Abstract
Background
Angiotensin-II (Ang-II) contributes to cardiac remodeling and left ventricular dysfunction. In contrast, exercise may have beneficial effects on left ventricular structure and function.
Methods and Results
We investigated the effects of low-intensity exercise training (ET) on in vivo cardiac function in hypertensive TG (mREN-2)27 rats (Ren-2) which develop left ventricular hypertrophy and dysfunction. Ren-2 rats and Sprague Dawley (SD) controls (4–5 weeks) began treadmill exercise every day for 5–6 weeks. Cardiac function was evaluated by echocardiography. Cardiac output and stroke volume were increased by ET in both 8-wk-old SD and Ren-2. Slope of mitral deceleration time, a non-invasive measure of diastolic function, was lower in the Ren-2 rats, but not changed by ET. LV collagen deposition, as assessed by hydroxyproline assay, was not affected by rat strain or ET at 10–11 weeks of age. Left ventricular B-type natriuretic peptide mRNA levels were higher in the Ren-2 rats (100%), but not affected by ET. Both α (~14.5 fold) and β (~2.5 fold) myosin heavy chain mRNA were higher in the LV of Ren-2 rats (p < 0.05), but were not changed by ET.
Conclusion
Low-intensity exercise training in Ren-2 rats, a model of Ang-II-mediated hypertension, maintains cardiac index and stroke volume in the presence of impaired diastolic function at 8 wks of age.
Keywords: Hypertension, heart, exercise
Introduction
The renin- angiotensin system (RAS) is an important mediator of both hypertension and cardiac remodeling. The transgenic TG(mRen2)27 rat (Ren-2), overexpresses the murine renin gene and exhibits increased angiotensin II (Ang II) levels in multiple extrarenal tissues (1).; this allows for investigation of the impact of chronic increases of Ang- II in the heart .Further, this model offers a unique approach to study the interaction of this genetic alteration of tissue RAS with environmental factors such as exercise.
Previous studies suggest that impaired cardiac function and maladaptation occur within 11 to 13 weeks of age in the Ren-2 rat (3,5–8). Both systolic and diastolic rates of pressure change (dP/dT) are reduced in 13-wk old Ren-2 rats when compared to age-matched, control Sprague Dawley(SD) rats (14). Maladaptation, characterized by re-expression of the fetal gene program and cardiac fibrosis, is commonly associated in left ventricular cardiac dysfunction (8; 9; 15). Interestingly, treatment with an Ang II type 1 receptor (AT1) antagonist, blocked the development of cardiac hypertrophy, reduced the ratio of β:α myosin heavy chain (MyHC) mRNA, and altered the expression of the extracellular matrix components in Ren-2 rats by 17 weeks of age. Increased levels of physical activity in rats can also serve as sufficient treatment for reducing hypertension and hypertensive heart disease. For example, in spontaneously hypertensive rats (SHR), 18 weeks of low intensity treadmill exercise (16–20 m/min) lowered systolic (SBP), diastolic (DBP), and mean arterial (MAP) blood pressures and resting cardiac output and heart rate, while high-intensity training (25–30 m/min for 60 min, 5 times / week) had no effect (19). Thus, low-intensity exercise regulates resting blood pressure and subsequent cardiac function in these animals. Additionally, six weeks of voluntary wheel exercise has been shown to significantly lower resting SBP in the TG(mRen2)27 rat from 198±5 to 167±5 mmHg (sedentary vs. trained, respectively) (10). However, cardiac function following exercise training in a model with selective Ang II-mediated hypertension, such as the Ren2 rat, has not been investigated.
The present investigation was designed to determine whether treadmill-running at a low intensity would delay cardiac decompensation in the hypertensive, insulin resistant Ren 2 rat. The onset of exercise was initiated at a time when the Ren 2 is beginning to develop significant elevations in blood pressure (4). It was hypothesized that exercise training would attenuate increases in blood pressure and preserve normal cardiac output and diastolic function. In addition, left ventricular collagen deposition, levels of α- and β-myosin heavy chain (MyHC) mRNA, and B-type natriuretic peptide (BNP) mRNA were assessed to determine if exercise training would delay the appearance of markers associated with maladaptive cardiac hypertrophy.
