Abstract
Children with cardiomyopathy carry significant risk of morbidity and mortality. New research and technology have brought about significant advancements to the diagnosis and clinical management of children with cardiomyopathy. However, currently heart transplantation remains the standard of care for children with symptomatic and progressive cardiomyopathy. Cardiovascular rehabilitation programs have yielded success in improving cardiac function, overall physical activity, and quality of life in adults with congestive heart failure from a variety of conditions. There is encouraging and emerging data on its effects in children with chronic illness and with its proven benefits in other pediatric disorders, the implementation of a program for with cardiomyopathy should be considered. Exercise rehabilitation programs may improve specific endpoints such quality of life, cardiovascular function and fitness, strength, flexibility, and metabolic risk. With the rapid rise in pediatric obesity, children with cardiomyopathy may be at similar risk for developing these modifiable risk factors. However, there are potentially more detrimental effects of inactivity in this population of children. Future research should focus on the physical and social effects of a medically supervised cardiac rehabilitation program with correct determination of the dosage and intensity of exercise for optimal benefits in this special population of children. It is imperative that more detailed recommendations for children with cardiomyopathy be made available with evidence-based research.
I. INTRODUCTION
Cardiomyopathies are diseases of the myocardium associated with cardiac dysfunction [1]. They can be classified into four categories: dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), arrythmogenic right ventricular cardiomyopathy (ARVC), and restrictive cardiomyopathy (RCM). Each condition can result in congestive heart failure (CHF), the need for heart transplantation, and sudden death [2]. For 57% to 68% of children who have a form of cardiomyopathy, the cause of the disease is unknown [3,4]. Although efforts must continue to identify distinct causes and effective treatments of cardiomyopathies, research in the past decade has shed light on the epidemiology and etiology of the different classifications of the disease.
DCM is the most common form of the disease, although its incidence ranges between 0.34 to 0.73 cases per 100,000 children [4–6]. It is characterized by increased left ventricular (LV) chamber size that reduces contractility and results in systolic dysfunction [2,7]. About half of patients with DCM die within 5 years of diagnosis if they do not undergo transplantation. The cause of death is split evenly between pump failure and sudden death [5]. DCM can be caused by genetics, viruses, and nutritional deficiencies, and understanding the specific etiology of DCM is an ongoing challenge. Although 30–40% of all patients with DCM have a genetic form, defining the etiology of DCM in children [2,5] is more challenging. In one study, only 34% of pediatric DCM cases had an identifiable cause. Myocarditis and neuromuscular disorders are associated with DCM more frequently than inborn errors of metabolism and malformation syndromes.
HCM is the second most common form of pediatric cardiomyopathy with a reported incidence between 0.32 and 0.47 cases per 100,000 children [8]. Clinically, HCM is identified by ventricular hypertrophy in the absence of a hemodynamic cause [4, 9]. The pathophysiology of HCM includes thickening of the left ventricular posterior wall and the interventricular septum. This anatomy results in hypercontractile systolic function and poor diastolic function. In addition, blood flow from the left ventricle to the aorta may be obstructed [9]. The majority of pediatric HCM cases are idiopathic, and known causes include familial isolated cardiomyopathy, inborn errors of metabolism, neuromuscular disorders, and malformation syndromes, with definitive etiologies more commonly established in girls [10].
ARVC and RCM occur less frequently than DCM and HCM. ARVC is characterized by the replacement of myocytes in the right ventricle with fatty, fibrous tissue [2,11]. RCM is characterized by restrictive filling in one or both ventricles during diastole. Systolic function and wall thickness, however, are normal or near-normal [1,11]. RCM is familial in some pediatric cases, but most cases are characterized as idiopathic [10]. RCM has the worst prognosis with the fewest treatment options [2].
While significant strides have been made in the treatment and survival of children with congenital heart disease, parallel ones have not been made for children with cardiomyopathy. Heart transplantation rates have not declined and remain the standard of care for children with progressive disease [12]. Nearly 40 percent of children who present with symptomatic cardiomyopathy receive a heart transplant or die [13, 14]. Furthermore, the time to transplant or death for children with cardiomyopathy has not improved during the past 35 years, and the most economically advanced nations have no better outcomes than developing nations [12]. Cardiomyopathies have an associated cost of nearly $200 million/year in adults and children in the United States alone [15]. Improvements in technology and medicine have contributed to an improved survival for children having heart transplants, however, it has not resulted in either a normal life span, or quality of life. Thus, exploring ancillary therapies, such as exercise interventions and rehabilitation that may not cure but may potentially improve cardiac function and quality of life are imperative to consider in children with all types of cardiomyopathy.
II. EXERCISE IN PEDIATRIC CARDIOMYOPATHY
A. The Overall Benefits of Exercise
Healthy People 2010 is a public health initiative which aims at increasing the quality and years of healthy life [16]. Active lifestyles are known to reduce the rates of illnesses, while sedentary habits place individuals at risk for the development of diseases such as cardiovascular disease, obesity, diabetes, and musculoskeletal injuries to name a few [17, 18]. While most of the documented improvements are reported in adults, there is emerging evidence that children may benefit as well. The growing rates of obesity in the recent years in children/adolescents are in part due to the increased sedentary lifestyles [17–23].
There is an associated downward spiral where children who have a chronic disease are more sedentary, thus placing them at greater risk for secondary diseases [24] (FIGURE 1). Several groups have set guidelines for physical activity in children [25–27]. Most of these guidelines were written with the normal healthy child in mind. As it relates to the healthy pediatric population, physical activity is usually recommended as adjunctive and preventative therapies, but restrictions are often set (by either the family or the health care provider) when a child has been diagnosed with a chronic illness. These limitations aim to decrease the risk for further or additional complications as a result of physical activity. However, data is limited on safety, efficiency, and effectiveness of exercise programs for children/adolescents with chronic illnesses. New and emerging data are beginning to show the effectiveness and safety of supervised exercise programs in children with chronic diseases [28].
Figure 1.
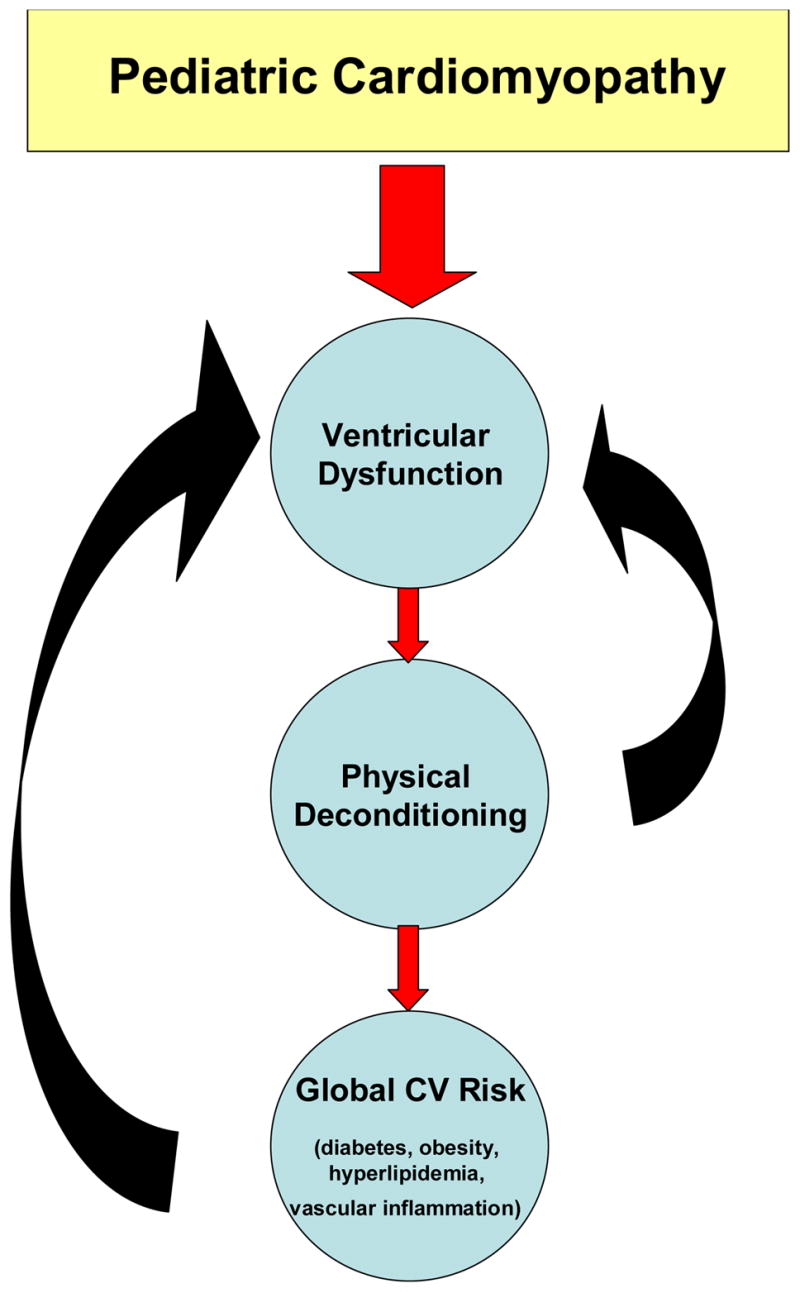
The downward vicious cycle of deconditioning in children with cardiomyopathy.
