Abstract
Objective
The specification and differentiation of hematopoietic stem cells into red blood cells requires precise coordination by multiple transcription factors. Most genes important for erythroid maturation are regulated by the Gata family of DNA-binding proteins. Previously, we identified three novel genes kelch-repeat containing protein (krcp), kiaa0650, and testhymin/glucocorticoid inducible transcript 1 (glcci1) to be expressed in erythroid cells in a Gata-independent manner, and we sought to further understand how these transcripts are regulated during zebrafish hematopoiesis.
Methods
We employed a loss-of-function approach, using combinations of antisense morpholinos to hematopoietic transcription factors and assayed for changes in gene expression in zebrafish embryos.
Results
Upon examination of embryos deficient for Gata1, Gata2, Biklf and/or Scl, we found distinct gene combinations were required for expression of the novel genes. While krcp expression was dependent upon Gata1 and Biklf, kiaa0650 expression was greatly reduced and glcci1 was maintained in Gata1/Gata2/Biklf-deficient embryos. As with the gata1 gene, kiaa0650 and krcp required Scl for blood expression. Although reduced, glcci1 was expressed in posterior blood precursors in the absence of Scl and Gata2.
Conclusions
This work identifies glcci1 as having Scl-independent expression in the posterior hematopoietic mesoderm, suggesting that its posterior expression is activated by factors upstream or parallel to Scl and Gata2. Additionally, these studies establish that blood gene expression programs are regulated by transcription factors acting in combination during erythroid maturation.
Introduction
During mammalian embryogenesis, blood cells develop on the yolk sac (YS) and predominantly give rise to erythrocytes. The formation of these blood cells requires the transcription factor stem cell leukemia gene (scl/tal1) and the lim-only 2 (lmo2) gene[1],[2],[3],[4]. In the mouse, transcripts of these genes are first detected in the YS at embryonic day 7.25 (E7.25)[5],[6], and are necessary for the specification of embryonic blood cells and proper angiogenesis[4],[7]. The zinc finger transcription factors GATA1 and GATA2, also expressed in the YS, are involved in blood cell proliferation and maturation events[5],[6]. In GATA1 mutant embryos, blood cells are formed, but are unable to differentiate, demonstrating an essential role for GATA1 in terminal erythroid maturation[8],[9]. GATA2 is necessary not only for maintaining erythroid cell number, but also for proper proliferation and survival of multipotential progenitor cells [9]. Additionally, there are erythroid lineage specific factors that have essential roles in red blood cell maturation. For instance, loss of the Erythroid Krüppel-like factor (EKLF), a CACC box zinc finger transcription factor, in mice results in disrupted adult β-globin expression, severe anemia, and ultimately death by E16[10],[11].
Similar to mammals, zebrafish embryonic hematopoiesis is detected by the expression of scl, lmo2 and gata2 at the 2-somite stage in blood and endothelial cell precursors in the posterior mesoderm[12]. The first genes in zebrafish to mark erythroid precursors, gata1 and the blood island-enriched Krüppel-like factor (biklf/klf4), are expressed by 4 somites [13],[14],[15]. By 12 somites, these cells express embryonic globins and migrate towards the midline to form the intermediate cell mass (ICM), a group of blood cells surrounded by axial vein endothelium. By 24 hours post fertilization (hpf), erythroblasts residing in the ICM express genes important for terminal erythroid maturation, such as the heme synthetic enzyme aminolevulinate synthase 2 (alas2), and eventually enter circulation[16]. Concomitant with erythropoiesis, myeloid cell development can be also detected by expression of the myeloid genes l-plastin and myeloperoxidase (mpo) in the ICM and an independent anterior location referred to as the rostral blood island (RBI)[17]. After 3 days post fertilization (dpf), adult hematopoiesis can be identified by ikaros and rag1 staining in the thymic primordium [18],[19],[20]. Expression of blood markers such as scl and c-myb, as well as morphologically identifiable blood cells, are also observed in the developing kidney[21].
There are several zebrafish mutant lines that harbor defects at specific stages of hematopoiesis, and their study has improved our understanding of embryonic blood cell development. The cloche mutant, whose gene has not been identified, has virtually undetectable expression of scl and gata1 and lacks blood and endothelial cells[22],[23],[24]. The spadetail (spt)/tbx16 mutant, whose primary defect is in posterior paraxial mesoderm formation, lacks early scl and gata1 expression in the posterior mesoderm at 4 somites[25],[26]. ICM blood cells are absent in spt, but unlike clo, these mutants begin expressing scl, gata2 and flk1 by 10 somites[27],[26]. The expression of these genes has been attributed to a later wave of angioblast development as spt mutants have endothelial cells that form severely disorganized vessels[26]. Consistent with the mouse scl knockout, Scl-deficient zebrafish have no gata1 and globin-expressing blood cells as well as defects in the formation of intersegmental vessels[28],[29]. These findings highlight the dual functions of scl, early in blood cell specification and later in endothelial cell organization. The moonshine (mon) mutant, which has a disruption in the transcriptional intermediary factor 1 γ(tif1γ) gene, has defects specific to the erythroid blood lineage. In this mutant, scl and gata1 expression are initiated normally, but by 18 somites most primitive erythroid cells are absent due to apoptosis[30]. Loss of zebrafish Gata1 also results in an absence of mature erythroid cells; however, most ICM precursors in Gata1-deficient embryos differentiate into myeloid cells that become granulocytes and macrophages[31],[32],[33]. Unlike Gata1, Gata2 deficiency does not cause a conversion to myelopoiesis, but results in a modest reduction in both myeloid and erythroid cell numbers, suggesting that like mammalian systems, Gata2 is required for multipotential cell survival and proliferation. Similar to mammalian EKLF, loss of Biklf also results in decreased numbers of red blood cells and a reduction in globin and gata1 expression[34].
We have previously shown in zebrafish that expression of three genes, testhymin (hereafter referred to as glucocorticoid inducible transcript 1 (glcci1)), kiaa0650, and kelch-repeat containing protein (krcp), occurs independently of Gata1 and Gata2[32]. To understand the complex process underlying the regulation of these and known blood genes, a combinatorial gene knock-down approach was employed using morpholinos to abolish gata1, gata2, biklf and scl protein expression. glcci1 was discovered to be a novel blood marker that is not expressed in ventral mesoderm or in endothelial cell precursors, but is specific to the hematopoietic system. Interestingly, glcci1 is expressed independently of Scl/Gata2 in the posterior hematopoietic precursors. Our studies begin to uncover how programs of gene expression are regulated by multiple hematopoietic transcription factors that ultimately coordinate erythroid differentiation.