Materials and Methods
Animal Care
Male TG(mRen2)27 rats and male Sprague-Dawley (SD) controls were received from Bowman Gray School of Medicine, Wake Forest University, Winston-Salem, NC at 3 wk of age. Animals were housed 2–3 per cage with a 12:12-h light-dark cycle in a room maintained at 20–22°C and had free access to rat chow and water. Nails were manicured as needed throughout the experiment in order to prevent potential foot injuries while running on the treadmill. Cardboard backstops were placed at the rear of the running belt, effectively promoting sufficient exercise without the need for electric shock, removing its effect on blood pressure. All experimental protocols were approved by the Animal Care and Use Committee at the Harry S. Truman Veterans Memorial Hospital, Columbia, MO.
Experimental Design
Rats were selected into a running group based upon acclimation to treadmill running and divided into 4 primary experimental groups including: Sedentary SD (SED-SD; n=5), Exercised SD (EX-SD; n=5), Sedentary TG(mRen2)27 (SED-Ren2; n=5), and Exercised TG(mRen2)27 (EX-Ren2; n=5). SD is the background strain from which Ren2 rats were developed (11). At 4 weeks of age, rats were familiarized with the treadmill for 2 weeks (pre-training) followed by 5–6 weeks of exercise training. Rats were run 7 days a week during both the pre-training and training days. Running time was increased during pre-training (2 weeks) until the rats were able to run consecutively for 60 min. During the 5-wk training program rats were given a 2-min daily warm-up and subsequently exercised for 60 min/day. Treadmill speed was progressively increased during this 5-week training period from 14.0 m/min up to 17.5 m/min.
Echocardiography
Rats were anesthetized with an intraperitoneal administration of ketamine (50 mg/kg) and diazepam (2.5 mg/kg). This drug combination has been previously shown to have minimal cardiorespiratory effects when compared to other suitable anesthetics (17). Echocardiography evaluated left ventricular systolic and diastolic function following the 3rd week of exercise training. The hair was removed from the left hemithorax using a depilatory cream to facilitate imaging. The rats were positioned in a right lateral decubitus position. Echocardiograms were performed using a GE Vivid 7 ultrasound system using a 10-mHz transducer and electrocardiographic monitoring. The echosonographer was blinded to the treatment groups. Two-dimensional imaging was used to guide pulsed-wave Doppler evaluation of mitral inflow velocities, and aortic outflow velocities from an apical 4-chamber orientation. M-mode echocardiography of was performed using the parasternal short-axis view of the left ventricle (LV). The guidelines of the American Society of Echocardiography were used for measurement of the LV end-diastolic and end-systolic diameters, and septal and posterior wall thickness. Fractional shortening, an index of contractility was determined from the measurements of LV chamber dimensions and calculated by LV internal diameter in diastole (LVIDd) – LV internal diameter in systole (LVIDs) / LVIDd. Images were captured digitally and a series of 6 consecutive cardiac cycles were measured and averaged for each individual measurement. Stroke volume was calculated using the following formula: aortic outflow velocity time integral × aortic valve cross sectional area. The aortic valve cross sectional area is calculated as follows: aortic diameter2 × 0.785. Cardiac output was calculated by multiplying stroke volume by heart rate. The cardiac index was calculated in order to normalize for the increase in cardiac output that occurs with changes in body size. Cardiac index was calculated as: cardiac output / body weight in grams. Rats were allowed to recuperate from the anesthetic for ~1 day before starting daily exercise again.
Blood pressure
SBP (mmHg) was measured in triplicate via tail cuff method in conscious rats before the daily exercise on a weekly basis using MC4000 blood pressure analysis system from Hatteras. Prior to the initial recorded blood pressures (week 1), rats were allowed to acclimate to the blood pressure system and measurements over several days. Tail cuff size was adjusted upward as needed for growth in tail diameter.
RNA isolation and cDNA synthesis
At 10–11 weeks of age, rats were deeply anesthetized and sacrificed. RNA was isolated from a section of the left ventricle using a method adapted from Chomczynski and Sacchi (3) and cDNA synthesis performed, as previously described by us (13).
