguing Mlt phenotype at all developmental stages at 15°C (total 18%, n=248) (Fig 1A). Temperature shift experiments with lin-12(n137n460gf) animals confirmed that Mlt is associated with increased lin-12/Notch activity same as the multi-vulva (Muv) phenotype described by Greenwald and colleagues [16]. A significant suppression of both Mlt and Muv phenotypes was observed in these worms when shifted to 25°C: ~2% Mlt, (n=675) and 6.3% Muv (n=64) vs. 18% Mlt and 100% Muv at 15°C (n>100) (P<0.05). We could not detect any Mlt defects in a loss-of-function and null mutant strain of lin-12 (Table 1 and data not shown).
Fig 1.
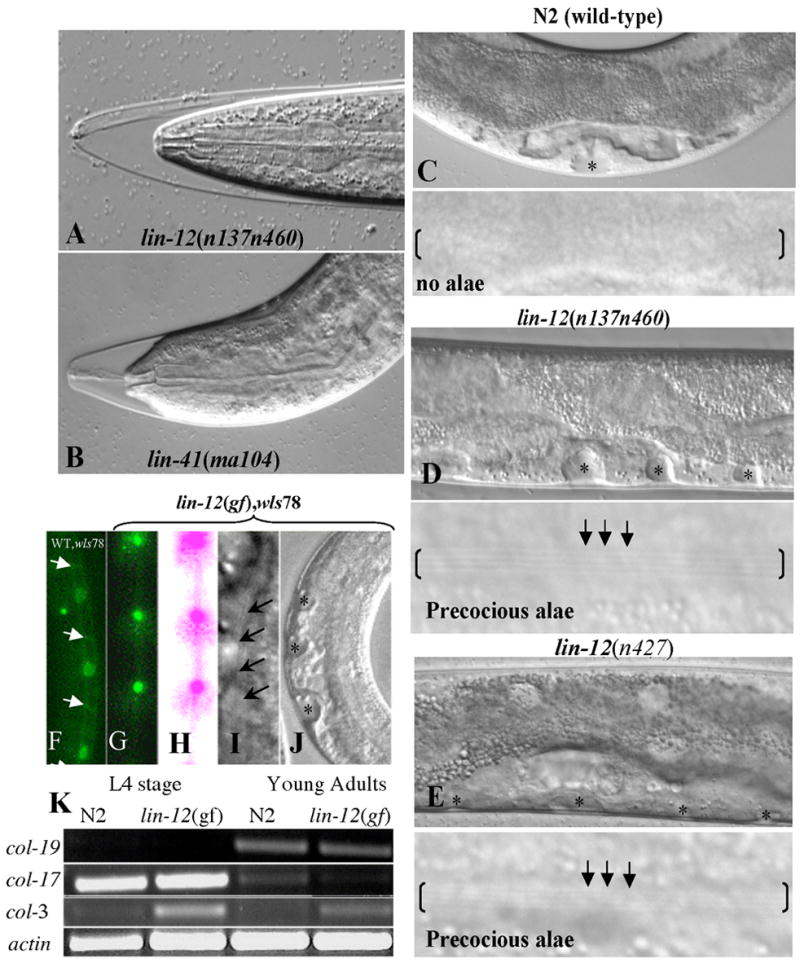
Activated lin-12/Notch mutants display precocious alae and seam cell phenotypes. A and B. The lin-12(n137n460gf) gain-of-function (A) and lin-41(ma104lf) loss-of-function (B) animals displaying molt (Mlt) phenotype at the transition from L4 to adult stage. C-E. A wild-type animal (N2 strain) (C) at the mid-late L4 stage with no alae (i.e., normal development) in contrast to lin-12(n137n460gf) (D) or lin-12(n427gf) mutants (E) at the L4 stage with vulva invagination (black asterisks) and precocious alae (black arrows and parenthesis). F. An N2, wIs78 animal expressing AJM-1::GFP (adherens junction marker; white arrows) and SCM::GFP (in seam cell nuclei) depicting un-fused seam cells at the L4 stage. G. A lin-12(n137n460gf), wIs78 animal expressing AJM-1::GFP and SCM::GFP demonstrating a fused seam cell phenotype at mid L4 stage with no obvious cell-cell junctions as indicated by the lack of junctional GFP signal. The fused seam cells visualized with GFP are shown in green (G) or after pseudo-coloring (pink) (H). I-J. lin-12(n137n460gf) L4 stage larvae displaying multiple invaginations of the forming multi-vulva and precocious alae (arrows). K. RT-PCR of developmental stage-specific collagen transcripts at L4 vs. adult stage in N2 (wild-type) and lin-12(n137n460gf) worms. The adult-specific col-19 marker [31] is highly expressed at the adult stage in both N2 and lin-12(gf) worms but is nearly undetectable at L4 stage. In contrast, L4-specific col-17 marker shows a reciprocal expression pattern. col-3 is expressed at all developmental stages but is higher at L4 and lower in adults [31]. All experiments were done at 15°C.