1. Types of Exercise
The individual sessions of a cardiac rehabilitation program should be divided into component parts; aerobic, resistance and flexibility training. All three of these components contribute differently to the general health of individuals. In the prescription of an individualized rehabilitation program, the F.I.T.T. Principle (Frequency, Intensity, Time, and Type) should be implemented [17, 18, 26], where each component of the exercise program satisfies this principle. Frequency refers to how often the exercise sessions are completed (e.g. two times per week), Intensity is the level of exertion during exercise (e.g. 60% of maximal heart rate), Time refers to the duration of the exercise session (e.g. 45 minutes), and type refers to the specific activity completed (e.g. treadmill). An example of the FITT principle applied to the aerobic component would consist of activity two times per week, in 30-minute sessions, at 50–75% of maximal heart rate, on a cycle ergometer [17, 18]. Similar to the food pyramid for nutrition, a physical activity pyramid indicates the amounts and types of physical activity that should be practiced (FIGURE 2).
Figure 2.
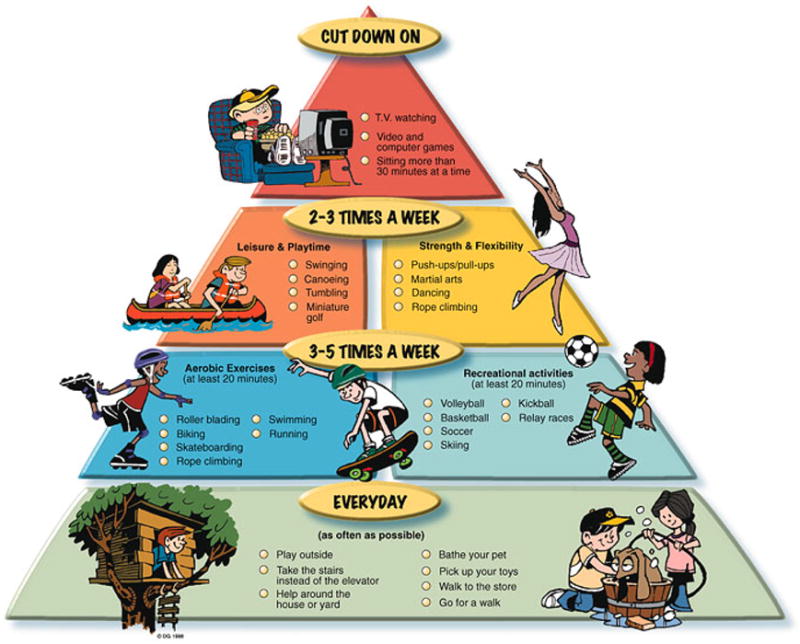
Children’s Activity Pyramid (Willenberg, B. Parent’s Pamphlets. Children’s Activity Pyramid. Retrieved from http://www.classbrain.com/artread/publish/article_31.shtml on August 27, 2007.)
a. Aerobic Training
Aerobic exercises are defined as “any activity that uses large muscle groups, can be maintained continuously, and is rhythmic in nature” [17]. Aerobic exercises are comprised of activities that cause the lungs and heart to function at higher levels in order to provide adequate substrates to working muscles over an extended period of time. According to the American Heart Association (AHA) and the American College of Sports Medicine (ACSM), aerobic exercises should be performed at least five days per week [26, 29]. The options for aerobic exercises are endless and the selection of activities that are fun and/or entertaining to the child should remain front and center when prescribing them. Fun activities will promote sustainability and compliance. Recent studies have shown promising results in children who use virtual reality game systems to augment physical activity [30, 31].
b. Resistance Training
Resistance training is composed of dynamic movements with progressive overload to increase and improve muscular strength [17, 18, 27, 32]. Previous studies may have been misinterpreted and caused great alarm regarding the safety and long-term adverse consequences of resistance training programs in children [33, 34]. Current evidence suggests that both prepubescent and adolescent children, can increase muscular strength by following a strength-training regimen without additional risk of injury [35–39]. Furthermore, exercise intervention studies in children, with a resistance-training component, have been published and adverse events have not been reported [36–38]. Authors acknowledge the potential for injury is greater in this population, but emphasize the need for trained personnel and close supervision of the pediatric participants. Resistance training is recommended 2–3 times per week incorporating all major muscle groups [26, 29].
For children and adolescents, the goal of a resistance-training program should aim to improve overall body strength with muscular hypertrophy deemphasized. Circuit training programs that emphasize aerobic conditioning while engaging in resistance training have been proven beneficial [39a, 40] in children. Careful selection of equipment is necessary and important as placing a small child on adult-sized equipment may lead an unnatural alignment of joints and muscle groups. In general, resistance-training machines are safer than free weights as they allow a fixed pattern of movement, and are easier to learn. Free weights require much more coordination and accessory strength for proper completion of the exercise thus placing a child at greater risk for injury.
Training protocols that prescribe a higher number of repetitions, rather that greater weight, are more effective for children during their first stages of resistance training [41]. There is no evidence that children lose flexibility with strength training [42]. Soon after beginning a strength-training program, it is common to appreciate significant improvements in strength. Initially there are improvements in motor learning, and efficiency of the task [43, 44] due to neuromuscular adaptations to exercise although no significant changes occur at the muscle fiber level. After the acute response, muscle hypertrophy [43] occurs. Hypertrophy is defined by increases in size of the muscle (through increase in myofibrils). Hypertrophy leads to more metabolically active tissue thus generating and utilizing more energy [43].
c. Flexibility Training
Flexibility training progressively increases the range of motion of a joint or set of joints over a period of time [17, 18]. Flexibility exercises can be completed in both dynamic or static positions. Benefits include increased range of motion, muscular relaxation, less chance of injury and less muscular soreness, to name a few [17, 45, 46]. Flexibility exercises should be preceded by warm-up activities to increase local blood flow to the working muscles [17,43]. Flexibility exercises should be performed 2–3 times per week and to include exercises for all major muscles groups. All stretches should be performed to the point of mild discomfort, with a sustained stretch for 30–60 seconds [17, 18].
2. Effects of Exercise on Children with Cardiomyopathy
There is a dearth of information regarding the effect of cardiac rehabilitation programs for children with cardiomyopathy. However, it is reasonable to expect that implementing an exercise rehabilitation program in the context of state-of-the-art medical therapies may provide added benefits for children with cardiomyopathy, since these children have lower functional capacities. The decrease in functional capacity is caused, in part, by their cardiovascular disease, but overall physical inactivity is also a contributing factor. Ideally, improved cardiac function and other cardiac biomarkers would provide the greatest evidence for the benefit of exercise rehabilitation in pediatric cardiomyopathy, but functional improvements such as quality of life and decreasing any concomitant cardiac risk factors are equally important.
B. The Effect of Exercise on Cardiomyopathy-Specific Outcomes
1. Aerobic Capacity and Rhythm Disturbances
Aerobic capacity, as determined by the metabolic stress test, is indicative of cardiorespiratory fitness. This test provides clinicians with information about the current fitness level and whether a child can safely participate in a cardiac rehabilitation program without becoming symptomatic or experiencing adverse events. The results may also be used as a marker to track progression. Furthermore, the rehabilitation program may be tailored to an individual based on the results by setting appropriate intensity ranges. Poor aerobic capacity is a cardiac risk factor and this test is used to assess this relationship. Cardiorespiratory fitness is most commonly measured by peak VO2. Normative ranges for children have been established that place the patients into categories based on their aerobic fitness [47]. Previous studies have linked tolerance to exercise with prognosis for patients with heart failure [48, 49]. Additionally, cardiorespiratory fitness has been presented to be a better prognostic indicator of health than the New York Association functional class [44].