Quantitative Real-time PCR
The quantitative PCR reactions were performed with an ABI 7000 Sequence Detection System (ABI) using Sybr Green chemistry (ABI), as previously described by us (13). All experimental samples were analyzed relative to GAPDH. The primer sequence used for rat B-type natriuretic peptide and rat GAPDH were as previously reported (18). (BNP Fw 5’ TGGGCAGAAGATAGACCGGA - 3′, BNP Rv 5′-ACAACCTCAGCCCGTCACAG - 3’; GAPDH Fw - 5′ TGCCAAGTATGATGACATCAAGAAG 3′, GAPDH Rv 5′ AGCCCAGGATGCCCTTTAGT 3′). Rat α- and β- MyHC primers also were constructed as per previous report (4) (Alpha Fw - 5′ TGTGAAAAGATTAACCGGAGTTTAAG 3′ , Alpha Rv - 5′ TCTGACTTGCGGAGGTATCG 3′ ; Beta Fw 5′ AAGTCCTCCCTCAAGCTCCTAAGT 3′, Beta Rv - 5′ TTGCTTTGCCTTTGCCC 3′). All sequences were verified using Primer Express 2.0 (ABI). PCR product formation was checked against a ladder for proper amplicon size using PCR Supermix (Invitrogen) before beginning Sybr Green.
Differences in gene expression were calculated using relative quantification to GAPDH via the comparative CT method from ABI (User Bulletin no. 2 ABI PRISM 7700 Sequence Detection System). GAPDH was found to be an appropriate normalizer by comparing the differences in raw CT values which did not differ between groups. GAPDH expression did not differ with strain (p=0.454), activity (p=0.254), or interaction (p=0.287). Standard curves for each target were run to verify equal efficiency of the PCR reaction.
Hydroxyproline Assay
The methods for the hydroxyproline assay are previously described (2). The amount of hydroxyproline was multiplied by the conversion factor 7.46 to calculate total collagen. Lastly, the data was expressed as total collagen normalized to milligrams of left ventricular dry weight (g of LV collagen / mg of LV dry weight).
Statistics
Two-way ANOVA was used for all statistical comparisons for echocardiography, blood pressures during the week of echocardiography, real-time PCR, and hydroxyproline assay. There were two possible main effects for these tests: a main effect for exercise training (sedentary and exercise) and a second main effect for strain (SD and Ren2). P < 0.05 was considered significant. A three-way ANOVA was used for initial and final blood pressures. A main effect included strain, exercise, or time (initial vs. final) where p < 0.05 was considered significant.
Results
Body Weight
Body weights were significantly greater (p < 0.05) in Ren2 compared to SD rats before and after the 5 weeks of treadmill exercise (Table 1). EX-SD and EX-Ren2 rats also exhibited lower body weights at the time of the echocardiography compared to SED-SD and SED-Ren2, respectively (Table 2).
Table 1.
Body weights, running speeds, and distances.
| Age (weeks) | SED-SD | EX-SD | SED-Ren2 | EX-Ren2 | p < 0.05 | |
|---|---|---|---|---|---|---|
| n | 5 | 5 | 5 | 5 | ||
| Initial body weight (g) | 3 | 81.7 ± 4.0 | 85.8 ± 5.9 | 136 ± 2.6 | 127 ± 2.9 | * |
| Final Body weight (g) | 10,11 | 253 ± 13 | 237 ± 18 | 298 ± 12.5 | 273 ± 6.7 | * |
| Total % weight gain | 210 ± 4.6 | 177 ± 15 | 119 ± 6.2 | 116 ± 4.1 | * | |
| Week 1 run speed (m/min) | 5 | 15.0 ± 0.18 | 15.0 ± 0.18 | |||
| Week 5–6 run speed (m/min) | 10,11 | 17.5 | 17.5 | |||
| Week 1 run distance (km) / day | 5 | 0.90 | 0.90 | |||
| Week 5–6 run distance (km) / day | 10,11 | 1.05 | 1.05 |
p < 0.05 for main effect of rat strain between Sprague-Dawley (SD) and Ren2 by 2-way ANOVA. Values for each group are mean ± SEM with n = 5 / group.
Table 2.