Materials and Methods
Zebrafish strains
Zebrafish were maintained and staged as described[35],[36]. Tü and TL strains were used for morpholino (MO) injection experiments and expression analysis. We incrossed heterozygous mutants of the following lines: sptb104, montg234, and clom39 [22],[25],[30]. The vltm651 line was genotyped as described[31].
Morpholino Injections
The gata1, gata1 mismatch, gata2 (also referred to as gata2E/I1), gata2 mismatch[32], scl, scl mismatch[28], biklf [34] MOs were used. A four basepair mismatch MO to biklf (5’-TGGAAATGATAGGGTACTGAGAAGG-3’) was designed. When the biklf mismatch MO was injected alone, globin expression was never affected, and when it was injected with the gata1 MO globin expression was not abolished (n=22; 100%). A second gata2 MO was designed against the splice donor site of exon 1 (gata2e1D: 5’- AATGAATCCTACCGTGCGCTTGTCC-3’), and it phenocopied the previously described gata2 MO when 1 nL was injected at 0.4 µM. Loss of globin expression was used as a control for the gata1/gata2 and the gata1/biklf MO injections and loss of gata1 was used as a control for the scl MO injections.
Reverse Transcription PCR
RNA was isolated from approximately 30 uninjected or gata2E/I1 MO-injected embryos as described[28], and cDNA was prepared using the Superscript II RT (Invitrogen). PCR was performed using EF1α control primers [28] and primers designed to gata2 (gata2.E2 forward: 5’-CACACATCATCCCATCCCGACCTA-3’ and gata2.E5 reverse: 5’- GGAATGGTGCGGGTGGCTGAATGT-3’). The uninjected embryo pool yielded a normal size product of ~690 base pairs (bp) while the gata2 E/I1 MO-injected embryo pool gave a ~540 kb product (Supplemental Figure 1A). After isolating, cloning, and sequencing the 540 bp product, it was determined that exon 3 (~150 bp) was absent in the gata2 E/I1 MO-injected amplified cDNA. Further sequence analysis revealed that deletion of exon 3 causes a frame shift into a premature stop codon in exon 4 and truncates the Gata2 protein at amino acid 337 (Supplemental Figure 1B). Since exon 3 and 4 contain both zinc fingers and the DNA-binding domain, their loss most likely renders the protein non-functional.
Whole mount in situ hybridizations
Whole mount in situ hybridization was performed as described[37]. Digoxigenin-labeled antisense RNA was transcribed from linearized plasmids using a Roche SP6/T7 RNA labeling kit. The following antisense riboprobes were generated as described: glcci1/testhymin, kiaa0650, krcp, carbonic anhydrase [32], gata1, gata2[13], biklf[38], scl[24], draculin[39], βe3 globin[40], and ikaros[19]. Embryos were mounted in glycerol and photographed using a Nikon Eclipse compound microscope.
Results
Expression analysis of glcci1, kiaa0650, and kcrp
From a zebrafish in situ hybridization screen for genes expressed in developing blood cells, three genes were discovered to not require Gata1 or Gata2 for their blood expression[32]. Further evaluation of glcci1, kiaa0650, and krcp revealed that these genes are expressed in two bilateral stripes of the posterior mesoderm at early stages of somitogenesis (Figure 1). A time-course analysis indicated that kiaa0650 and glcci1 are first expressed in blood precursors at the 3-somite stage, while krcp transcripts are not detected until the 5 somites. Expression of all three genes is maintained in the blood cells as they migrate medially to form the ICM at 24 hpf (Figure 1). glcci1 and kiaa0650 are expressed in the circulating primitive blood precursors until 36–48 hpf (Figure 1, arrowheads). krcp is also expressed at these stages, but its expression is decreased in the ICM after 20 hpf and found in fewer circulating blood cells than glcci1 or kiaa0650. Diffuse expression of glcci1, kiaa0650 and krcp was observed in regions of the brain at 24 hpf and 36 hpf (Figure 1). krcp was expressed in the somites and the tailbud from early somitogenesis to 24 hpf, and after 36 hpf, krcp transcripts were found in the fin buds and in the gut endoderm (Figure 1 and data not shown). Virtually no expression of these genes was detected in the blood, kidney or thymus between 48 hpf and 5 dpf although modest levels of kiaa0650 were found in the ventral tail region between 3–5 dpf in a pattern similar to scl (data not shown)[24].
Figure 1. glcci1, kiaa0650, krcp are expressed in erythroid blood cell precursors.