Cardiac rhythm during physical activity is measured with a simultaneous electrocardiogram (ECG). The electrical activity of the heart is measured while the patient is under physiological stress. Increased physiological stress may elicit arrhythmias that can be absent in the resting state. The heart tissue of children with cardiomyopathy can disrupt the electrical conduction system in the heart, leading to arrhythmias. Baseline knowledge of a predisposition to arrhythmia is important, as uncontrolled arrhythmias are an absolute contraindication for exercise. Furthermore, certain types of arrhythmias, such as ventricular arrhythmias in HCM, are the primary cause of sudden cardiac death in these children [17, 50]. Other modalities, such as Holter monitoring, can give added information on rhythm disturbances. The advantages of the Holter monitor is that the electrical activity is recorded over 24 hours while the child goes about his/her normal activities. The results may indicate arrhythmias not present during a stress test and also the frequency of these rhythm disturbances.
The acute response to an aerobic exercise session requires cardiorespiratory adaptations. The heart has to increase its metabolism to meet the greater demands placed on the body. The response is short lived and once the child is rested the heart will return to its baseline metabolic rate. Peak VO2 does not change in response to acute cardiovascular training. Changes in VO2 are typically expected after weeks of training [51] (the training response [52]). Many studies have failed to show improvements acutely upon limited exercise sessions, while other reports indicate physiological changes in response to acute exercise [53, 54]. For example, in children with hypertrophic cardiomyopathy, the myocardium may have limited ability to respond to the metabolic demands imposed by acute bouts of exercise (55).
Improvements in cardiorespiratory fitness are generally realized when aerobic exercises are engaged in for an extended period of time at predetermined aerobic ranges of intensity [17, 18]. Previous intervention studies have found increases in VO2 in persons with a heart conditions after moderate intensity aerobic conditioning [56–58]. The duration of the interventions varied from 3–6 months, and 2–3 sessions per week. In the pediatric population with cardiac disease, studies have shown ambiguous results [59, 60]. The type of exercises that are completed along with the intensity and duration in which it is completed are of utmost importance to improve in metabolic fitness. More intervention studies are needed to determine that appropriate duration and intensity levels that may produce beneficial results in children with cardiomyopathy.
2. Cardiac Function (echocardiography)
Echocardiography is a diagnostic test commonly used to assess cardiac function. It uses ultrasound waves to create images of the heart chambers, valves and surrounding structures [61]. Important echocardiographic outcomes for children with cardiomyopathy include end diastolic dimension, end systolic dimension, fractional shortening, end diastolic posterior wall thickness and left ventricular mass [5, 9, 10]. Guidelines have been delineated and Z-scores created to categorize children with diseased or normal hearts [62]. Functionally, a decreased shortening fraction will precede a decrease in ejection fraction. As a result, contractility will decrease indicating an overall deterioration of the health of the heart [63]. These values are used clinically to place children into either a conservative treatment approach or transplantation [5]. Towbin et al reported survival based on variables from echocardiography at diagnosis [5]. Echocardiography provides the means for proper determination of clinical care and longitudinal measure to assess the effects of an exercise rehabilitation program.
During a bout of exercise, the increased physical demands require the heart to meet the metabolic requirements of the active tissue to maintain the level of exercise. As a result, there is an immediate increase in heart rate and stroke volume, resulting in an increase in cardiac output [43]. This increase occurs until a steady state is reached and heart rate, stroke volume and cardiac output plateau. In children with DCM who have lower stroke volumes [64], the associated diastolic dysfunction may prevent the increase in stroke volume [55]. This abnormal response helps explain the decreased exercise capacity in children with cardiomyopathy. Remodeling to the left ventricle, as a result of a single bout of exercise, has not been shown [65].
For children with cardiomyopathy, abnormal cardiac function remains the greatest concern for overall success of an exercise rehabilitation program. Left ventricular filling is the limiting factor for meeting adequate demands during exercise [66]. However, in other disorders such as children with congenital heart disease, there is an improvement in stroke volume as a result of a cardiac rehabilitation program [67]. A report in adults with ischemic cardiomyopathy suggests that exercise training can improve exercise capacity [68]. Other studies of adults with idiopathic dilated cardiomyopathy showed a significant increase in exercise time and peak oxygen consumption and a decrease in left ventricular end-diastolic dimension and end-systolic dimension. Patients with idiopathic DCM either had improved ventricular function or did not worsen with the exercise program [69, 70].
3. Vascular Reactivity
Flow mediated vasodilation (FMD) is a method of assessing of endothelial function that is performed commonly at the brachial artery. Abnormal endothelial function may result in vasoconstriction and increased afterload in the heart. Clinically, endothelial dysfunction is an important finding, as studies have suggested endothelial dysfunction to be an independent predictor of cardiac events [71–73]. Endothelial dysfunction may present in the early stages of cardiovascular disease [74]. Patients with congestive heart failure have impaired vasodilation as a result of increased sympathetic tone, activity of the renin-angiotensin system, and high endothelin levels [75–77].
Vasodilation is, in part, mediated by nitric oxide (NO) that is released by the endothelium. Endothelin-1, a chemical mediator produced by the endothelial cell and cardiac myocytes, is inversely related to nitric oxide (NO) levels. An increase in NO decreases endothelin-1 which inhibits vasoconstriction thus limiting afterload. This may lead to an overall beneficial effect by reducing vasculature resistance. Patients with congestive heart failure have higher levels of endothelin-1 that correlate with increased vascular tone as described above [78]. Children who, at baseline, present with increased endothelin-1 measures may likely have decreased vasodilation capabilities.
Regular physical activity can improve endothelium-dependent vasodilation in patients with heart failure [79]. Adult exercise studies have also shown improvements in NO release thereby improving endothelial function [80]. Previous studies have attempted to find a relationship between a single bout of aerobic training and endothelium-dependent vasodilation. After acute exercise, endothelium-dependent vasodilation improves [81–83]. The causal factor of acute dilation in this situation is the availability of NO [80, 81]. This response causes increased delivery of blood to the distal tissues. Acutely, improvements in vasodilation correlate with better exercise capacity [84, 85]. The mechanisms of the effect of exercise in changing endothelin-1 levels remain unclear. Several studies have indicated the beneficial responses to an exercise program in endothelium-dependent vasodilation [81, 86]. However, other studies show that endothelin-1 levels remained lower four weeks after the end of intervention [87].
4. Metabolic factors
Global assessment of cardiovascular risk including lipid profiles (total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides), and cardiac biomarkers are important components of the care of patients with cardiomyopathy. Adult studies have demonstrated improvements in all of these factors with the implementation of an exercise rehabilitation program. Cardiac specific markers such as endothelin-1 (see prior section), pro-brain naturetic protein (BNP), tumor necrosis factor (TNF)-alpha, and highly sensitive C-reactive protein (hsCRP) should be monitored. Brain natriuretic peptide is associated with left ventricular dysfunction [14]. Pro-BNP is the precursor to BNP and is used clinically to determine ventricular strain. Adult studies including patients with chronic heart failure have had significant reduction in pro-BNP measures as a result of aerobic training [88]. hsCRP is another inflammatory marker which is predictive of myocardial infarction or other vascular events. Studies have indicated improvements in this marker as a result of a structured cardiac rehabilitation program [89].
The effects of a single bout of exercise may change some of these biomarkers. However, researchers have found conflicting results with many of the positive changes occurring in people who have higher than normal baseline values of these biomarkers [90]. A single bout of aerobic exercise can reduce triglycerides and increase HDL-cholesterol [91]. These results have been demonstrated in individuals who are highly fit and participate in a prolonged bout of exercise, neither of these conditions are often found in children with cardiomyopathy. Plasma volume expansion may be one potential explanation for these acute responses [91]. Other studies have shown improved glucose control acutely after exercise with results lasting for several days [92]. Studies involving a single bout to one week of training [93, 94] have demonstrated similar results. There seems to be a dose response, however, where the greater the duration and intensity of exercise, the greater the improvement. Acute bouts of exercise can also increase interleukin-6 (a pro-inflammatory cytokine), but appear to cause no damage and levels subsequently decrease post-exercise [95, 96].