Resting echocardiographic measurements determined at 8 weeks of age.
| SED-SD | EX-SD | SED-Ren2 | EX-Ren2 | p < 0.05 | |
|---|---|---|---|---|---|
| Body Weight (BW) - (g) | 185 ± 3.7 | 178 ± 10 | 240 ± 4.2 | 215 ± 6.3 | * † |
| Heart rate (beats /minute) | 519 ± 49 | 556 ± 6.5 | 470 ± 21 | 507 ± 34 | |
| aortic diameter (µm) /BW (g) | 12.1 ± 0.42 | 13.2 ± 0.75 | 8.97 ± 0.65 | 10.9 ± 0.32 | |
| Interventr. septum diastole (µm) /BW (g) | 11.6 ± 0.58 | 10.7 ± 0.58 | 10.1 ± 0.57 | 11.6 ± 0.63 | |
| LV internal diameter diastole (µm) /BW (g) | 28.9 ± 2.1 | 31.2 ± 1.4 | 27.5 ± 1.1 | 30.0 ± 1.1 | |
| LV posterior wall diastole (µm) /BW (g) | 13.5 ± 1.1 | 13.0 ± 0.79 | 11.3 ± 0.52 | 12.5 ± 0.75 | |
| Interventr. septum systole (µm) /BW (g) | 17.3 ± 1.3 | 16.8 ± 1.6 | 15.7 ± 0.58 | 17.2 ± 0.99 | |
| LV internal diameter systole (µm) /BW (g) | 13.2 ± 2.4 | 16.4 ± 0.74 | 15.0 ± 1.3 | 14.9 ± 1.0 | |
| LV posterior wall systole (µm) /BW (g) | 19.4 ± 1.6 | 18.4 ± 2.7 | 15.6 ± 0.72 | 17.6 ± 1.2 | |
| % fractional shortening | 55.8 ± 4.8 | 51.0 ± 4.1 | 45.3 ± 2.6 | 46.6 ± 2.5 | |
| LV outflow max. (m/sec) | 1.07 ± 0.048 | 1.12 ± 0.088 | 1.01 ± 0.029 | 1.15 ± 0.049 | |
| LV outflow mean (m/sec) | 0.662 ± 0.038 | 0.692 ± 0.044 | 0.618 ± 0.038 | 0.700 ± 0.044 | |
| Mitral valve inflow max. (m/sec) | 1.13 ± 0.078 | 1.23 ± 0.052 | 1.11 ± 0.046 | 0.990 ± 0.053 | * |
| Mitral valve inflow mean (m/sec) | 0.700 ± 0.073 | 0.750 ± 0.058 | 0.700 ± 0.025 | 0.596 ± 0.022 |
p < 0.05 for main effect of rat strain between Sprague-Dawley (SD) and Ren-2 by 2-way ANOVA.
p < 0.05 for main effect of exercise within SD and Ren-2 by 2-way ANOVA. Values for each group are mean ± SEM with n = 5 / group.
Echocardiography
Echocardiography was performed after 3 weeks of exercise training (8 weeks of age) and 2–3 weeks prior to sacrifice. Septal wall thickness during diastole, left ventricular internal diameter during diastole, septal wall thickness during systole, and left ventricular internal diameter during systole were not different between Ren-2 and SD rats when normalized to body weight (Table 2). Also, left ventricular posterior wall thickness (µm) per body weight (g) during diastole and systole were not different. Ren-2 rats exhibited a lower maximum mitral valve inflow velocity (m/sec) (Table 2). There was no significant differences between strain or exercise condition for fractional shortening (p>0.05).