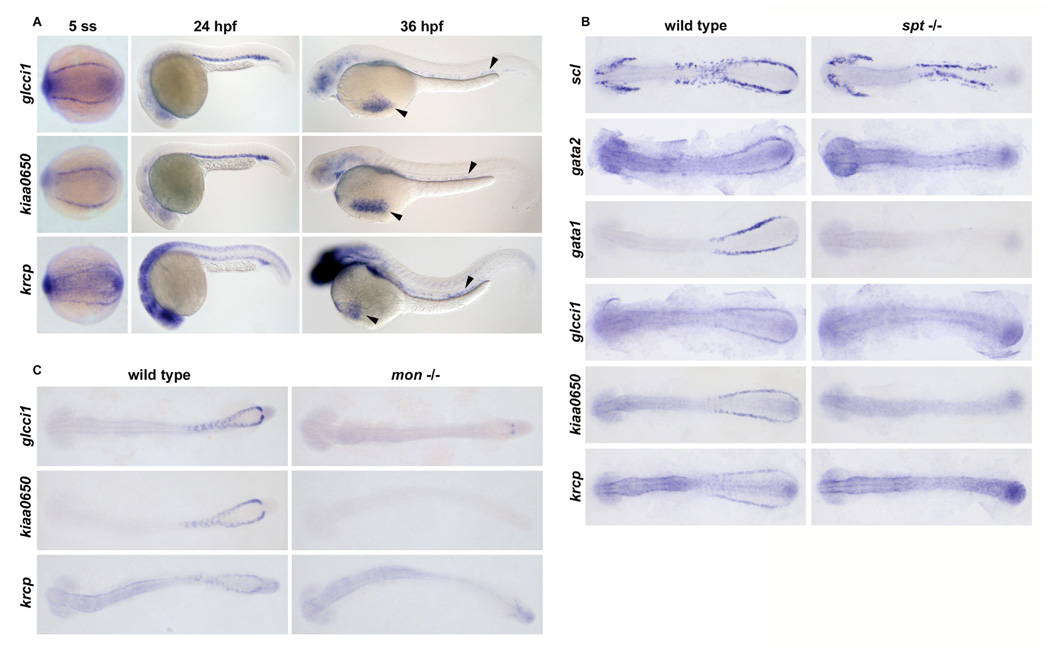
(A) Wild type embryos exhibiting expression of glcci1, kiaa0650, krcp in blood cells in the posterior mesoderm at 5 somites (dorsal view, left panels), in the ICM blood cells at 24 hpf (lateral view, middle panels), and in circulating blood cells in the vessels and over the yolk (arrowheads, lateral view, right panels). (B) Flat mount images of embryos are oriented with anterior on the left and posterior on the right. Expression of gata1 (100%, n=9), glcci1 (100%, n=7), kiaa0650 (100%, n=3), and krcp (100%, n=6) was lost in sptb104 mutant embryos compared with wild-type and heterozygous siblings at 12 somites. Expression of scl (100%, n=5) and gata2 (100%, n=7) was present but disorganized in putative angioblasts of sptb104 mutant embryos compared with wild-type and heterozygous siblings. (C) At 18 somites, normal expression of glcci1 (5/27), kiaa0650 (7/37), and krcp (6/27) in ICM blood cell precursors of wild-type and heterozygous siblings was virtually absent in montg234 mutant embryos. Numbers in parentheses represent the number of embryos lacking blood expression out of total number of embryos in an incross clutch.
To test if these genes were present in blood and/or angioblasts, we analyzed their expression in cloche (clo) mutants, which specifically lack both hematopoietic and endothelial cell precursors. ICM expression of glcci1, kiaa0650, and krcp was absent in clo mutants, indicating these genes are expressed in blood or endothelium (data not shown). To determine that these genes were expressed in erythroid blood precursors and not endothelial cells, their expression was examined in spt and mon mutants. Upon inspection of spt embryos, a mutant that lacks blood but retains endothelial cells, we found that expression of all three genes was lost at 5 and 12 somites, indicating that their expression is confined to the hematopoietic compartment (Figure 1B and data not shown). ICM expression of glcci1, kiaa0650, and krcp was also virtually lost at 18 somites in the mon mutants compared to their wild type and heterozygous siblings (Figure 1C). At this time, mon mutant embryos have lost most erythroid precursors due to cell death[30]. Taken together, these results indicate that glcci1, kiaa0650, and krcp are expressed in blood precursors destined to become erythrocytes.
Loss of Biklf and GATA factors selectively affects erythroid gene expression
To determine which hematopoietic transcription factors are mediating blood cell expression of glcci1, kiaa0650, and krcp in the absence of GATA factors, loss-of-function experiments were performed using morpholino (MO) antisense oligonucleotides. biklf is important for globin and gata1 expression in zebrafish[34], and is expressed independently of Gata1 and Gata2[32]. Therefore, we sought to determine whether biklf could regulate the blood expression of these genes. First, we examined the effect of loss of Biklf and Gata1 on the blood program, using well-characterized blood markers. Loss of Gata1 alone caused reduced numbers of ICM cells expressing the erythroid markers globin, carbonic anhydrase, and gata1 compared to controls at 20 somites (Figure 2). Alternatively, expression of ikaros, a transcription factor found in myeloid and erythroid lineages, was unaltered compared to controls. This result is consistent with the ICM cells of Gata1-deficient embryos converting to the myeloid lineage[32].
Figure 2. Loss of Biklf and GATA factors selectively effects erythroid gene expression.
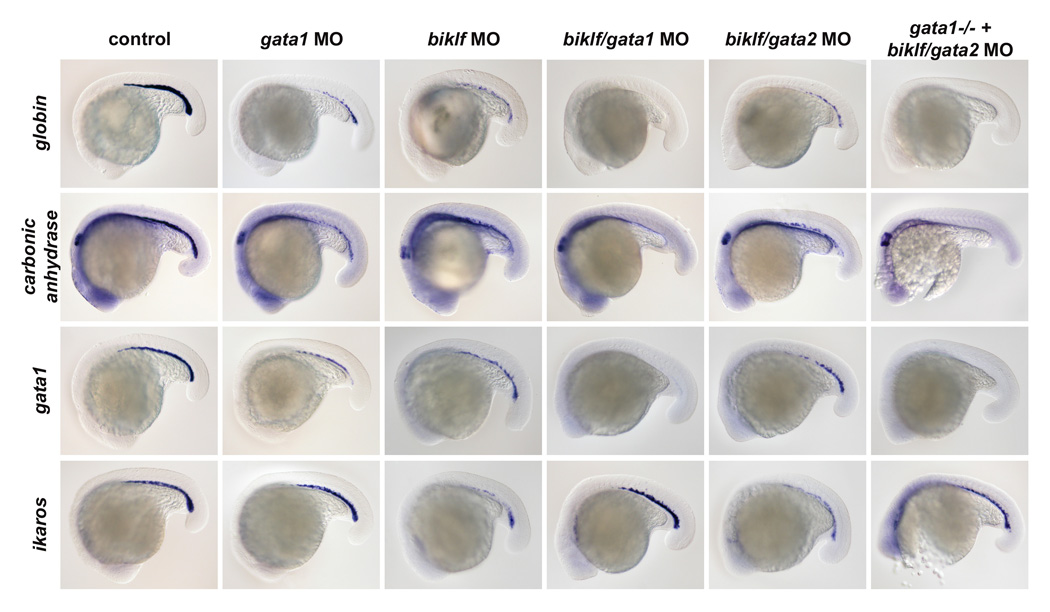
Wild type ICM blood expression of globin, carbonic anhydrase, gata1, and ikaros in control embryos at 20 hpf (left column). There is a decrease in the number of cells expressing globin (100%, n=67), carbonic anhydrase (100%, n=22), and gata1 (97%, n=34) in gata1 and biklf MO-injected embryos. While expression of ikaros is maintained in the myeloid ICM cells of gata1 MO-injected embryos (100%, n=13), the number of ikaros-expressing ICM blood cells is reduced in biklf MO-injected embryos (100%, n=36). There is a loss of globin (100%, n=39), carbonic anhydrase (63%, n=16), and gata1 (100%, n=29) expression in the ICM blood cells of gata1/biklf MO-injected and gata2/biklf MO-injected gata-/- embryos. Relatively normal ikaros expression is observed in ICM cells of gata1/biklf MO-injected and gata2/biklf MO-injected gata-/- embryos (100%, n=8). A decrease in the number of ICM cells expressing globin (100%, n=53), carbonic anhydrase (60%, n=30), gata1 (100%, n=24), and ikaros (100%, n=45) is found in gata2/biklf MO-injected embryos.