For those patients enrolled in a long-term exercise rehabilitation program, most research indicates improvements in these cardiac-specific biomarkers that are also predictive of morbidity and mortality in patients with congestive heart failure [88, 97–99]. In chronic heart failure, proinflammatory cytokines and other markers of inflammation are present in higher amounts. Studies have shown variable positive changes with exercise rehabilitation programs [89, 100, 101], although most have not found a deleterious effect (with increased inflammatory biomarkers) of chronic exercise. However, not all effects of exercise have proven to be beneficial for a child with a chronic disease. If the intensity of the exercise program is great enough, it can stimulate inflammatory cytokines leading to a catabolic state and may adversely affect the child [102].
III. CASE STUDY
We present the results of a 12 week structured exercise intervention for 2 children with DCM as an example of both the safety and effectiveness of exercise training to improve a variety of clinically important outcomes. Two children with primary dilated cardiomyopathy completed a 12-week exercise intervention program in our hospital-based exercise laboratory. Patients were required to be on a stable dose of medications (at least three months) and required clearance to participate by a pediatric cardiologist. Patients were excluded if they were classified as NYHA Class IV, were unable to achieve maximal exertion on an exercise stress test, or were unable to understand or perform the exercise regimen for any reason including, but not limited to, cognitive or orthopedic problems. The program consisted of twice weekly resistance and flexibility training with an aerobic component over 12 weeks. The aim was to assess if the patients could complete the program safely and to determine whether the exercise program could demonstrate gains in strength, aerobic function, body composition, and quality of life. We hoped to show that such a program can safely end the cycle of inactivity and deconditioning in children with cardiomyopathy.
Baseline testing was conducted to measure anthropometry (height, weight, BMI, skin-fold thickness, dual X-ray absorptiometry (DXA) scanning), left ventricular and overall cardiovascular function (echocardiography, flow-mediated vasodilation), aerobic function (exercise stress testing), strength (1-repetition maximum), quality of life (Functional Status [103, 104] and Child Health Questionnaire [105]). At the end of the 12-week exercise program, patients were retested for all baseline measures.
Patient A
Patient A is a seven year old female with idiopathic DCM diagnosed in the first year of life. After completing the intervention on schedule, her left ventricular and cardiovascular function, as measured by echocardiography and FMD, respectively, remained unchanged. There were slight decreases in left ventricular end diastolic dimension (LVEDD) z-score (5.045 to 4.685, Δ = −0.360) and left ventricular end systolic dimension (LVESD) z-score (6.130 to 5.885, Δ = −0.245), although all of these measurements were above the 99th percentile, indicative of dilated left ventricles. Fractional shortening, a measure of left ventricular function, remained unchanged (25% at baseline to 24% post-exercise). Similarly, peak dilation of her brachial artery, as measured by flow-mediated vasodilation (FMD), also remained unchanged. Remarkably, this rigorous training program did not cause deterioration in cardiac or vascular function, suggesting for this patient, the intervention was safe.
Patient A, did realize positive gains in body composition. She experienced a decrease in body fat (34.3% to 32.8%, Δ = −4%) along with an increase in body mass index (BMI) (z-score: 1.18 to 1.52, Δ = 0.32), indicating an overall increase in muscle mass. Most notably, strength and flexibility improved as much as 46% (FIGURE 3). Quality of life and functional status improved as well (General Health component of the Functional Status II(R) questionnaire 77.1 to 80; 4% increase and the physical component of the Child Health Questionnaire Parent Form 50 (CHQ-PF50) 48.2 to 52.1%). The exercise intervention also resulted in a more active lifestyle with increased daily physical activity (from 2 hours/day at baseline to 2.5 hours/day post-exercise) and decreased time spent watching television, videos or computer (from 5 to 4 hours/day).
Figure 3.
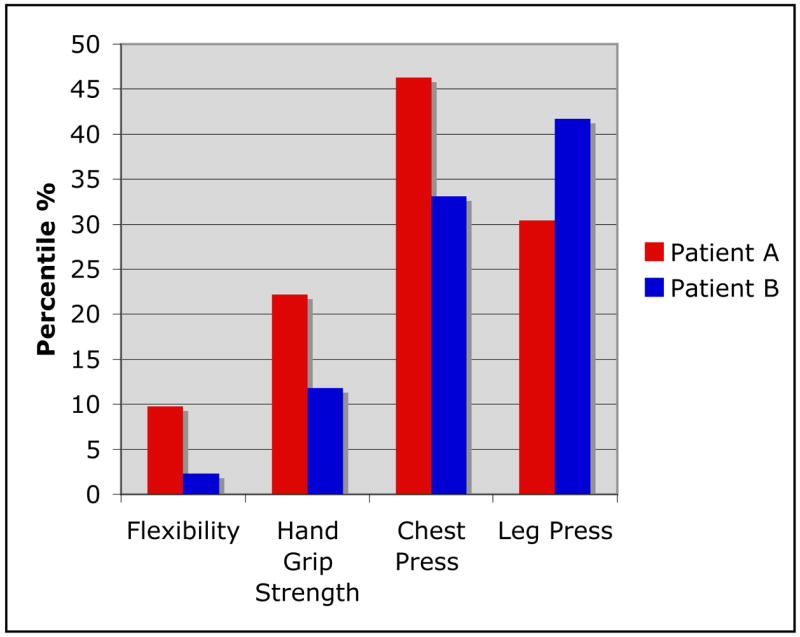
Percent change from baseline to completion of intervention for Patients A and B.
Patient B
Patient B also completed the 12-week program on schedule. He is a seven year old male with idiopathic DCM. Similar to Patient A, there were negligible changes in ventricular function, LVEDD (4.451 to 4.568, Δ = 0.117) and LVESD z-scores (4.564 to 4.922, Δ = 0.358) and fractional shortening (28.5% to 27%). Patient B’s FMD showed increases in peak dilation (4.12 to 7.37%, 79% increase) and overall improved vascular reactivity. The modified Bruce protocol was used to measure aerobic capacity and showed that Patient B had significant improvements in maximal aerobic capacity (VO2) from a baseline measure of 30.7 ml/kg/min to 44.3 ml/kg/min at the end of the intervention, and increases in anaerobic threshold (AT), 30.3 to 33.9 ml/kg/min.
Other improvements in response to this exercise intervention included a decrease in BMI (z-score: 2.71 to 2.40, Δ = −0.31) and body fat (43.8% to 41.7%, Δ = −5%). His strength increased significantly (FIGURE 3). Further, Patient B improved his scores on all three quality of life surveys (FSII(R): 80 to 82.9, 4% increase; CHQ-PF50 physical component: 49.2 to 51.2; and CHQ-PF50 psychosocial component: 58.2 to 63.6 %. Finally, daily physical activity time improved from 1 to 1.5 hours/day (33% increase), and sedentary time fell (6 to 5 hours/day, 17% decrease) as a response to the intervention.
IV. RECOMMENDATIONS
A. Screening
In a national study of young competitive athletes who died suddenly, 115 children had a pre-participation medical evaluation, but the cardiovascular abnormality that caused sudden death was found in only one athlete [106]. Screening procedures and policies to clear children for participation in high school sports vary widely by state. Some require a thorough history and physical administered by a physician, but other states have far less stringent requirements. Thus, detection of potentially harmful cardiovascular defects may be compromised in some states [107].
According to the AHA’s 2006 guidelines, pediatric cardiomyopathy patients can be classified based on risks during stress testing. The lower risk group consists of patients with stable DCM without uncompensated congestive heart failure (CHF) or documented arrhythmia. The higher risk group, however, includes DCM (and restrictive cardiomyopathy) patients with CHF or arrhythmia as well as hypertrophic cardiomyopathy patients who have symptoms, greater than mild left ventricular outflow tract obstruction, and arrhythmia [108].
A thorough and detailed medical history and interview is critical in the pre-exercise screening of children with cardiomyopathy. A medical history may also expose comorbidities would be essential to treat prior to the prescription of a cardiac rehabilitation program. An interview may reveal definite contraindications to exercise such as unresolved orthopaedic complications or history of unstable arrhythmias [17]. The proper diagnosis of a child will create options in the modes of appropriate treatment.