Since body weights of the EX-Ren-2 and EX-SD were significantly lower at the time of echocardiography compared to their sedentary counterparts (10.4% and 4.0%, respectively), cardiac output and stroke volume were normalized to body weights. During the 3rd week of exercise training, resting cardiac output [stroke volume (ml) × heart rate (beats/min)] normalized to body weight (g), also known as cardiac index, was significantly greater in both the 8 wk-old EX-SD (21%) and EX-Ren2 (57%) rats compared to their respective sedentary groups (p < 0.05; Figure 1). Additionally, there was a significant main effect of rat strain on cardiac index which was greater in the SD compared to the Ren-2 (p < 0.05). Resting stroke volume normalized to body weight (ml/g) was also 13% and 43% higher following exercise training in EX-SD (1011 ± 75 × 10−6) vs. SED-SD (897 ± 63 × 10−6) and EX-Ren2 (930 ± 82 × 10−6) vs. SED-Ren2 (649 ± 72 × 10−6) rats, respectively (p < 0.05). Stroke volume was also larger in the SD strain compared to the Ren-2 (p < 0.05). Mitral valve deceleration time (msec) which is the time from the peak velocity of blood flow to the end of flow in diastole was not significantly different (strain p = 0.067, exercise p = 0.550; data not shown). However, mitral valve deceleration slope (m/sec2), which is the change in velocity over the change in time, was shown to be significantly lower in the Ren-2 rats [SED-Ren2 (37.8 ± 2.50 and EX-Ren2 (34.1 ± 4.2)] compared to the SD strain [SED-SD (46.8 ± 6.1) and SED-EX (46.2 ± 2.9)] (p < 0.05). There was no significant difference in mitral valve deceleration with treadmill running in either Ren-2 or SD groups (p>0.05).
Figure 1.
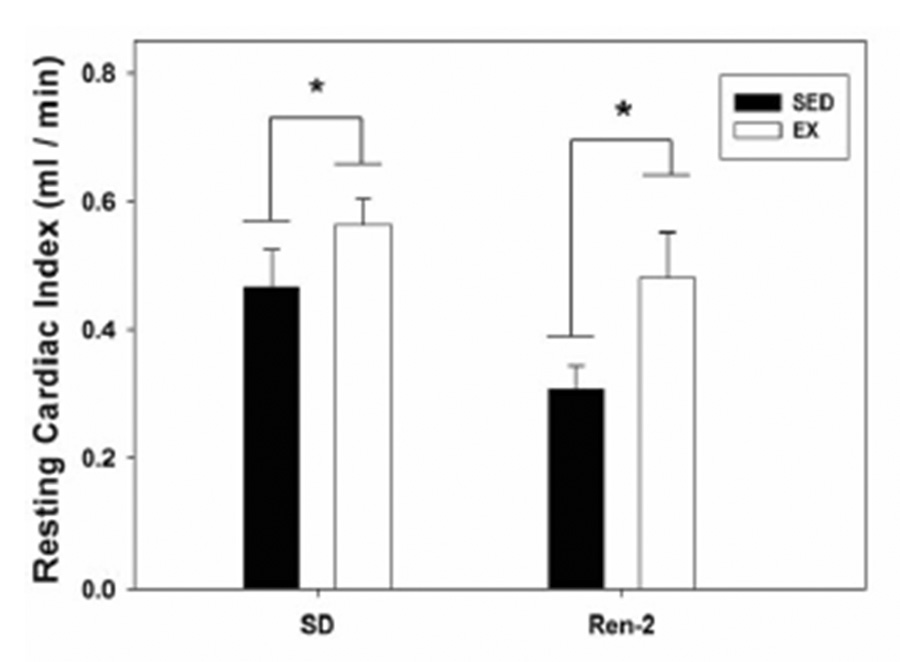
Effect of exercise training on resting cardiac output (ml/min) normalized to body weight (g) [cardiac index] in 8-wk-old Sprague-Dawley (SD) and Ren-2 rats. * indicates for main effect (p < 0.05) of exercise within SD and Ren-2 by 2-way ANOVA. Values for each group are mean ± SEM with n = 5 / group.
Blood Pressures and Heart Rates
Pre-training, resting systolic blood pressure (SBP; mmHg) was not significantly different between sedentary and exercise groups in either rat strain (p=0.184) [SED-SD (162 ± 7); EX-SD (147 ± 5); SED-Ren2 (181 ± 3); and EX-Ren2 (179 ± 6)]. Ren-2 rats had a significantly higher pre-training SBP than SD (p<0.05).