Similar to loss of Gata1, loss of Biklf caused a reduction in the number of cells expressing globin, carbonic anhydrase, and gata1 at 20 somites (Figure 2, Supplemental Figure 1). We next determined whether genes previously found to require Gata1 for expression were dependent on Biklf. Biklf MO-injected embryos were examined for the expression of SH3 domain binding protein-5 (SH3BP5), epsin/mitotic phosphoprotein and biliverdin reductase, Gata1-dependent genes. While loss of Biklf reduced the number of blood cells expressing SH3BP5, epsin, and biliverdin reductase compared to controls at 24 hpf, it did not completely eliminate their ICM expression as in gata1 MO-injected embryos[32] (data not shown). This result suggests that Biklf is not required for expression of these Gata1-dependent genes, and instead may regulate erythroid cell number. In contrast to Gata1 deficiency, loss of Biklf caused a decrease in ikaros staining at 24 hpf compared to controls and did not result in a myeloid cell expansion in the ICM (Figure 2; Supplemental Figure 1). These results indicate that Biklf is required for maintaining proper ICM blood cell number and for erythroid differentiation, but not important for determining myeloerythroid fates (Supplementary Figure 1). These results are consistent with a study demonstrating reduced gata1 and globin expression in biklf MO-injected embryos[34].
To determine the effect of loss of Biklf and Gata1 and/or Gata2 on blood development, we examined gene expression in embryos deficient for specific combinations of these factors. Similar to Gata1/Gata2-deficient embryos, Biklf/Gata1-deficient embryos lost globin, carbonic anhydrase and gata1 transcripts in the ICM at 20 hpf, indicating that both genes are necessary for expression (Figure 2). As loss of Gata2 causes a reduction in blood cells, a combined loss of Gata2 and Biklf also resulted in a reduction in the number of ICM blood cells expressing globin, carbonic anhydrase, and gata1 (Figure 2). Since injection of all three MOs simultaneously was toxic, the biklf and gata2 MOs were injected into vlad tepes/gata1 heterozygous mutant incrosses and embryos were analyzed. Interestingly, the number of ikaros-expressing or cmyb-expressing ICM cells in Biklf/Gata1- and Biklf/Gata1/Gata2- deficient embryos resembles that of gata1 morphants rather than the biklf MO-injected embryos (Figure 2, data not shown). This result is consistent with the Gata1-deficient ICM containing myeloid precursor cells, and suggests that biklf acts downstream of gata1 in the erythroid lineage. Biklf/Gata1/Gata2-deficient embryos have less cmyb- or ikaros-staining cells in the ICM compared with Gata1- and Biklf/Gata1-deficient embryos, demonstrating that loss of Gata2 affects both myeloid and erythroid cell number.
As loss of Biklf, Gata1 and Gata2 affected erythroid gene expression at 20 somites, we investigated if their loss affected hematopoietic precursor genes at earlier stages. draculin, a gene expressed in erythroid and myeloid cell precursors, and scl, lmo2, and gata2, genes found in blood and endothelial cell precursors, were present in Biklf-, Biklf/Gata1-deficient embryos at 12 somites, and their expression was reduced in the Biklf/Gata2- and Biklf/Gata1/Gata2-deficient embryos (Figure 3B). This result is likely due to decreased blood cell number caused by loss of Gata2. Compared to control embryos at 12 somites, expression of biklf was reduced in the blood precursors of all MO-injected embryos especially the Biklf/Gata1/Gata2-deficient embryos (Figure 3B). The hatching gland expression of biklf was completely lost, demonstrating that it is required for hatching gland formation and providing an internal control for the morpholino injections. Expression of the previously identified erythroid genes zinc finger-like gene 2 (znfl2) and nuclear receptor coactivator 4 (ncoa4) were also examined in these morphants[41]. ncoa4 expression was lost in Gata1-deficient embryos at 24 hpf, suggesting it requires Gata1 (Supplemental Figure 2). At 12 somites, znfl4 expression was present in Biklf morphants, reduced in Gata1 morphants and absent in Gata1/Gata2-deficient embryos, suggesting it requires Gata1 and Gata2 but not Biklf for expression (Figure 3B and Supplemental Figure 2). Taken together, loss of Gata1/Gata2, Gata1/Biklf, or Gata1/Gata2/Biklf abolishes most erythroid gene expression as demonstrated by the loss of genes such as globin and znfl2, whereas expression of erythroid precursor genes such as biklf, erythroid and myeloid precursor genes such as draculin, biklf, ikaros, and cmyb or the early hematopoietic and endothelial markers, scl, lmo2 and gata2, are maintained.
Figure 3. Loss of Gata1, Gata2 and Biklf has differing effects of krcp, kiaa0650 and glcci1 expression.