Medications become the primary mode of therapy for children with cardiomyopathy. Previous studies show children with cardiomyopathy are on a variety of medications with different actions including anticongestive agents (82 %), angiotensin-converting enzyme inhibitors (64%), antiarrhythmic medications (38%), L-carnitine supplements (15%) and dietary supplement (16%) [5]. A list of the most commonly prescribed drugs and their possible effects on physical activity are presented in TABLE 1. The effects of various medications on response to exercise programs should be considered and discussed with the patient prior to the beginning of any exercise program.
Table 1.
Commonly Prescribed Medications for Children with Cardiomyopathy and Effects on Physical Activity*
| Class | % Use5 | Indications | Reaction to physical exertion |
|---|---|---|---|
| Angiotensin-converting enzyme inhibitors | 64 | Congestive heart failure, hypertension | ↓blood pressure, no effect on exercise capacity |
| Antiarrhythmics | 38 | Prevention of ventricular arrythmias | ↑heart rate, ↓blood pressure, prolong QRS and QT intervals |
| L-carnitine | 15 | Cardioprotectant, may lower triglyceride | No effect on exercise capacity |
| Antithrombotics | 19 | Reduce risk of thromboembolic events | No effect on hear rate, blood pressure or exercise capacity |
| Inotropes | 16 | Mild to moderate heart failure, ↓ ejection fraction | ↓ exercise capacity |
| Calcium channel blockers | 3 | Hypertension, chronic stable angina, vasospastic angina | ↓ or ↑ in hear rate, ↓ blood pressure |
| Beta-blockers | 4 | Angina pectoris, hypertension | ↓ heart rate, blood pressure |
Adapted from PDR 16th edition and American College of Sports Medicine Guidelines for Exercise Testing and Prescription17.
B. The Exercise Program
The goals of a pediatric cardiac rehabilitation program are to augment the functional capacity of the child, improve the child’s quality of life, improve body composition (to increase the percent of lean body mass over fat mass), to increase overall physical activity outside of the program, to educate the child and family to adopt a healthy lifestyle to reduce the risk of progressive cardiovascular disease, and finally to reduce the risk of future cardiovascular disease resulting in the need for more medical treatment and potential hospitalizations.
In the past, physical activity was not prescribed as a form of treatment, but with the current knowledge of the benefits of exercise, it is becoming a regular part of the medical regime for many patients with chronic disease. Exercise is also indicated for the prevention of many diseases [26, 29]. In adults, cardiac rehabilitation programs are now the standard care for patients following a cardiac event. Yet, little is known on the effect of a structured exercise program for children with chronic illness and specifically, cardiomyopathy. To date there are case reports and small series citing the clinical improvements with exercise in children, but very few, if any, in pediatric cardiomyopathy. However, the benefits of exercise in adults with chronic disease warrant the incorporation and adaptation of similar programs for children. Most children should, given the appropriate medical recommendations, become involved in activities that maximize the benefits of exercise.
Several organizations report guidelines for physical activity in children for the maintenance and prevention of illness [26, 29]. Prescribing exercise to a child with cardiovascular disease has long caused apprehension. The Bethesda Conference developed recommendations as they relate to people with cardiovascular abnormalities [108]. Specifically, hypertrophic, and arrythmogenic cardiomyopathy have been associated with an increased risk of sudden death during exercise in the child and in young adulthood [108, 109]. Although the incidence of these two types of cardiomyopathy are very rare, the risk versus benefit of strenuous exercise does not justify itself. Recommendations should extend to all children who are asymptomatic, regardless of the type of cardiomyopathy, to participate in some form of physical activity. A modest level of physical activity should be considered, yet should be carefully followed by medically trained personnel who have experience with children, who have hypertrophic and arrhythmogenic cardiomyopathy. For other forms of cardiomyopathy, the child should be carefully monitored at baseline and through the progression of the program as outlined below.
1. Structure of the Pediatric Exercise Rehabilitation Program
A pediatric cardiac rehabilitation program should optimize the use game-like and child-friendly activities rather than rely on the traditional adult gym equipment (treadmills, stationary bicycles, etc) [110]. For example, one activity-promoting video game, in which children must keep up with prescribed dance movements, has been shown to increase energy expenditure more than walking on a treadmill [30].
The cardiac rehabilitation program for children with cardiomyopathy will require greater supervision than that needed for a healthy pediatric population, primarily because of the increased risk for a cardiac event. The over-riding philosophy, not necessarily always evidence-based, suggests that these children are at a higher risk for sudden death with exercise. Thus, safety is a priority that should be addressed prior to beginning any exercise rehabilitation program. For this special population, a medically-oriented, team approach will optimize program success. The program should be physician-directed by a pediatric cardiologist along with a physician who has expertise in exercise physiology and nutrition. A coordinated team approach that includes additional staffing such as exercise physiologists, nurse practitioners, registered nurses, dietitians, and social workers is optimal. Experience in evaluating not only medical conditions, but a comprehensive assessment of exercise capacity, nutrition, and psychosocial factors is required. All staff should have the training to handle medical emergencies and the exercise area should be fully equipped with the necessary emergency equipment. The exercise physiologist should have prior experience with children and should be able to provide pediatric advanced life support (PALS). To make the program as safe as possible, the staff to patient ratio should be no greater than 1 to 4 at any time.
There should be open communication between the exercise physiologist and the physician to discuss patient progress and any pertinent changes in condition. All programs should be individualized to the patient based on the results of the baseline tests. The metabolic stress test, cardiac biomarkers and echocardiogram are the measures that have the most clinical importance in determining safety of participating in the program. The exercise physiologist will develop a program using the results of the metabolic stress test. During the stress test, the patient will reach a maximal safe level of activity. The exercise routine will be set to work within the ‘safe range’ based on heart rate, Rate of Perceived Exertion (RPE) and/or symptoms experienced. All subsequent visits in the exercise program the patient will have a routine working within the previously tested safe levels where the patient is asymptomatic. The patients will be able to participate in a structured program that will still yield the benefits of physical activity. The program should be divided into two phases; intensive phase and maintenance phase and is depicted in FIGURE 4:
Figure 4.
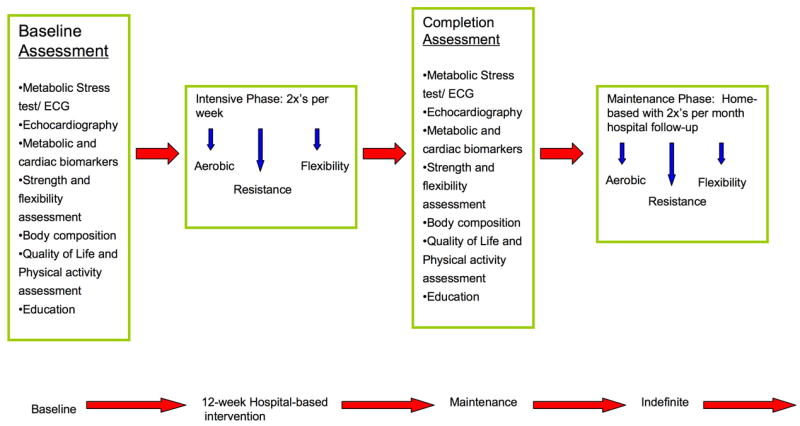
Recommended structure of an exercise rehabilitation program for children with cardiomyopathy.
a. Intensive phase
The program should include at least 24 visits, spanning 12 weeks of training. Three visits per week would be optimal, however two is acceptable. An individualized exercise plan for each child should be developed from the information obtained on the pre-program metabolic exercise test, baseline resistance training capacity, physical exam and past medical history. A metabolic stress test is completed with the use of a metabolic analyzer and a piece of exercise equipment usually a treadmill or cycle ergometer. The testing requires the patient to continuously exercise with incremental stages until volitional exhaustion or the clinician determines a clinical end-point has been reached. The patients are also equipped with an ECG unit. Baseline cardiac function should also be measured (through echocardiography). These baseline values should then be used to determine initial exercise prescriptions as well as to monitor changes as a result of physical activity intervention. During this baseline measurement, indications for treatment may become evident. Optimization of medical therapies is indicated prior to the prescription of any cardiac rehabilitation program. Lastly, an initial assessment of strength is important for any individual beginning a resistance-training program. A baseline value is used not only to gauge increases in strength but more importantly to set the appropriate resistance for the specific exercises to be completed. Prior to any assessment of maximal strength, the patient should have a familiarization period. This is usually done one week prior to testing that will also allow the child to recover from any muscle soreness. Furthermore, familiarization permits motor performance to improve.