Table 1.
Precocious phenotype of activated lin-12 gain-of-function (gf) mutant worms and interactions with the let-7 heterochronic pathway components.
| % of animals with adult lateral alae | ||||
|---|---|---|---|---|
| Genotype | L4 larva (L3 molt) | Adult (L4 molt) | Alae phenotype | Other phenotypes |
| N2 (wild-type) | 0% (48)* | 100 % (56) | Normal | - |
| lin-12(n137gf) | 35% (60) | 100% (30) | Precocious | Muva, Eglb |
| lin-12(n137n460gf) | 54% (106) | 100% (37) | Precocious | Mltc, Muv, Egl Dpyd |
| lin-12(n427gf) | 51 % (80) | 100% (29) | Precocious | Muv, Egl |
| Lin-12(427gf), lag-1(om13lf) | 0% (17) | 100% (20) | Normal | Egl, Embe, Lvaf |
| lin-12(n676gf) | 30% (40) | 100 % (33) | Precocious | Egl |
| lin-12(n302gf) | 29% (31) | 100% (10) | Precocious | Egl |
| lin-12(n676n930lf) | 0% (30) | 100% (10) | Normal | Egl, Vulg |
|
| ||||
| let-7(n2853lf) | 0% (20) | 0% (20) | Retarded | Mlt, Egl |
| let-7(mn112null); unc-3(e151lf) | 0% (20) | 0% (20) | Retarded | Mlt, Let |
| lin-41(ma104lf) | 46% (57) | 100% (25) | Precocious | Mlt, Egl, Dpy |
| lin-29(n333lf) | 0% (15) | 0% (31) | Retarded | Mlt, Egl, |
|
| ||||
| let-7(n2853lf); lin-12(n137n460gf) | 0% (20) | 0% (20) | Retarded | Mlt, Muv, Egl |
| let-7(mn112null); lin-12(n137n460gf) | 0% (16) | 0% (25) | Retarded | Mlt, Muv, Egl |
| lin-41(ma104lf); lin-12(n137n460gf) | 50% (32) | 100% (16) | Precocious | Mlt, Muv, Egl |
| lin-29(n333lf); lin-12(n137n460gf) | 0% (30) | 0% (25) | Retarded | Mlt, Muv, Egl |
Number animals scored.
Multi-vulva,
Egg-laying defect,
Molting defect,
Dumpy,
Embryonic lethal,
Larval arrest,
Vulvaless.
In the RNAi screen (Liu, Solomon & Band, unpublished), we identified the heterochronic gene lin-41, which encodes a RING-finger, B-box and Coiled-Coil (RBCC) protein. We found that reduction of lin-41 mRNA levels in lin-12(n137n460gf) animals by RNAi feeding enhanced the Mlt phenotype (from 18% to 44% Mlt at 15°C, n=760), without affecting the Muv phenotype (100% Muv). Notably, the Mlt defect occurred predominantly at the L4 to adult transition and most of the Mlt animals were trapped in their old cuticle. A similar but weaker defect was observed in lin-41(ma104) (13% Mlt, n=54) (Fig 1B) and in wild-type animals fed with lin-41 RNAi construct (5% Mlt, n>100). The enhanced Mlt phenotype observed in lin-12(n137n460gf); lin-41(RNAi) animals suggested that lin-12 and lin-41 may cooperate to regulate molting.