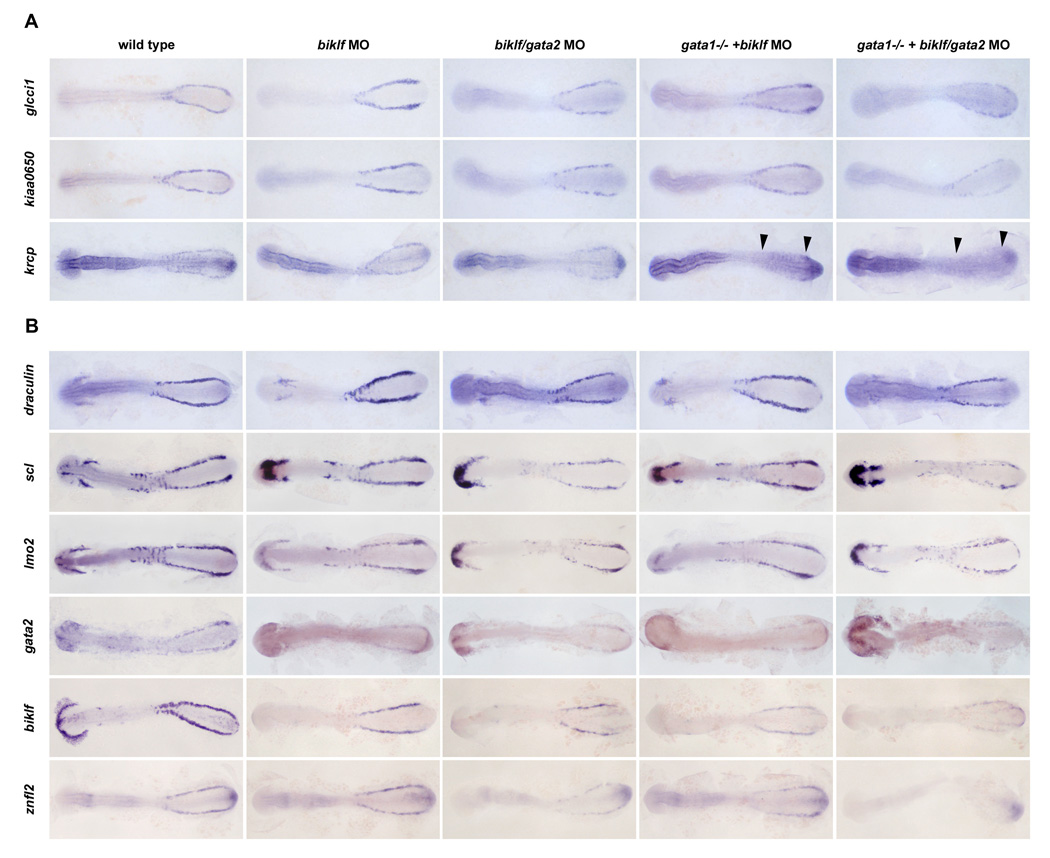
(A) In control embryos at 12 somites, glcci1, kiaa0650, and krcpare expressed in ICM blood cell precursors. Expression of glcci1 in the ICM precursors was not lost in biklf (100%, n=21), gata1/biklf (100%, n=43), gata2/biklf (100%, n=7) or gata2/biklf MO-injected gata-/- embryos (100%, n=5). Similarly, kiaa0650 expression was not absent in biklf (100%, n=18), gata1/biklf (100%, n=63), or gata2/biklf (100%, n=9) injected embryos, but was greatly reduced in gata2/biklf MO-injected gata-/- embryos (70%, n=10). krcp was expressed in biklf (100%, n=10) and gata2/biklf (70%, n=20), but unlike glcci1 not in biklf MO-injected gata1-/- embryos (100%, n=11) or gata2/biklf MO-injected gata-/- embryos (100%, n=2). (B) While reduced in biklf/gata2 MO-injected and biklf/gata2 MO-injected gata-/- embryos, draculin expression was present in all MO-injected embryos (100%, n=54). scl, lmo2 and gata2 are expressed in both the anterior and posterior blood and endothelial cell precursors in wild type embryos. Expression of scl (100%, n=25), lmo2 (100%, n=27) and gata2 (100%, n=28) was maintained in biklf MO-injected and biklf MO-injected gata-/- embryos. scl (100%, n=33), lmo2 (100%, n=40) and gata2 (97%, n=31) expression was reduced in biklf/gata2 MO-injected and biklf/gata2 MO-injected gata-/- embryos. Expression of biklf was reduced in the blood precursors and absent in the hatching gland of biklf MO-injected, biklf/gata2 MO-injected, biklf MO-injected gata-/-, and biklf/gata2 MO-injected gata-/- embryos (100%, n=63). znfl2 expression was maintained in biklf MO-injected embryos (100%, n=18), reduced in biklf/gata2 MO-injected (100%, n=26) and biklf MO-injected gata-/- embryos (100%, n=4), and virtually absent in biklf/gata2 MO-injected gata-/- embryos (100%, n=10).
Loss of key hematopoietic transcription factors has a differential effect on glcci1, kiaa0650 and krcp expression
To determine how loss of combinations of Biklf and Gata1 and/or Gata2 affected expression of glcci1, kiaa0650, and krcp, in situ hybridizations were performed on 12 somite-stage MO-injected vlt mutant embryos. Hematopoietic expression of krcp was virtually absent in the Gata1/Biklf-deficient embryos (Figure 3A, arrowheads) and expression of kiaa0650 was markedly reduced in Biklf/Gata1/Gata2-deficient embryos compared with the Gata2/Biklf-deficient or Biklf-deficient animals alone (Figure 3A). This suggests that Gata1 and Biklf are necessary for krcp expression and Gata1, Gata2, and Biklf are necessary for robust kiaa0650 expression in the blood. In contrast, glcci1 was expressed in the absence of Biklf, Gata1, and Gata2, demonstrating that Biklf and GATA factors are not required for blood expression (Figure 3A). This also demonstrates that the novel gene, glcci1 is unique in that it is regulated in a manner similar to the early endothelial markers such as scl, lmo2, and gata2, yet it is expressed specifically in the erythroid blood lineage.
Based on this data, we hypothesized that genes such as scl that are involved in early blood cell specification may be important regulators of glcci1 and kiaa0650 expression. To test this, a scl MO was injected, and expression was examined. Scl-deficiency results in a loss of gata1 and biklf expression[28]. We found that expression of krcp was lost in scl MO-injected embryos (Figure 4), consistent with krcp dependence on gata1 and biklf. Expression of kiaa0650 was virtually ablated in scl MO-injected embryos compared to control MO-injected embryos, indicating that it is also dependent on Scl for blood expression (Figure 4). glcci1 expression was reduced but not lost in Scl-deficient embryos, indicating that Scl is not required for posterior blood precursor expression. Previous studies have shown that some hematopoietic genes such as gata2 are expressed in the blood of scl MO-injected embryos[28]. To eliminate the possibility that Gata2 may be regulating posterior expression of glcci1 in the absence of Scl, scl and gata2 MOs were co-injected and expression of glcci1 was examined in 12 somite-stage embryos. Although expression of glcci1 was restricted to the posterior axis of the embryo compared with control MO-injected animals, its expression persisted, suggesting that factors upstream or parallel to Scl and Gata2 regulate its expression in this region (Figure 4). Interestingly, draculin expression, while significantly reduced in scl and scl/gata2 morphants, remained in some posterior hematopoietic precursor cells (Figure 4). Taken together, these findings demonstrate how genes with similar expression patterns can be dependent on different factors for expression, and identify glcci1 as having an Scl-independent expression domain in the more posterior blood cell precursors.