The exercise sessions (lasting approximately one to one and one half hours) should consist of aerobic exercise (pediatric friendly games, bicycle or treadmill), as well as resistance training with appropriate warm-up and cool-down periods. The training equipment should be evaluated and meet the size requirement of the child. Appropriately sized equipment is more effective and safer for the child. The exercise sessions should be supervised directly by an exercise physiologist with a pediatric cardiologist on-site. Blood pressure and heart rate should be monitored periodically throughout the exercise session.
The patient’s progress should be reviewed weekly (more often as needed) and progressive increases should be made in the child’s exercise workload as tolerated and with physician approval. The child’s progress should be reviewed with the exercise physiologist and dietitian on a monthly or as needed basis. Education is an important component of the program. A variety of health related topics should be discussed during weekly educational sessions. Prior to the completion of the 12-week intensive phase, an exercise and nutrition plan should be developed for the child and family to ease the transition from the hospital-based program to home. This plan may include lifting free weights at home with an aerobic activity prescription.
b. Maintenance Phase
The maintenance phase of the program should consist of 2 exercise visits per month for 6 months. The child should be instructed on how to maintain daily exercise and nutrition logs. Exercise and nutrition plans should be re-evaluated and reviews with any of the staff should be scheduled as needed. This continued support is important to both maintain the child’s accomplishments and further improve function in the home environment. This will increase the chances of lifetime maintenance of a healthy lifestyle.
c. Exclusion criteria
Children with any condition or situation that would interfere with compliance or adherence to the program or that could potentially be harmful to the child should be excluded. Such conditions would include 1. impaired cognitive functioning such that the child is unable to follow instructions, 2. an unstable medical conditions: including but not limited to untreated congestive heart failure, unstable arrhythmias, 3. Under 5 years of age or size too small to use equipment safely,
d. Outcomes
The outcome measures should be based on the programmatic goals and be obtained before and immediately following the 12-week intensive program, as well as following the 6-month maintenance phase. These outcomes include 1. functional capacity (VO2 max [aerobic capacity with evaluation of rhythm disturbances] and 1-RM [muscular strength], 2. echocardiographic evaluation of ventricular function, 3. Cardiac biomarkers to assess ventricular health (pro-BNP, cardiac troponins, and hs-CRP), 4. quality of life and physical activity (and inactivity) assessments, 5. body composition (to determine lean and fat mass), and 6. education – knowledge questionnaires designed by the staff,
V. SUMMARY
The effects of exercise rehabilitation for children with a variety of chronic medical conditions is now only starting to be discovered. Exercise programs that include aerobic, resistance and flexibility training have proved to be safe and effective in improving disease-specific outcomes for children as young as 6 years. Two children with dilated cardiomyopathy presented here underwent a structured exercise program and showed benefits in improving cardiovascular fitness and strength, yet showed no deterioration in ventricular function. We recommend a careful and medically-supervised approach for exercise rehabilitation in children with cardiomyopathy. Larger, prospective studies on the functional and metabolic responses to exercise in children with cardiomyopathy are warranted in order to optimize their quality of life and functional status as well as lower their overall cardiovascular risk.
Acknowledgments
Supported by NIH NHLBI grant RO1 HL53392 and the Children’s Cardiomyopathy Foundation, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation. 1996;93:841–2. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 2.Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–33. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2006;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 4.Nugent AW, Daubeney PE, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 5.Towbin JA, Lowe AM, Colan SD, et al. Incidence, Causes, and Outcomes of Dilated Cardiomyopathy in Children. JAMA. 2006;296(15):1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 6.Arola A, Jokinen E, Ruuskanen O, et al. Epidemiology of Idiopathic Cardiomyopathies in Children and Adolescents; A Nationwide study in Finland. Am J of Epi. 1997;146(5):385–393. doi: 10.1093/oxfordjournals.aje.a009291. [DOI] [PubMed] [Google Scholar]
- 7.Towbin JA, Solaro RJ. Genetics of dilated cardiomyopathy: More genes that kill. J Am Coll Cardiol. 2004;44:2041–2043. doi: 10.1016/j.jacc.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348(17):1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 9.Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and Cause-Specific Outcomes of Hypertrophic Cardiomyopathy in Children: Findings From the Pediatrics Cardiomyopathy Registry. Circulation. 2007;115:773–781. doi: 10.1161/CIRCULATIONAHA.106.621185. [DOI] [PubMed] [Google Scholar]
- 10.Cox GF, Sleeper LA, Lowe AM, et al. Factors associated with establishing a causal diagnosis for children with cardiomyopathy. Pediatrics. 2006;118:1519–1531. doi: 10.1542/peds.2006-0163. [DOI] [PubMed] [Google Scholar]
- 11.Thiene G, Basso C, Calabrese F, Angelini A, Valente M. Twenty years of progress and beckoning frontiers in cardiovascular pathology Cardiomyopathies. Cardiovascular Pathology. 2005;14:165–169. doi: 10.1016/j.carpath.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Boucek MM, Edwards LB, Keck BM, et al. The Registry of the International Society for Heart and lung Transplantation: Sixth Official Pediatric Report-2003. J Heart Lung Transplant. 2003;22:636–652. doi: 10.1016/s1053-2498(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 13.Bilgic A, Ozbarlas N, Ozkutlu S, et al. Cardiomyopathies in children: clinical, epidemiological and prognostic evaluation. Jpn Heart J. 1990;31:789–797. doi: 10.1536/ihj.31.789. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE. Ventricular dysfunction clinical research in infants, children and adolescents. Prog Pediatr Cardiol. 2000;12:1–28. doi: 10.1016/s1058-9813(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 15.Evans RW. Economic and social costs of heart transplantation. Heart Transplant. 1982;1:243–51. [PubMed] [Google Scholar]
- 16. [January 23, 2007];Healthy People 2010. Retrieved from http://www.healthypeople.gov.
- 17.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 7. Baltimore, MD: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 18.Nieman DC. A health-Related Approach. 4. Mayfield Publishing Company; Mountain View, California: 1999. Exercise Testing and Prescription. [Google Scholar]
- 19.Ford ES, Li C. Physical Activity or Fitness and the Metabolic Syndrome. Expert Rev Cardiovasc Ther. 2006;4:897–915. doi: 10.1586/14779072.4.6.897. [DOI] [PubMed] [Google Scholar]
- 20.Walter U, Kramer S, Robl M. Physical (in)activity in Childhood and Adolescence. Dtsch Med Wochenschr. 2005;130:2876–2878. doi: 10.1055/s-2005-923319. [DOI] [PubMed] [Google Scholar]
- 21.Amisola RV, Jacobson MS. Physical Activity, Exercise, and Sedentary Activity: Relationship of the Causes and Treatment of Obesity. Adolesc Med. 2003;14:23–35. [PubMed] [Google Scholar]
- 22.Epstein LH, Paluch RA, Gordy CC, Dorn J. Decreasing Sedentary Behaviors in Treating Pediatric Obesity. Arch Pediatr Adolesc Med. 2000;154:220–226. doi: 10.1001/archpedi.154.3.220. [DOI] [PubMed] [Google Scholar]
- 23.Epstein LH, Roemmich JN, Paluch RA, Raynor HA. Physical activity as a substitute for sedentary behavior in youth. Ann Behav Med. 2005;29:200–209. doi: 10.1207/s15324796abm2903_6. [DOI] [PubMed] [Google Scholar]
- 24.Lunt D, Briffa T, Briffa NK, Ramsay J. Physical Activity Levels of Adolescents with Congenital Heart Disease. Australian Journal of Physiotherapy. 2003;49:43–49. doi: 10.1016/s0004-9514(14)60187-2. [DOI] [PubMed] [Google Scholar]
- 25.United states Department of Health and Human Services. Physical Activity and Health: a report of the Surgeon General. Atlanta GA: US Department of Health and Human Services, Centers for Disease Controls and Prevention, National Center for Chronic Disease Prevention and Health Promotion; [Google Scholar]
- 26.Physical Activity. [February 14, 2007];American Heart Association Scientific Position. Retrieved from http://www.americanheart.org/presenter.jhtml?identifier=4563.