Activated lin-12/Notch mutants display heterochronic defects
Molting defects were previously reported in retarded and precocious heterochronic pathway mutants [11], and recently let-7 was shown to be involved in the regulation of molting [17]. Therefore, based on the genetic interaction observed between lin-12 and lin-41, and the fact that lin-41 is a target of let-7, we hypothesized that Mlt and heterochronic defects may be linked. To determine whether lin-12/Notch gf animals display heterochronic defects, we analyzed several lin-12/Notch (gf) mutants and found that they indeed displayed a phenotype of precocious alae and fused seam cells at the L3 molt phase (Fig 1 and Table 1). To verify the identity of the different developmental stages analyzed, we performed RT-PCR for developmental stage-specific markers and examined the gonad morphology, and found that they indeed correspond to the appropriate developmental stages (Fig 1K and data not shown). We also analyzed a partial loss-of-function strain and null mutant strain of lin-12 and could not detect any retarded developmental phenotypes in these animals (Table 1 and data not shown). To address the possibility that our negative results with loss-of-function and null animals reflect redundant roles of the two Notch receptors, lin-12 and glp-1, we depleted the glp-1 mRNA from lin-12(null) and lin-12(lf) animals. Unfortunately, we could not analyze the F1 progeny because of larval arrest and/or embryonic lethality. Alternatively, our results could be explained by a redundant role of lin-12 in development timing in C. elegans. However, it is possible that the observed effects represent an aberrant effect of gain of function lin-12 alleles and that normal lin-12does not play a physiological role in developmental timing.
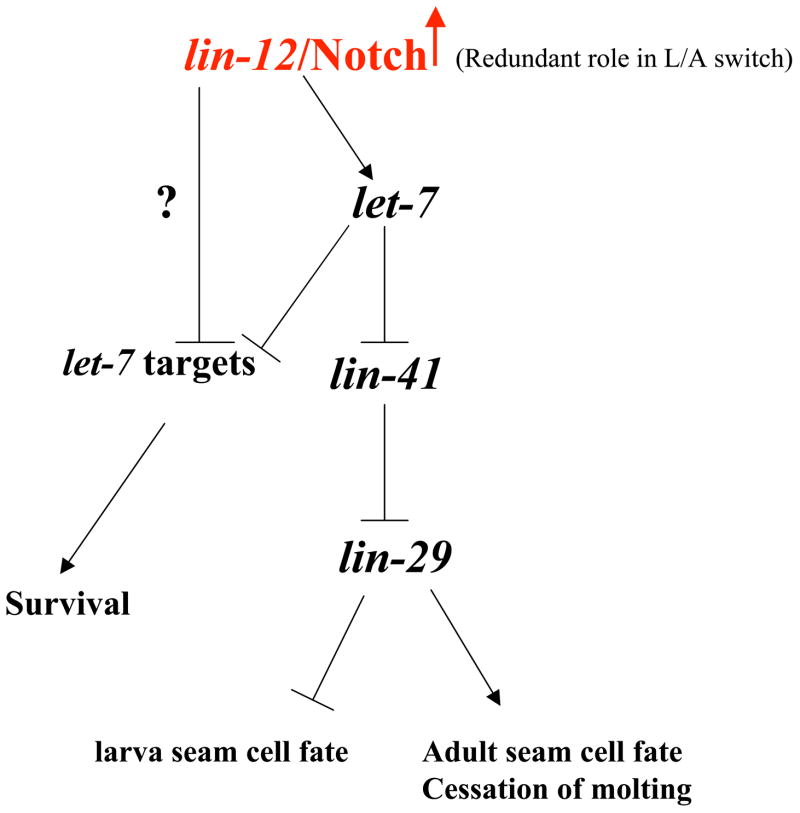
Next, we examined if lin-12 and let-7 pathways interact genetically. For this analysis, we used the lin-12(gf) strain lin-12(n137n460gf) since it showed the most penetrant Mlt and heterochronic defects (Table 1). Our results demonstrate that the precocious alae in the L4 stage observed in lin-12(gf) animals were fully suppressed in null and loss-of-function mutant backgrounds of let-7 and lin-29, respectively (Table 1). In addition, the precocious alae phenotype in double mutant lin-12(n137n460gf); lin-41(ma104) was comparable to that in the single mutant lin-12(n137n460gf) (Table 1). Our result indicates that lin-41 may act downstream of lin-12 in regulating alae formation. Taken together, we propose a genetic model wherein lin-12 functions upstream of, or in parallel to, the let-7 late larval development pathway in C. elegans (Fig. 5). Furthermore, our data suggest that lin-12/Notch might regulate let-7 expression in C. elegans
Fig 5.