During the week of echocardiography (after 3 weeks of exercise training), SBP in Ren2 rats was significantly higher than SD (p<0.05) and EX-Ren2 was greater than SED-Ren2 (p<0.05) (data not shown). After 5 weeks of treadmill running, no significant differences in resting SBP (mmHg; p=0.478) were observed in EX-SD (146 ± 9) or EX-Ren-2 (228 ± 5) rats compared to respective sedentary groups [SED-SD (136 ± 6); and SED-Ren2 (228 ± 6). A significant interaction for strain×time (p < 0.05) occurred for SBP. Final resting SBP in EX-Ren2 (67%) and SED-Ren2 (56%) were significantly higher compared to the EX-SD and SED-SD, respectively (p<0.05).
Post-training resting heart rates were higher in the SD compared to the Ren2 (p=0.028; data not shown), whereas there was not a significant difference before training. There were no differences in resting heart rate between sedentary and exercise trained groups either pre- or post-training (p=0.821 and p=0.889, respectively; data not shown).
B-type Natriuretic Peptide
Left ventricular B-type natriuretic peptide (BNP) mRNA was significantly higher by 2-fold in 10–11-wk-old Ren-2 rats than in SD rats (p<0.05). GAPDH mRNA was used to normalize all mRNA data since it was unchanged by the treatments or strains. Data were then expressed relative to EX-SD. Exercise failed to have an effect on BNP mRNA levels in either the SD or Ren-2 (p=0.81) [SED-SD (0.93 ± 0.12); SED-EX (1.0 ± 0.17); SED-Ren2 (2.13 ± 0.32); and EX-Ren2 (2.11 ± 0.38)].
Myosin Heavy Chain and Collagen
α-myosin heavy chain mRNA levels, normalized to GAPDH, in the left ventricle were significantly higher by ~14.5 fold in 10–11-wk-old Ren-2 rats compared to SD (p < 0.05), but was not affected by exercise training in either strain (p=0.693) [SED-SD (1.04 ± 0.09); EX-SD (1.0 ± 0.06); SED-Ren2 (14.7 ± 1.7); and EX-Ren2 (14.6 ± 2.6)]. β-myosin heavy chain mRNA levels were significantly higher by ~2.5-fold (p < 0.05) in the Ren-2, but not changed by exercise in either rat strain (p=0.388) [SED-SD (1.05 ± 0.18); EX-SD (1.0 ± 0.18); SED-Ren2 (2.74 ± 0.40); and EX-Ren2 (2.32 ± 0.43)].
Left ventricular collagen deposition, as determined by hydroxyproline assay, did not significantly differ between the four groups for either strain of rat (p=0.613) or exercise (p=0.398) [SED-SD (18.3 ± 2.0); EX-SD (19.4 ± 1.0); SED-Ren2 (21.2 ±0.9); and EX-Ren2 (17.8 ± 1.3)].
Discussion
To our knowledge, this is the first study to evaluate the effects of low-intensity exercise training on cardiac function in an animal model of Ang-II overexpression in cardiac tissue. Exercise training of transgenic Ren 2 rats was begun at a time prior to cardiac decompensation because it was hypothesized that exercise would attenuate a relative decrease in cardiac output. The novel findings are that introduction of low-intensity treadmill running at a young age (3–4 weeks of age) did not exhibit a decline in relative cardiac function at 8 weeks of age as experienced by the age-matched sedentary group. Echocardiography revealed resting cardiac index (↑ 57%) and stroke volume (↑ 43%) to be significantly higher for the EX-Ren2 compared to their SED-Ren2 counterparts. Although not significant, the tendency for resting heart rate to be higher in both exercised groups compared to their respective sedentary groups possibly contributed to the improved cardiac output. Interestingly, EX-Ren2 and SED-SD had similar values for resting cardiac index and stroke volume, suggesting that exercise can maintain cardiac systolic function during the early development of hypertension in the Ren-2 model. In addition, mitral deceleration time slope (m/sec2), a non-invasive estimate of diastolic function, was significantly reduced in the Ren-2 compared to SD rats. Further, diastolic dysfunction was not prevented by exercise training in the Ren-2 rats, indicating that the initial preservation of cardiac index and stroke volume occurred in the presence of diastolic dysfunction.