Figure 4. Posterior blood precursor expression of glcci1 is not dependent on Scl and Gata2.
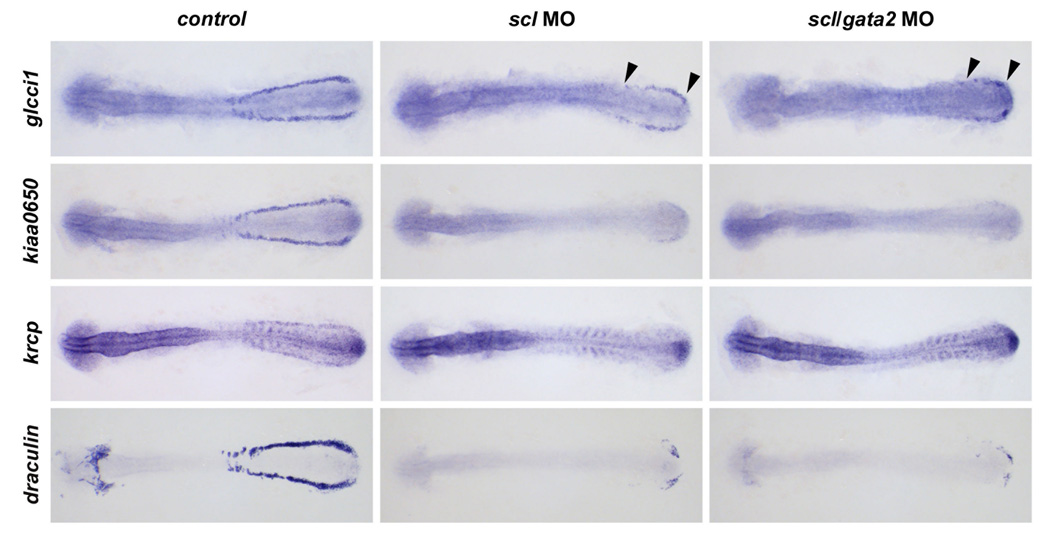
Compared with control MO-injected embryos, expression of kiaa0650 was virtually lost in the ICM precursors of both scl (39/39 gone) and scl/gata2 (18/18 gone) MO-injected embryos at 12 somites. Similarly, krcp expression was absent in the posterior region of scl (100%, n=38) and scl/gata2 (100%, n=18) MO-injected embryos. glcci1 was reduced but not absent in scl (100%, n=41) and scl/gata2 (89%, n=37) MO-injected embryos at 12 somites. Expression of draculin was significantly reduced in scl MO-injected embryos (100%, n=21) and scl/gata2 MO-injected embryos (100%, n=16) at 12 somites compared with control MO-injected embryos.
Discussion
While most erythroid genes are dependent on Gata1 or Gata2 for expression, our in situ hybridization screen discovered three novel genes that did not require Gata1 or Gata2 for their hematopoietic expression[32]. Similar to gata1 and biklf, these genes were expressed in blood but not endothelial cell precursors, and specific transcription factors were necessary for their expression. Expression of krcp required Gata1 and Biklf, consistent with its expression initiating later than gata1 and biklf. Expression of kiaa0650, was found to be dependent upon Gata1, Gata2, and Biklf together as well as Scl alone. Its regulation, therefore, resembles that of biklf, which also had significantly reduced expression in Biklf/Gata1/Gata2-deficient embryos and requires Scl for expression. Unlike these Scl-dependent genes, expression of glcci1 is present but reduced in Scl and Scl/Gata2-deficient embryos. While there is the possibility that this results from hypomorphic Scl function rather than a complete loss-of-function situation, efficient knockdown of RNA levels was demonstrated at the concentration used[28]. The phenotype was also assessed in parallel with scl MO-injected sibling controls, in which a complete loss of gata1 was always observed. Additionally, the persistent posterior expression of glcci1 most resembles that of draculin expression in scl/gata2 MO-injected embryos and may indicate similar regulatory mechanisms[28],[29]. Unlike glcci1, however, draculin expression is much more reduced in the scl morphants. In addition, it is expressed in ventral mesoderm during gastrulation and in myeloid cell precursors at later stages. Therefore, glcci1 represents a unique gene that is specific to the blood lineage and not dependent on Scl and Gata2 for its early posterior hematopoietic expression.
Given the unique expression pattern of glcci1, analysis of its regulatory regions may give insight into which transcription factors are crucial for blood specification. Examination of 1 kb sequence of the 5’UTR of glcci1 revealed the presence of Ets-, C/ebp-, Oct-1 and Gata-binding sites. The Ets domain transcription factors fli1a, fli1b, ets1, mef, and ets1-related protein are expressed during early embryogenesis. However, fli1b and ets1-related protein are only expressed in endothelial cell precursors, making them unlikely regulators of glcci1 [42],[43],[44]. Alternatively, fli1a, ets1, and mef are expressed in domains coinciding with lmo2 expression, and ets1, in particular, can activate the lmo2 promoter[44]. lmo2 is also expressed in the absence of Scl and Gata2 [28] (data not shown); however, in contrast to glcci1, lmo2 is expressed in endothelial and blood cells. CCAAT/enhancer binding proteins (C/ebp), transcription factors, are unlikely regulators of glcci1 expression as c/ebpα, c/ebp β, and c/ebp1 are found in hematopoietic cells of the myeloid lineage[15],[45]. Interestingly, Oct-1 can cooperate with Smads to activate the zebrafish gata2 promoter, and although these factors were shown to regulate gata2 in ventral mesoderm, glcci1 induction may depend upon a similar mechanism[46]. Ultimately, the examination of the regulation of genes such as glcci1 that are only expressed in blood cell precursors and not myeloid or endothelial cells is crucial for gaining insight into how cell fate decisions are made during development.