The proposed genetic model for lin-12/Notch in regulating larva-to-adult switch (L/A) in C. elegans. We propose that lin-12/Notch positively regulates the let-7 microRNA in a non-cell autonomous manner in the L4 stage to execute the L/A switch. Temporal up-regulation of let-7 will ensure the proper down-regulation of lin-41 mRNA and activation of the transcription factor lin-29. Upon LIN-29 activation, seam cells will exit the cell cycle and will differentiate into adult seam cells. In this final molt phase (L4), an adult cuticle and alae are formed, and molting cycle ceases. In addition, we propose that rescue of lethality in let-7 mutants by activated lin-12/Notch suggests the possibility that lin-12/Notch may also regulate other let-7 targets independent of the let-7 microRNA.
lin-12/Notch positively regulates the expression of let-7
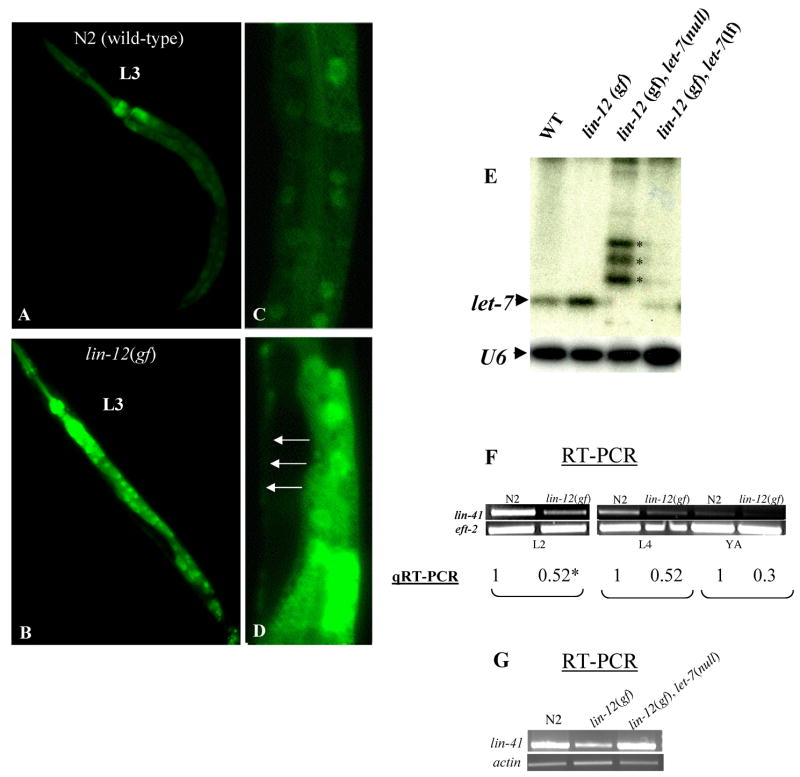
To determine whether lin-12/Notch regulates let-7 expression, we generated the lin-12(n137n460gf); zaEx5 strain carrying a reporter construct for let-7 expression (plet-7::GFP) and analyzed GFP expression levels at different developmental stages. This strain exhibited precocious GFP expression in seam cells as well as enhanced GFP expression in the intestine at the L3 stage (90%, n=67) (Fig. 2). On the other hand, wild-type animals never showed GFP expression in seam cells before the L3 to L4 transition, as reported previously [18]. A brighter GFP signal was also detected in lin-12(n137n460gf); zaEx5 L4 and adult animals in hypodermal and intestinal cells (80–90%, n>100, and data not shown). In addition to the in vivo observations, Northern blot analysis demonstrated a 2-fold increase in mature let-7 microRNA levels in lin-12(gf) compared to wild-type worms (Fig 2E). Because of the negative regulatory role of let-7 on lin-41 mRNA levels [4], we expected lower levels of lin-41 mRNA in lin-12(gf) worms. Indeed we found a substantial reduction in lin-41 mRNA levels in L2, L4 and young adults stages in lin-lin-12(gf) worms, and this phenotype was reversed in the let-7 null background (Fig 2G). The lower lin-41 mRNA level in lin-12(gf) animals is expected to lead to a higher level of LIN-29 activity to specify premature larva to adult transition. Taken together, our results provide evidence that lin-12/Notch may negatively regulates lin-41 via the up-regulation of the let-7 microRNA.
Fig. 2.