Unexpectedly, fractional shortening was not influenced by exercise, which contrasts with the observation of increased cardiac output in the exercised groups. Fractional shortening is calculated from single line measurement through the left ventricle, and is therefore a relatively crude index of contractility. Cardiac index, a more global measure of cardiac function, is modulated by various factors which it might have been enhanced by exercise training.
Factors commonly associated with cardiac decompensation and remodeling, such as ratios of α- and β- MHC mRNA levels, collagen accumulation and natriuretic peptide synthesis, were unaltered following exercise training in the Ren-2 model. Thus, exercise doesn’t appear to maintain cardiac index through such remodeling events. It seems possible that other factors aside from changes in MyHC transcription, collagen deposition, and cardiac morphology are responsible for improved LV function. Another likely candidate involves factors that influence contractility due to significantly improved stroke volume observed following exercise training. It has been shown that Ang II exerts a negative inotropic effect on cardiomyocytes by diminishing calcium transits which, in turn, leads to myocardial dysfunction and remodeling (6; 12). Also, endurance exercise training in rats demonstrates improved cardiac myocyte contractility through increased sensitivity to Ca+2. In response to the same [Ca+2], 50% of maximal tension was elevated in single myocytes following exercise training (5).
Other studies have also shown that low-intensity exercise or voluntary running attenuates blood pressure increases in hypertension prone rats (7; 16). It was previously reported (11) that low-intensity treadmill exercise (16–20 m/min, 60 min, 5 times / week) for 18 weeks significantly reduced SBP, DBP, and MAP in the SHR. Interestingly, within the same study a high-intensity exercise training group (25–30 m/min) failed to show any difference in blood pressure measurements suggesting an exercise intensity-dependent effect. Additionally, another study (10) demonstrated that 6-weeks of voluntary wheel running reduced SBP by 16% in Ren-2 rats, supporting similar results observed in SHR. Results from the current study show that SBP blood pressure in the Ren-2 rat did not change in response to 5–6 weeks of low-intensity treadmill exercise. This may be, in part, related to the forced nature of the exercise and a consequent adverse physiological stress. Or, that the total amount (distance ~1 km/d) of running performed at a relatively low intensity (vs. 6–7 km/d voluntary) was insufficient to overcome the Ang II-mediated hypertension. Differences in response between SHR and Ren-2 rats most likely result from multiple factors including differences in the etiology of the hypertension, age (11 vs. 16–18 wks of age), and/or the intensity (low vs. high), duration or distance run, and/or the method of exercise (voluntary vs. forced treadmill).
Limitations of this study include changes in cardiac hemodynamics after 3 weeks of exercise training may have been different if the echocardiography was performed after 5 weeks of exercise training. This is important to consider since exercise had no apparent effect on blood pressures after 5–6 weeks in either SD or Ren-2 rats, but after 3 weeks EX-Ren2 rats exhibited higher blood pressures than the SED-Ren2 animals. In addition, the low number of animals in the study results in a limitation of statistical power. Another potential limitation is the possibility that different strains and exercise levels could impact body fat thereby affecting loading conditions to the heart under anesthesia. A further limitation is that echocardiographic measures are typically more conclusive when coupled with invasive measurements such as catheterization of the LV. For example, the lack of diastolic filling pressure determined via an indwelling catheter was not measured and is considered a limitation of the study.
In summary, these results demonstrate that low-intensity treadmill exercise beginning at an early age in the Ren-2 rats serves to maintain cardiac output and stroke volume without improvements in diastolic function at 8 wks of age. However, typical characteristics of maladaption, such collagen deposition and altered MyHC expression ratios, were unaffected via exercise training in RAS-mediated cardiac dysfunction. Thus, it seems likely that improved ventricular performance due to exercise in RAS-mediated hypertension may occur through other existing mechanisms or factors.
Acknowledgements
Research was supported by Veterans Affairs (VA) ARCD award (CSS), NIH 5RO1 – HL073101–02 and VA Merit 0018 (JRS), and anonymous gift (FWB).