- 27.Pate RR, Pratt M, Blair SN, et al. Physical Activity and Public Health. A Recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 28.Durstine JL, Painter P, Franklin BA, Morgan D, et al. Physical activity for the chronically ill and disabled. Sports Med. 2000;30:207–219. doi: 10.2165/00007256-200030030-00005. [DOI] [PubMed] [Google Scholar]
- 29.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health. Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 30.Lanningham-Foster L, Jensen TB, Foster RC, et al. Energy expenditure of sedentary screen time compared with active screen time for children. Pediatrics. 2006;118:1831–1835. doi: 10.1542/peds.2006-1087. [DOI] [PubMed] [Google Scholar]
- 31.Warburton DE, Bredin SS, Horita LT, et al. The health benefits of interactive video game exercise. Appl Physiol Nutr Metab. 2007;32:655–663. doi: 10.1139/H07-038. [DOI] [PubMed] [Google Scholar]
- 32.TR Baechle, RW Earle. Essentials of strength training and conditioning/National Strength and Conditioning Association. 2. Champaign, IL: Human Kinetics; 2000. [Google Scholar]
- 33.Maffulli N. Intensive training in young athletes. The orthopaedic surgeon’s viewpoint. Sports Med. 1990;9:229–243. doi: 10.2165/00007256-199009040-00004. [DOI] [PubMed] [Google Scholar]
- 34.Maffulli N, Bruns W. Injuries in young athletes. Eur J Pediatr. 2000;159:59–63. doi: 10.1007/s004310050011. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Pediatrics. Strength training by children and adolescents. Pediatrics. 2001;107:1470–1472. doi: 10.1542/peds.107.6.1470. [DOI] [PubMed] [Google Scholar]
- 36.Malina RM. Weight training in youth-growth, maturation, and safety: An evidence-based review. Clin J Sports Med. 2006;16:478–487. doi: 10.1097/01.jsm.0000248843.31874.be. [DOI] [PubMed] [Google Scholar]
- 37.Guy JA, Micheli LJ. Strength training for children and adolescents. J Am Acad Orthop Surg. 2001;9:29–36. doi: 10.5435/00124635-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Sewall L, Micheli LJ. Strength training for children. J Pediatr Orthop. 1986;2:143–146. doi: 10.1097/01241398-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Annesi JJ, Westcott WL, Faigenbaum AD, Unruh JL. Effects of a 12-week physical activity protocol delivered by YMCA after-school counselors (Youth Fit for Life) on fitness and self-efficacy changes in 5–12-year-old boys and girls. Res Q Exerc Sport. 2005;76:468–476. doi: 10.1080/02701367.2005.10599320. [DOI] [PubMed] [Google Scholar]
- 39a.Gotshalk LA, Berger RA, Kraemer WJ. Cardiovascular responses to a high-volume continuous circuit resistance training protocol. J Strength and Conditioning Research. 2004;18:760–764. doi: 10.1519/14954.1. [DOI] [PubMed] [Google Scholar]
- 40.Blundell SW, Shepherd RB, Dean CM, Adams RD, Cahill BM. Functional strength training in cerebral palsy: a pilot study of a group circuit training class for children aged 4–8 years. Clin Rehabil. 2003;17:48–57. doi: 10.1191/0269215503cr584oa. [DOI] [PubMed] [Google Scholar]
- 41.Faigenbaum AD, Loud RL, O’Connell J, Glover S, Westcott WL. Effects of different resistance training protocols on upper-body strength and endurance development in children. J Strength Cond Res. 2001;15:459–465. [PubMed] [Google Scholar]
- 42.Guy JA, Micheli LJ. Strength training for children and adolescents. J Am Acad Orthop Surg. 2001;9:29–36. doi: 10.5435/00124635-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Powers SK, Howley ET. Exercise physiology: Theory and application to fitness and performance. 4. New York NY: McGraw-Hill Companies; 2001. [Google Scholar]
- 44.Kisner C, Colby LA. Therapeutic Exercise Foundations and Techniques. 4. F.A. Davis Company; Philadelphia, USA: [Google Scholar]
- 45.Faigenbaum AD, Bellucci M, Bernieri A, Bakker B, Hoorens K. Acute effects of different warm-up protocols on fitness performance in children. J Strength and Conditioning Research. 2005;19:376–381. doi: 10.1519/R-15344.1. [DOI] [PubMed] [Google Scholar]
- 46.Faigenbaum AD, Westcott W. Strength & power for young athletes. Champaign, IL: Human Kinetics; 2000. [Google Scholar]
- 47.The physical fitness specialist certification manual, The Cooper institute for Aerobics Research, Dallas Tx, revised 1997.
- 48.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Progress in Cardiovascular Disease. 1995;38:1–22. doi: 10.1016/s0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 49.Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise VO2 for optimal cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 50.Jurynec J. Hypertrophic cardiomyopathy: A review of etiology and treatment. J Cardiovasc Nursing. 2007;22:65–73. doi: 10.1097/00005082-200701000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Jennings GL, Deakin G, Korner P, et al. What is the dose-response relationship between exercise training and blood pressure? Annals of Medicine. 1991;23:313–318. doi: 10.3109/07853899109148066. [DOI] [PubMed] [Google Scholar]
- 52.Haskell WL. Health consequences of physical activity: understanding and challenges regarding dose-response. Med Sci Sports and Exerc. 1994;26:645–660. doi: 10.1249/00005768-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Hagberg JM, Monatin SJ, Martin WH. Blood pressure and hemodynamic responses after exercise in older hypertensives. J Appl Physiol. 1987;63:270–276. doi: 10.1152/jappl.1987.63.1.270. [DOI] [PubMed] [Google Scholar]
- 54.Yiannis TE, Amalia YE, Dimitios B. A single bout of brisk walking increase basal very low-density lipoprotein triacylglycerol clearance in young men. Metabolism. 2007;56:1037–1043. doi: 10.1016/j.metabol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Lele SS, Thomson HL, Seo H, Belenkie I, McKenna WJ, Frenneaux MP. Exercise capacity in hypertrophic cardiomyopathy: Role of stroke volume limitation, heart rate, and diastolic filling characteristics. Circulation. 1995;92:2886–2894. doi: 10.1161/01.cir.92.10.2886. [DOI] [PubMed] [Google Scholar]
- 56.Klecha A, Kawecka-Jaszcz K, Bacior B. Physical training in patients with chronic heart failure of ischemic origin: effect on exercise capacity and left ventricular remodeling. Eur J Cardiovasc Prev Rehabil. 2007;14:85–91. doi: 10.1097/HJR.0b013e3280114f12. [DOI] [PubMed] [Google Scholar]
- 57.Roditis P, Dimopoulos S, Sakellariou D, et al. The effect of exercise training on the kinetics of oxygen uptake in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2007;14:304–311. doi: 10.1097/hjr.0b013e32808621a3. [DOI] [PubMed] [Google Scholar]
- 58.Belardinelli R, Capestro F, Misiani A, Scipione P, Georgiou D. Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil. 2006;13:818–825. doi: 10.1097/01.hjr.0000230104.93771.7d. [DOI] [PubMed] [Google Scholar]
- 59.Moalla W, Maingourd Y, Gauthier R. Effect of exercise training on respiratory muscle oxygenation in children with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:604–611. doi: 10.1097/01.hjr.0000201515.59085.69. [DOI] [PubMed] [Google Scholar]
- 60.Fredriksen PM, Kahrs N, Blaasvaer S, et al. Effect of physical training in children and adolescents with congenital heart disease. Cardiol Young. 2000;10:107–114. doi: 10.1017/s1047951100006557. [DOI] [PubMed] [Google Scholar]
- 61.Taber’s Cyclopedic Medical Dictionary. 20. F.A. Davis; Philadelphia Pa: [Google Scholar]
- 62.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 63.Sutton P. Measurements in Cardiology. Parthenson Publishing; Nashville, TN: 1999. [Google Scholar]
- 64.Riggs TW. Abnormal right ventricular filling in patients with dilated cardiomyopathy. Pediatr Cardiolo. 1993;14:1–4. doi: 10.1007/BF00794835. [DOI] [PubMed] [Google Scholar]
- 65.Whyte GP, George K, Sharma S, et al. Cardiac fatigue following prolonged endurance exercise of different distances. Med Sci Sports Exerc. 2000;21:1067–1072. doi: 10.1097/00005768-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Libonati JR. Myocardial diastolic function and exercise. Med Sci Sports Exerc. 1999;31:1741–1747. doi: 10.1097/00005768-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Opocher F, Varnier M, Sanders SP, et al. Effects of aerobic training in children after the Fontan operation. Cardiol. 2005;95:150–152. doi: 10.1016/j.amjcard.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 68.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Effects of exercise training on left ventricular filling at rest and during exercise in patients with ischemic cardiomyopathy and severe left ventricular systolic dysfunction. Am Heart J. 1996;132:61–70. doi: 10.1016/s0002-8703(96)90391-9. [DOI] [PubMed] [Google Scholar]