Up-regulation of let-7 microRNA expression in lin-12/Notch gain-of-function mutant worms. A–D. N2, plet-7::GFP reporter animals at L3 stage display weak GFP expression in intestinal cells with undetectable GFP in seam cells (A and C). In contrast, lin-12(n137n460gf), plet-7::GFP worms display brighter GFP signals in intestinal cells and precocious GFP signals in seam cells (B, D) (white arrows). C-D. C and D are higher magnification of mid-body section of worms depicted in A and C respectively. E. Elevated let-7 microRNA expression in lin-12/Notch gf worms. Total RNA was isolated form young adult animals of the N2 strain, or lin-12(n137n460gf), the doubles lin-12(n137n460lf);let-7(mn112null) and lin-12(n137n460gf);let-7(n2853lf) mutants, and subjected to Northern blot analysis for let-7 microRNA and U6 (loading control). Note that let-7 expression is 2–3-fold higher in lin-12(gf) single mutants compared to wild-type animals but weak or undetectable in let-7(n2853) and let-7(mn112) null mutant background, respectively. The identity of the bands above the mature let-7 microRNA (see asterisks) present in lanes 3 and 4 is unknown. F. RT-PCR (upper line) and real-time PCR (qRT-PCR) (lower line) quantification of the relative levels of lin-41 mRNA in lin-12(gf) worms at L2, L4 and young adults (YA) stages in comparison to wild-type animals. The average fold-change in mRNA levels (see experimental procedure for qRT-PCR analyses) in each developmental stage is marked with an asterisk. G. RT-PCR analysis to demonstrate that the observed reduction of lin-41 mRNA levels in the lin-12(n137n460gf) animals is reversed in a let-7 null mutant background.
To determine if let-7 regulation by lin-12/Notch is cell-autonomous, we analyzed LIN-12 expression in seam cell using a strain carrying a functional lin-12::LacZ fusion construct [19]. However, we were unable to detect LacZ staining in seam cells although we observed the typical staining of vulva precursor cells and sheath cells around the tip of the gonad (data not shown). Our inability to detect the expression of LIN-12 in seam cells is likely to be due to either a very low level of expression and/or short temporal expression of LIN-12 in these cells, as is the case in certain other tissues types and cells known to require LIN-12 activity such as rectum, anal depressor muscle cells, rectal sphincter cells and some L1 intestinal cells [20].
Heterochronic phenotypes in lin-12/Notch gain-of-function mutants are lag-1 dependent
To determine if the canonical lin-12 signaling pathway is involved in late larval developmental timing in C. elegans, we tested whether the precocious alae in lin-12(gf) worms are dependent on lag-1. LAG-1 is a conserved transcription cofactor that serves as a major effector of lin-12 signaling [23]. We generated the lin-12(n427gf);lag-1(om13) and lin-12(n137n460gf);lag-1(om13) double mutants and found that both show a very slow growth rate with embryonic and larval arrest. Since the n137n460 allele showed a higher penetrance of embryonic lethality, we analyzed the lin-12(n427gf);lag-1(om13) mutant and found a complete suppression of the Muv and the precocious alae phenotypes but not of the Egl phenotype (Table 1). These results suggest that lin-12/Notch act in a canonical lag-1 dependent manner to control alae formation in C. elegans.
The lethality phenotype of let-7 mutants is suppressed by lin-12/Notch gf mutant
The let-7 microRNA is essential in C. elegans and the let-7(mn112) null homozygous animals die by a characteristic bursting of the vulva [8]. A similar phenotype was also described for the temperature sensitive let-7(n2853) mutant [8]. Previous studies have shown that the lethality of let-7 mutants can be rescued by crossing with specific let-7 target gene mutants, such as lin-41, hbl-1, let-60/Ras and daf-12 [8, 10]. To our surprise, we found that lin-12(n137n460gf) mutant rescued the lethality phenotypes of let-7(n2853) and let-7(mn112) mutants (Fig 3 and Table 2). The previous studies [8, 10] suggest that dysregulated expression of let-7 target genes contributes to the lethal and sterile phenotypes observed in let-7 mutants. Rescue of lethality in let-7 mutants by activated lin-12 suggests the possibility that lin-12 may also regulate let-7 targets independent of let-7 gene product (Table 2). This hypothesis can explain the rescue of the let-7 mutants and it is currently under investigation.
Fig. 3.