Phylogenetic analysis of the amino acid sequence of the three novel genes revealed orthology to mammalian genes. While no known structural motifs exist in Glcci1, there is strong synteny and moderate identity with human (54%) and murine (62%) orthologs. Mouse glcci1 was originally identified in a screen for genes regulated by glucocorticoids, but no mammalian expression pattern has been reported[47]. Zebrafish Kiaa0650 is syntenic with the mouse and human SMC hinge domain containing protein, and has 43% and 45% identity with them, respectively. Zebrafish Krcp shares modest identity with mouse (33%) and human (33%) F-box only protein 42 and was not found to be syntenic with any kelch domain-containing gene. Overall, the degree of conservation, among the Glcci1 and Kiaa0650 orthologs, suggests that the functional role of these genes may be preserved among vertebrates and future studies are aimed at determining the expression of their mammalian counterparts.
The specification and differentiation of HSCs into mature blood cells involves the activation of transcription factors that regulate expression of lineage-specific genes. The function of these genes has been well established, however, the mechanisms governing lineage-specific gene expression remain unclear. Additionally, several of these transcription factors have redundant or overlapping functions, and only by eliminating the function of multiple genes simultaneously can their roles in blood development be elucidated. Our study sought to dissect how the complex regulation of erythroid gene expression is orchestrated by a subset of known essential transcription factors. Zebrafish provided an ideal system as they permitted the knockdown of multiple genes at one time in an embryo. Using this approach, we identified glcci1 to be expressed early and specifically in the erythroid blood lineage and have a Scl-independent expression domain in the most posterior hematopoietic precursors. We also established that despite their similar blood expression patterns, krcp, kiaa0650, and glcci1 required distinct combinations of known transcription factors for their erythroid gene expression during development.
Supplementary Material
(A) RT-PCR analysis of uninjected and gata2 MO-injected embryos reveals that uninjected embryos had a normal product size of ~690 bps while the gata2E/I1 MO-injected embryos had a ~540 bp product. (B) A cartoon depicts the exons of the gata2 gene, the site of the primers used to amplify cDNA (arrows), and the morpholino (black bar). Sequencing of the 540 bp product revealed that exon 3 was absent in the gata2E/I1 MO-injected cDNA, which resulted in a frame shift into a premature stop codon in exon 4 (asterisk). Gata2 protein is predicted to be truncated at amino acid 337 and missing exons 3 and 4, which contain both zinc fingers and the DNA-binding domain.
The number of globin-expressing cells is reduced in biklf MO-injected embryos (100%, n=17) compared to controls at 28 hpf. Expression of mpo in biklf MO-injected embryos (100%, n=15) resembles that of controls at 28 hpf. There is also reduction in the number of o-dianisidine-staining cells in biklf MO-injected embryos (100%, n=30) compared with controls at 36 hpf.
(A) At 24 hpf, expression of ncoa4 is lost in the ICM of Gata1-deficient embryos (100%, n=14). (B) Expression of znfl2 is reduced in Gata1- deficient (100%, n=3) and Gata2-deficient embryos (100%, n=18) and virtually absent in Gata1/Gata2-deficient embryos (100%, n=6).
Acknowledgements
L.I.Z. is supported by a grant from the National Institutes of Health (R01 HL48801-13). B.T. and C.T. are supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Association pour la Recherche contre le Cancer, the Ligue de Recherche contre le Cancer and the National Institute of Health (R01 RR15402). We thank K. Pfaff, N. Paffett- Lugassy and J. Rivera-Feliciano for critical reading of the manuscript, and A. Davidson for helpful advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, Rabbitts TH. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 2.Robb L, Lyons I, Li R, Hartley L, Kontgen F, Harvey RP, Metcalf D, Begley CG. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci U S A. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Fujiwara Y, Orkin SH. Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev. 1998;12:473–479. doi: 10.1101/gad.12.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver L, Palis J. Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood. 1997;89:1154–1164. [PubMed] [Google Scholar]
- 6.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Pannell R, Forster A, Rabbitts TH. The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice. Proc Natl Acad Sci U S A. 2000;97:320–324. doi: 10.1073/pnas.97.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V, Orkin SH, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 9.Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 10.Miller IJ, Bieker JJ. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins AC, Sharpe AH, Orkin SH. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 12.Davidson AJ, Zon LI. The 'definitive' (and 'primitive') guide to zebrafish hematopoiesis. Oncogene. 2004;23:7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- 13.Detrich HW, 3rd, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, Pratt S, Ransom D, Zon LI. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci U S A. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawahara A, Dawid IB. Expression of the Kruppel-like zinc finger gene biklf during zebrafish development. Mech Dev. 2000;97:173–176. doi: 10.1016/s0925-4773(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 15.Lyons SE, Shue BC, Lei L, Oates AC, Zon LI, Liu PP. Molecular cloning, genetic mapping, and expression analysis of four zebrafish c/ebp genes. Gene. 2001;281:43–51. doi: 10.1016/s0378-1119(01)00774-0. [DOI] [PubMed] [Google Scholar]
- 16.Wingert RAaZ LI. Hematopoietic Stem Cells. Georgetown, TX: Georgetown, TX; 2003. [Google Scholar]
- 17.Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, Langenau DM, Delahaye-Brown A, Zon LI, Fleming MD, Look AT. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- 18.Willett CE, Cherry JJ, Steiner LA. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics. 1997;45:394–404. doi: 10.1007/s002510050221. [DOI] [PubMed] [Google Scholar]
- 19.Trede NS, Zapata A, Zon LI. Fishing for lymphoid genes. Trends Immunol. 2001;22:302–307. doi: 10.1016/s1471-4906(01)01939-1. [DOI] [PubMed] [Google Scholar]