Grants and Support: This manuscript was supported by Veterans Affairs (VA) ARCD award (CSS), NIH 5RO1 – HL073101-02 and VA Merit 0018 (JRS), and anonymous gift (FWB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension. 1995;25:1014–1020. doi: 10.1161/01.hyp.25.5.1014. [DOI] [PubMed] [Google Scholar]
- 2.Chiariello M, Ambrosio G, Cappelli-Bigazzi M, Perrone-Filardi P, Brigante F, Sifola C. A biochemical method for the quantitation of myocardial scarring after experimental coronary artery occlusion. J Mol Cell Cardiol. 1986;18:283–290. doi: 10.1016/s0022-2828(86)80410-2. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 5.Diffee GM, Seversen EA, Titus MM. Exercise training increases the Ca(2+) sensitivity of tension in rat cardiac myocytes. J Appl Physiol. 2001;91:309–315. doi: 10.1152/jappl.2001.91.1.309. [DOI] [PubMed] [Google Scholar]
- 6.Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–432. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann P, Friberg P, Ely D, Thoren P. Effect of spontaneous running on blood pressure, heart rate and cardiac dimensions in developing and established spontaneous hypertension in rats. Acta Physiol Scand. 1987;129:535–542. doi: 10.1111/j.1748-1716.1987.tb08094.x. [DOI] [PubMed] [Google Scholar]
- 8.Izumo S, Lompre AM, Matsuoka R, Koren G, Schwartz K, Nadal-Ginard B, Mahdavi V. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987;79:970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinnick TR, Youngblood EB, O'Keefe MP, Saengsirisuwan V, Teachey MK, Henriksen EJ. Exercise Effects on Muscle Insulin Signaling and Action: Selected Contribution: Modulation of insulin resistance and hypertension by voluntary exercise training in the TG(mREN2)27 rat. J Appl Physiol. 2002;93:805–812. doi: 10.1152/japplphysiol.00236.2002. [DOI] [PubMed] [Google Scholar]
- 11.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 12.Palomeque J, Sapia L, Hajjar RJ, Mattiazzi A, Vila PM. Angiotensin II-induced negative inotropy in rat ventricular myocytes: role of reactive oxygen species and p38 MAPK. Am J Physiol Heart Circ Physiol. 2006;290:H96–H106. doi: 10.1152/ajpheart.00324.2005. [DOI] [PubMed] [Google Scholar]
- 13.Pattison JS, Folk LC, Madsen RW, Childs TE, Booth FW. Transcriptional profiling identifies extensive downregulation of extracellular matrix gene expression in sarcopenic rat soleus muscle. Physiol Genomics. 2003;15:34–43. doi: 10.1152/physiolgenomics.00040.2003. [DOI] [PubMed] [Google Scholar]
- 14.Pinto YM, Buikema H, van Gilst WH, Scholtens E, van Geel PP, de Graeff PA, Wagner J, Paul M. Cardiovascular end-organ damage in Ren-2 transgenic rats compared to spontaneously hypertensive rats. J Mol Med. 1997;75:371–377. doi: 10.1007/s001090050123. [DOI] [PubMed] [Google Scholar]
- 15.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–1041. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd RE, Kuehne ML, Kenno KA, Durstine JL, Balon TW, Rapp JP. Attenuation of blood pressure increases in Dahl salt-sensitive rats by exercise. J Appl Physiol. 1982;52:1608–1613. doi: 10.1152/jappl.1982.52.6.1608. [DOI] [PubMed] [Google Scholar]
- 17.Sumitra M, Manikandan P, Rao KV, Nayeem M, Manohar BM, Puvanakrishnan R. Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine and thiopentone anesthesia in male Wistar rats--a comparative analysis. Life Sci. 2004;75:1887–1896. doi: 10.1016/j.lfs.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Tavi P, Pikkarainen S, Ronkainen J, Niemela P, Ilves M, Weckstrom M, Vuolteenaho O, Bruton J, Westerblad H, Ruskoaho H. Pacing-induced calcineurin activation controls cardiac Ca2+ signalling and gene expression. J Physiol. 2004;554:309–320. doi: 10.1113/jphysiol.2003.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veras-Silva AS, Mattos KC, Gava NS, Brum PC, Negrao CE, Krieger EM. Low-intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats. AJP - Heart and Circulatory Physiology. 1997;273:H2627–H2631. doi: 10.1152/ajpheart.1997.273.6.H2627. [DOI] [PubMed] [Google Scholar]