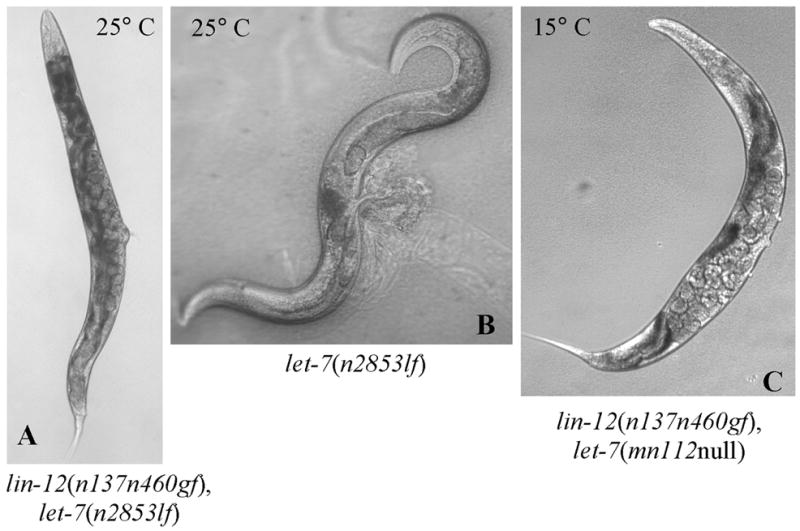
lin-12(n137n460gf) mutant suppresses the lethality and sterility phenotypes of let-7 mutations. A–B. lin-12(n137n460gf); let-7(n2853lf) Egl double mutant (A) with fertilized eggs and progeny in contrast to let-7(n2853lf) animals which die as a result of characteristic bursting of the vulva at 25°C (B). C. A viable lin-12(n137n460gf), let-7(nmn112) null animal showing an Egl phenotype with progeny developing within the parental worm at 15°C, and also at 25°C (see table 2).
Table 2.
Activated lin-12/Notch mutant suppresses the lethality and sterility phenotypes of let-7 mutations
| Genotype | No. of progeny a | Fertile wormsb |
|---|---|---|
| N2 (wild-type) | 280±12 | 100 % |
| lin-12(n137n460gf) | 21±2 | 100 % |
| let-7(n2853lf) | 0 | 2–5 % |
| lin-12(n137n460gf), let-7(n2853lf) | 23±3 | 100 % |
| let-7(nm112null), unc-3(e151) | 0 | 2–5 % |
| lin-12(n137n460gf), let-7(nm112), unc-3(e151) | 32±3 | 100 % |
|
| ||
| lin-41(ma104lf) | 30c | 100 %c |
| lin-41(ma104lf), let-7(nm112), unc-3(e151) | 19 c | 95 % c |
Average number of live progeny from ten adults worms at 25°C.
Percent of worms (n >100) with fertile eggs scored at 25°C.
From Slack et al. Molecular cell, 5 659–669, 2000.
Activated Notch1 regulates the let-7a microRNA in human cells
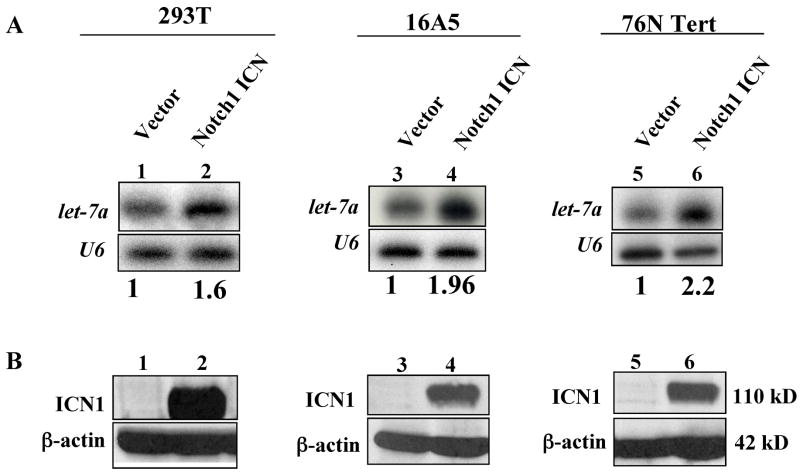
The fact that lin-12/Notch regulates seam cell fate via a conserved transcription cofactor (lag-1) and a phylogentically conserved microRNA (let-7), raises the possibility that a crosstalk between Notch receptors and the let-7 microRNA might be conserved in higher metazoans. To test if let-7 regulation by Notch receptor is conserved in humans, we introduced the intracellular domain (ICN) of mammalian Notch1 into human cell lines and analyzed the effect on human let-7a expression. Over-expression of Notch1 ICN resulted in a ~2 fold increase in let-7a RNA levels in the human embryonic kidney cell line 293T, and the immortalized human mammary epithelial cell lines 16A5 and 76NTert (Fig 4). These results suggest a conserved regulation of let-7 by lin-12/Notch receptors across evolution. Notch signaling regulates important cell fate decisions in mammals, including stem cell maintenance, cell differentiation and proliferation, and dysregulation of Notch pathway is strongly associated with cancer. For example, activating mutations of Notch1 are found in more than 50 % of human T cell acute lymphoblastic leukemia [24], and activated ICN is found at high levels in breast cancer cell lines and tissues [25]. On the other hand, in an animal model, Nicolas et al. [26] demonstrated that Notch1 functions as a tumor suppressor in mammalian skin cells. Specifically, Notch1 deficiency in mouse epidermis and primary keratinocytes resulted in basal-cell carcinomas. It was also suggested that let-7 may function as a tumor suppressor in human lung cancer by down-regulating the expression of Ras [27]. In addition, let-7 was shown to inhibit cell growth of human lung adenocarcinoma and colon cancer cell lines [28, 29]. Thus, it is conceivable that the novel Notch-let-7 axis defined here could play an important role in cell fate determination in higher organisms.