- 20.Willett CE, Kawasaki H, Amemiya CT, Lin S, Steiner LA. Ikaros expression as a marker for lymphoid progenitors during zebrafish development. Dev Dyn. 2001;222:694–698. doi: 10.1002/dvdy.1223. [DOI] [PubMed] [Google Scholar]
- 21.Willett CE, Cortes A, Zuasti A, Zapata AG. Early hematopoiesis and developing lymphoid organs in the zebrafish. Dev Dyn. 1999;214:323–336. doi: 10.1002/(SICI)1097-0177(199904)214:4<323::AID-AJA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Stainier DYR, Weinstein BM, Detrich HW, III, Zon LI, Fishman MC. cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- 23.Liao W, Bisgrove BW, Sawyer H, Hug B, Bell B, Peters K, Grunwald DJ, Stainier DY. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development. 1997;124:381–389. doi: 10.1242/dev.124.2.381. [DOI] [PubMed] [Google Scholar]
- 24.Liao EC, Paw BH, Oates AC, Pratt SJ, Postlethwait JH, Zon LI. SCL/Tal-1 transcription factor acts downstream of cloche to specifiy hematopoietic and vascular progenitors in zebrafish. Genes Dev. 1998;12:621–626. doi: 10.1101/gad.12.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development. 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- 26.Rohde LA, Oates AC, Ho RK. A crucial interaction between embryonic red blood cell progenitors and paraxial mesoderm revealed in spadetail embryos. Dev Cell. 2004;7:251–262. doi: 10.1016/j.devcel.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, Oates AC, Fritz A, Gates MA, Amores A, Bahary N, Talbot WS, Her H, Beier DR, Postlethwait JH, Zon LI. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 28.Dooley KA, Davidson AJ, Zon LI. Zebrafish scl functions independently in hematopoietic and endothelial development. Dev Biol. 2005;277:522–536. doi: 10.1016/j.ydbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105:3502–3511. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- 30.Ransom DG, Bahary N, Niss K, Traver D, Burns C, Trede NS, Paffett-Lugassy N, Saganic WJ, Lim CA, Hersey C, Zhou Y, Barut BA, Lin S, Kingsley PD, Palis J, Orkin SH, Zon LI. The zebrafish moonshine gene encodes transcriptional intermediary factor 1gamma, an essential regulator of hematopoiesis. PLoS Biol. 2004;2:E237. doi: 10.1371/journal.pbio.0020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons SE, Lawson ND, Lei L, Bennett PE, Weinstein BM, Liu PP. A nonsense mutation in zebrafish gata1 causes the bloodless phenotype in vlad tepes. Proc Natl Acad Sci U S A. 2002;99:5454–5459. doi: 10.1073/pnas.082695299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev Cell. 2005;8:109–116. doi: 10.1016/j.devcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes J, Hagen A, Hsu K, Deng M, Liu TX, Look AT, Kanki JP. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev Cell. 2005;8:97–108. doi: 10.1016/j.devcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Kawahara A, Dawid IB. Critical role of biklf in erythroid cell differentiation in zebrafish. Curr Biol. 2001;11:1353–1357. doi: 10.1016/s0960-9822(01)00398-0. [DOI] [PubMed] [Google Scholar]
- 35.Westerfield M. The Zebrafish Book. Eugene, OR: Eugene, OR; 1993. [Google Scholar]
- 36.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 37.Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- 38.Oates AC, Pratt SJ, Vail B, Yan Y, Ho RK, Johnson SL, Postlethwait JH, Zon LI. The zebrafish klf gene family. Blood. 2001;98:1792–1801. doi: 10.1182/blood.v98.6.1792. [DOI] [PubMed] [Google Scholar]
- 39.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 40.Brownlie A, Hersey C, Oates AC, Paw BH, Falick AM, Witkowska HE, Flint J, Higgs D, Jessen J, Bahary N, Zhu H, Lin S, Zon L. Characterization of embryonic globin genes of the zebrafish. Dev Biol. 2003;255:48–61. doi: 10.1016/s0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 41.Weber GJ, Choe SE, Dooley KA, Paffett-Lugassy NN, Zhou Y, Zon LI. Mutant-specific gene programs in the zebrafish. Blood. 2005;106:521–530. doi: 10.1182/blood-2004-11-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown LA, Rodaway AR, Schilling TF, Jowett T, Ingham PW, Patient RK, Sharrocks AD. Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech Dev. 2000;90:237–252. doi: 10.1016/s0925-4773(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 43.Sumanas S, Lin S. Ets1-Related Protein Is a Key Regulator of Vasculogenesis in Zebrafish. PLoS Biol. 2005;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu H, Traver D, Davidson AJ, Dibiase A, Thisse C, Thisse B, Nimer S, Zon LI. Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish. Dev Biol. 2005;281:256–269. doi: 10.1016/j.ydbio.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Lyons SE, Shue BC, Oates AC, Zon LI, Liu PP. A novel myeloid-restricted zebrafish CCAAT/enhancer-binding protein with a potent transcriptional activation domain. Blood. 2001;97:2611–2617. doi: 10.1182/blood.v97.9.2611. [DOI] [PubMed] [Google Scholar]
- 46.Oren T, Torregroza I, Evans T. An Oct-1 binding site mediates activation of the gata2 promoter by BMP signaling. Nucleic Acids Res. 2005;33:4357–4367. doi: 10.1093/nar/gki746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapman MS, Qu N, Pascoe S, Chen WX, Apostol C, Gordon D, Miesfeld RL. Isolation of differentially expressed sequence tags from steroid-responsive cells using mRNA differential display. Mol Cell Endocrinol. 1995;108:R1–R7. doi: 10.1016/0303-7207(95)03481-l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) RT-PCR analysis of uninjected and gata2 MO-injected embryos reveals that uninjected embryos had a normal product size of ~690 bps while the gata2E/I1 MO-injected embryos had a ~540 bp product. (B) A cartoon depicts the exons of the gata2 gene, the site of the primers used to amplify cDNA (arrows), and the morpholino (black bar). Sequencing of the 540 bp product revealed that exon 3 was absent in the gata2E/I1 MO-injected cDNA, which resulted in a frame shift into a premature stop codon in exon 4 (asterisk). Gata2 protein is predicted to be truncated at amino acid 337 and missing exons 3 and 4, which contain both zinc fingers and the DNA-binding domain.
The number of globin-expressing cells is reduced in biklf MO-injected embryos (100%, n=17) compared to controls at 28 hpf. Expression of mpo in biklf MO-injected embryos (100%, n=15) resembles that of controls at 28 hpf. There is also reduction in the number of o-dianisidine-staining cells in biklf MO-injected embryos (100%, n=30) compared with controls at 36 hpf.
(A) At 24 hpf, expression of ncoa4 is lost in the ICM of Gata1-deficient embryos (100%, n=14). (B) Expression of znfl2 is reduced in Gata1- deficient (100%, n=3) and Gata2-deficient embryos (100%, n=18) and virtually absent in Gata1/Gata2-deficient embryos (100%, n=6).