- 69.Webb-Peploe KM, Chua TP, Harrington D, Henein MY, et al. Different responses of patients with idiopathic and ischaemic dilated cardiomyopathy to exercise training. Int J Cardiol. 2000;74:215–224. doi: 10.1016/s0167-5273(00)00293-x. [DOI] [PubMed] [Google Scholar]
- 70.Myers J, Wagner D, Schertler T, et al. Effects of exercise training on left ventricular volumes and function in patients with nonischaemic cardiomyopathy: application of magnetic resonance myocardial tagging. Am Heart J. 2002;144:719–725. doi: 10.1067/mhj.2002.124401. [DOI] [PubMed] [Google Scholar]
- 71.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 72.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 73.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes. Further evidence for the existence of the ‘vulnerable patient’. Circulation. 2004;110:1926–1932. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- 74.Ross R. The pathogenesis of atherosclerosis: a perspective of the 1990’s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 75.Zelis R, Longhurst J, Capone RJ, et al. A comparison of regional blood flow and oxygen utilization during dynamic forearm exercise in subjects and patients with congestive heart failure. Circulation. 1974;50:137–143. doi: 10.1161/01.cir.50.1.137. [DOI] [PubMed] [Google Scholar]
- 76.Zelis R, Sinoway LI, Musch TI, et al. Regional blood flow in congestive heart failure: concept of compensatory mechanisms with short and long time constants. Am J Cardiol. 1988;62:2E–8E. doi: 10.1016/s0002-9149(88)80002-x. [DOI] [PubMed] [Google Scholar]
- 77.McMurray JJ, Ray SG, Abdullah I, et al. Plasma endothelin in chronic heart failure. Circulation. 1992;85:1374–1379. doi: 10.1161/01.cir.85.4.1374. [DOI] [PubMed] [Google Scholar]
- 78.Kiowski W, Sutch G, Hunziker P, et al. Evidence for endothelin-1 mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- 79.Linke A, Schoene N, S Gielen, et al. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–397. doi: 10.1016/s0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- 80.Hambretch R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 81.Haram PM, Adams V, Kemi OJ, Brubakk AO, et al. Time-course of endothelial adaptation following acute and regular exercise. European Journal of Cardiovascular Prevention and Rehabilitation. 2006;13:585–591. doi: 10.1097/01.hjr.0000198920.57685.76. [DOI] [PubMed] [Google Scholar]
- 82.Benjamin EJ, Larson MG, Keyes MJ. Clinical correlates and heritability of flow-mediated dilation in the community: The Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 83.Johnson LR, Rush JW, Turk JR, Price EM, Laughlin MH. Short-term exercise training increases Ach-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol. 2001;90:1102–1110. doi: 10.1152/jappl.2001.90.3.1102. [DOI] [PubMed] [Google Scholar]
- 84.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 85.Green DJ, Maiorana A, O’Driscoll G, et al. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarkson P, Montogomery HE, Mullen MJ, Donald AE, et al. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 87.Maeda S, Miyauchi T, Kakiyama T, et al. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci. 2001;69:1005–1016. doi: 10.1016/s0024-3205(01)01192-4. [DOI] [PubMed] [Google Scholar]
- 88.Maria Sarullo F, Gristina T, Brusca I, Milia S, et al. Effect of physical training on exercise capacity, gas exchange and N-terminal pro-brain natriuretic peptide levels in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:812–817. doi: 10.1097/01.hjr.0000238396.42718.61. [DOI] [PubMed] [Google Scholar]
- 89.Caulin-Glaser T, Falko J, Hindman L, La Londe M, Snow R. Cardiac rehabilitation is associated with an improvement in C-Reactive Protein levels in both men and women with cardiovascular disease. J Cardiopulmonary Rehabil. 2005;25:332–336. doi: 10.1097/00008483-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Carlson LA, Mossfeldt F. Acute effects of prolonged heavy exercise on the concentration of plasma lipids and lipoproteins in man. Acta Physiol Scand. 1964;62:51–59. doi: 10.1111/j.1748-1716.1964.tb03951.x. [DOI] [PubMed] [Google Scholar]
- 91.Thompson PD, Crouse SF, Goodpaster B, Kelley D, Niall Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33:S438–S445. doi: 10.1097/00005768-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 92.Delvin JT, Horton ES. Effects of prior high-intensity exercise on glucose metabolism in normal and insulin resistant men. Diabetes. 1985;34:973–979. doi: 10.2337/diab.34.10.973. [DOI] [PubMed] [Google Scholar]
- 93.Perseghin GT, Price TB, Petersen KF. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin resistant subjects. N Eng J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 94.Rogers MA, Yamamoto C, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Improvement in glucose tolerance after one week of exercise in patients with mild NIDDM. Diabetes Care. 1988;11:613–618. doi: 10.2337/diacare.11.8.613. [DOI] [PubMed] [Google Scholar]
- 95.Gleeson M. Interleukins and exercise. J Physiol. 2000;529:1. doi: 10.1111/j.1469-7793.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pederson BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration and adaptation. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 97.Cheng V, Kazanagra R, Garcia A, et al. A rapid bed side test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37:386–391. doi: 10.1016/s0735-1097(00)01157-8. [DOI] [PubMed] [Google Scholar]
- 98.Koglin J, Pehlvivanli S, Schwaiblmair M, et al. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001;38:1934–1941. doi: 10.1016/s0735-1097(01)01672-2. [DOI] [PubMed] [Google Scholar]
- 99.Conraads VM, Beckers P, Vaes J, Martin M, et al. Combined endurance/resistance training reduces NT-proBNP levels in patients with chronic heart failure. European Heart J. 2004;25:1797–1805. doi: 10.1016/j.ehj.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 100.LeMaitre JP, Harris S, Fox KA, Denvir M. Changing in circulating cytokines after 2 forms of exercise training in chronic stable heart failure. Am Heart. 2004;147:100–105. doi: 10.1016/j.ahj.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Pedersen BK, Fischer CP. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care. 2007;10:265–271. doi: 10.1097/MCO.0b013e3280ebb5b3. [DOI] [PubMed] [Google Scholar]
- 102.Cooper DM, Nemet D, Galassetti P. Exercise, stress, and inflammation in the growing child: from bench to the playground. Pulmonology. 2004;16:286–292. doi: 10.1097/01.mop.0000126601.29787.39. [DOI] [PubMed] [Google Scholar]
- 103.Stein REK, Jessop DJ. Manual for the Functional Status II(R) Measure: PACTS Papers. Bronx, NY: Albert Einstein College of Medicine; 1991. [Google Scholar]
- 104.Stein REK, Jessop DJ. Functional Status II(R): a measure of child health status. Med Care. 1990;28:1041–1055. doi: 10.1097/00005650-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 105.Sleeper LA, Anderson P, Hsu DT. Pediatric Heart Network Investigators. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–33. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 107.Glover DW, Maron BJ. Profile of preparticipation cardiovascular screening for high school athletes. JAMA. 1998;279:1817–1819. doi: 10.1001/jama.279.22.1817. [DOI] [PubMed] [Google Scholar]
- 108.Paridon SM, Alpert BS, Boas SR, et al. Clinical stress testing in the pediatric age group: a statement from the American Heart Association Council on Cardiovascular Disease in the Young, Committee on Atherosclerosis, Hypertension, and Obesity in Youth. Circulation. 2006;113:1905–1920. doi: 10.1161/CIRCULATIONAHA.106.174375. [DOI] [PubMed] [Google Scholar]
- 109.Priori SG, Aliot E, Blomstrom-Lundqvist C, et al. European Society of Cardiology. Update of the guidelines on sudden cardiac death of the European Society of Cardiology. Eur Heart J. 2003;24:13–15. doi: 10.1016/s0195-0668x(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 110.Tomassoni TL. Role of exercise in the management of cardiovascular disease in children and youth. Med Sci Sports Exerc. 1996;4:406–413. doi: 10.1097/00005768-199604000-00003. [DOI] [PubMed] [Google Scholar]