Fig 4.
Overexpression of activated Notch1 up-regulates the let-7a microRNA expression in human epithelial cell lines. A. The indicated cell lines were retrovirally transduced with intracellular domain of Notch1 (Notch1 ICN) or control vector and total RNA isolated from subconfluent cell monolayers followed by Northern analyses of the let-7a mature microRNA expression. The autoradiograms were scanned and the intensity of bands quantified using the LabWork™ imaging analysis software (UVP). The relative let-7a expression in ICN-over-expressing cells relative to their vector controls are shown below the lanes. A comparable RNA loading between control and ICN lanes is shown by U6. The results are representative of two independent experiments. B. Western blot analysis of Notch1 ICN and β-actin (loading control) proteins in aliquots of the cell samples used for Northern blotting in A.
Conclusions
The role of the canonical lin-12/Notch signaling pathway in the regulation of cell fates, cell proliferation and cell death during development has been well established. Our study revealed that activated lin-12/Notch alleles can affect late larval development timing in C. elegans by upregulating the let-7 microRNA expression. We were not able to answer the question whether lin-12/Notch has a normal role in developmental timing because of lethality issues, and potentially redundant roles of the two Notch receptors (lin-12 and glp-1). However, the observations that activated lin-12 mutants display precocious alae at L4 larval stage with a precocious and elevated expression of let-7, and the ability to rescue the lethal phenotype of let-7 mutations with lin-12 gf mutants collectively suggest that lin-12/Notch has a redundant role in developmental timing in C. elegans. Our finding that activated human Notch1 can regulate let-7a expression in human cell lines suggests an evolutionarily-conserved cross-talk between let-7 and the canonical lin-12/Notch signaling pathway. Future studies aimed at understanding the molecular mechanisms by which lin-12/Notch signaling regulates let-7 could provide mechanistic insights relevant to developmental biology and may help understand the mechanisms of cellular transformation and tumor progression in higher organisms.
Acknowledgments
This work was supported by: the NIH Grants CA116552, CA87986, CA76118, CA99900 and CA99163 to H.B., and CA94143, CA96844 and CA81076 to V.B.; DOD Breast Cancer Research Grants DAMD17-02-1-0303 (H.B.) and DAMD17-02-1-0508 (V.B.); the NCI Cancer Center of Nanotechnology Excellence Grant NCI 1U54 CA119341-01 (H.B. and V.B.); the Jean Ruggles-Romoser Chair of Cancer Research (H.B.), the Duckworth Family Chair of Breast Cancer Research (V.B.) and an ENH Institutional Research Career Development Award (M.N.). G.C.B. is a recipient of DOD Breast Cancer Research trainee award. We thank Dr. Warren Pear for Notch1 ICN expression constructs and Dr. Frank Slack, Dr. Amy Pasquinelli and Dr. Iva Greenwald for protocols, strains and plasmids. We thank Drs. Greg Beitel, Susanna Garcia, Lei Duan and Srikumar Raja for helpful suggestions and discussion. We thank Theresa Stiernagle and the Caenorhabditis Genetic Center for strains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasquinelli AE, McCoy A, Jimenez E, Salo E, Ruvkun G, Martindale MQ, Baguna J. Expression of the 22 nucleotide let-7 heterochronic RNA throughout the Metazoa: a role in life history evolution? Evol Dev. 2003;5:372–378. doi: 10.1046/j.1525-142x.2003.03044.x. [DOI] [PubMed] [Google Scholar]
- 2.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer S, Voinnet O. Viruses, microRNAs and cancer. Oncogene. 2006;25:6211–6219. doi: 10.1038/sj.onc.1209915. [DOI] [PubMed] [Google Scholar]
- 4.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210:279–289. doi: 10.1002/jcp.20869. [DOI] [PubMed] [Google Scholar]
- 6.Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell. 2001;1:453–465. doi: 10.1016/s1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 7.Abbott AL. Heterochronic genes. Curr Biol. 2003;13:R824–825. doi: 10.1016/j.cub.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 9.Lin SY, Johnson SM, Abraham M, Vella MC, Pasquinelli A, Gamberi C, Gottlieb E, Slack FJ. The C elegans hunchback homolog, hbl-1, controls temporal patterning and is a probable microRNA target. Dev Cell. 2003;4:639–650. doi: 10.1016/s1534-5807(03)00124-2. [DOI] [PubMed] [Google Scholar]
- 10.Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell. 2000;5:659–669. doi: 10.1016/s1097-2765(00)80245-2. [DOI] [PubMed] [Google Scholar]
- 11.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 12.Bettinger JC, Lee K, Rougvie AE. Stage-specific accumulation of the terminal differentiation factor LIN-29 during Caenorhabditis elegans development. Development. 1996;122:2517–2527. doi: 10.1242/dev.122.8.2517. [DOI] [PubMed] [Google Scholar]
- 13.Rougvie AE, Ambros V. The heterochronic gene lin-29 encodes a zinc finger protein that controls a terminal differentiation event in Caenorhabditis elegans. Development. 1995;121:2491–2500. doi: 10.1242/dev.121.8.2491. [DOI] [PubMed] [Google Scholar]
- 14.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- 16.Greenwald I, Seydoux G. Analysis of gain-of-function mutations of the lin-12 gene of Caenorhabditis elegans. Nature. 1990;346:197–199. doi: 10.1038/346197a0. [DOI] [PubMed] [Google Scholar]
- 17.Hayes GD, Frand AR, Ruvkun G. The mir-84 and let-7 paralogous microRNA genes of Caenorhabditis elegans direct the cessation of molting via the conserved nuclear hormone receptors NHR-23 and NHR-25. Development. 2006;133:4631–4641. doi: 10.1242/dev.02655. [DOI] [PubMed] [Google Scholar]
- 18.Johnson SM, Lin SY, Slack FJ. The time of appearance of the C. elegans let-7 microRNA is transcriptionally controlled utilizing a temporal regulatory element in its promoter. Dev Biol. 2003;259:364–379. doi: 10.1016/s0012-1606(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson HA, Greenwald I. Spatial and temporal patterns of lin-12 expression during C. elegans hermaphrodite development. Genetics. 1995;141:513–526. doi: 10.1093/genetics/141.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson HA, Fitzgerald K, Greenwald I. Reciprocal changes in expression of the receptor lin-12 and its ligand lag-2 prior to commitment in a C. elegans cell fate decision. Cell. 1994;79:1187–1198. doi: 10.1016/0092-8674(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 21.Lopez SL, Paganelli AR, Siri MV, Ocana OH, Franco PG, Carrasco AE. Notch activates sonic hedgehog and both are involved in the specification of dorsal midline cell-fates in Xenopus. Development. 2003;130:2225–2238. doi: 10.1242/dev.00443. [DOI] [PubMed] [Google Scholar]
- 22.Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133:1871–1880. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen S, Kodoyianni V, Bosenberg M, Friedman L, Kimble J. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H) Development. 1996;122:1373–1383. doi: 10.1242/dev.122.5.1373. [DOI] [PubMed] [Google Scholar]
- 24.Weng AP, Ferrando AA, Lee W, Morris JP, 4th, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 25.Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- 26.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 27.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull. 2006;29:903–906. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 29.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 30.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Kirch S, Ambros V. The Caenorhabditis elegans heterochronic gene pathway controls stage-specific transcription of collagen genes. Development. 1995;121:2471–2478. doi: 10.1242/dev.121.8.2471. [DOI] [PubMed] [Google Scholar]
- 32.Band V, Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989;86:1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimri GP, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol Cell Biol. 2000;20:273–285. doi: 10.1128/mcb.20.1.273-285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]