Abstract
3,4-Methylenedioxymethamphetamine (MDMA or Ecstasy) stimulates the transporter-mediated release of monoamines, including serotonin (5-HT). High-dose exposure to MDMA causes persistent 5-HT deficits (e.g., depletion of brain 5-HT) in animals, yet the functional and clinical relevance of such deficits are poorly defined. Here we examine functional consequences of MDMA-induced 5-HT depletions in rats. Male rats received binges of 3 ip injections of MDMA or saline, one injection every 2 h; MDMA was given at a threshold pharmacological dose (1.5 mg/kg × 3, low dose) or at a 5-fold higher amount (7.5 mg/kg × 3, high dose). One week later, jugular catheters and intracerebral guide cannulae were implanted. Two weeks after binges, rats received acute iv challenge injections of 1 and 3 mg/kg MDMA. Neuroendocrine effects evoked by iv MDMA (prolactin and corticosterone secretion) were assessed via serial blood sampling, while neurochemical effects (5-HT and dopamine release) were assessed via microdialysis in brain. MDMA binges elevated core temperatures only in the high-dose group, with these same rats exhibiting ~50% loss of forebrain 5-HT two weeks later. Prior exposure to MDMA did not alter baseline plasma hormones or dialysate monoamines, and effects of iv MDMA were similar in saline and low-dose groups. By contrast, rats pretreated with high-dose MDMA displayed significant reductions in evoked hormone secretion and 5-HT release when challenged with iv MDMA. As tolerance developed only in rats exposed to high-dose binges, hyperthermia and 5-HT depletion are implicated in this phenomenon. Our results suggest that MDMA tolerance in humans may reflect 5-HT deficits which could contribute to further dose escalation.
Keywords: Ecstasy, serotonin, dopamine, transporter, neuroendocrine, microdialysis
(±)-3,4-Methylenedioxymethamphetamine (MDMA, or Ecstasy) is an illicit drug that stimulates transporter-mediated release of monoamines from neurons (White et al., 1996, Green et al., 2003). High-dose administration of MDMA to rats causes impairments in central serotonin (5-HT) neurons, including inhibition of tryptophan hydroxylase (Stone et al., 1987), depletion of tissue 5-HT (Commins et al., 1987) and loss of 5-HT transporter (SERT) binding sites (Battaglia et al., 1987). The spectrum of MDMA-induced deficits can last for months, prompting general acceptance that the drug is a 5-HT neurotoxin (Ricaurte et al., 2000, Green et al., 2003). Nevertheless, there are unresolved issues surrounding MDMA neurotoxicity in rats (Wang et al., 2005), especially with regard to functional consequences and their potential clinical relevance (Easton and Marsden, 2006, Baumann et al., 2007).
Functional correlates of MDMA-induced 5-HT deficits in rats have been examined, but findings are often ambiguous. For example, rats pretreated with high-dose MDMA are reported to exhibit tolerance or reverse tolerance (i.e., sensitization) to locomotor effects of the drug (Kalivas et al., 1998, Brennan and Schenk, 2006). Similarly, neuroendocrine studies show that rats given neurotoxic doses of MDMA can display a reduction or enhancement in pituitary hormone responses to serotonergic drug challenge (Poland et al., 1997, Baumann et al., 2007). Our preliminary data indicate that neuroendocrine responses to MDMA are uniformly blunted in rats previously exposed to the drug, in agreement with findings in human Ecstasy users (Gerra et al., 1998, Gerra et al., 2000, Verkes et al., 2001). Importantly, tolerance to the subjective effects of MDMA is a common complaint in human drug users and has been implicated in dangerous dose escalation (Verheyden et al., 2003, Parrott, 2005).
Evaluating the clinical relevance of MDMA toxicity data from rats is complicated because humans take much lower doses than those given to rats. In laboratory studies, humans are typically given doses of 1–2 mg/kg (po) which are well tolerated (Cami et al., 2000, Harris et al., 2002), whereas rats receive non-contingent doses of 10–20 mg/kg (sc or ip) which cause hyperthermia and even death (Green et al., 2003). Many investigators have cited principles of interspecies scaling (i.e., allometric scaling) to support the view that high doses of MDMA in rats are equivalent to recreational doses in humans (Ricaurte et al., 2000, Green et al., 2003). However, allometric relationships are not always valid for extrapolating doses across species, especially for drugs that are extensively metabolized like MDMA (Lin, 1998, de la Torre and Farre, 2004). The biotransformation of MDMA in vivo generates a number of metabolites, including the N-demethylated analog, 3,4-methylenedioxyamphetamine (MDA), which is a potent monoamine releaser. The extent of MDA formation varies between species, with rats forming much more of this metabolite when compared to humans. In any case, behaviorally-relevant doses of MDMA appear to be equivalent in rats and humans, as both species can readily discriminate 1.5 mg/kg under controlled laboratory conditions (Schechter, 1988, Johanson et al., 2006).
The purpose of the present investigation was three-fold. First, we wished to employ an MDMA binge regimen in rats based on threshold pharmacological doses. In vivo neurochemical effects of a discrimination training dose of MDMA (1.5 mg/kg, ip) were examined, and this dose was used as a reference point for designing a repeated injection regimen. Second, we sought to determine functional consequences of MDMA binge exposure by implementing a neuroendocrine challenge paradigm in rats, similar to the approach used in clinical studies. Finally, it was of interest to see whether neuroendocrine changes observed after MDMA binges were reflective of neurochemical changes in brain regions involved with locomotor and rewarding effects of abused drugs. To this end, in vivo microdialysis was carried out in the n. accumbens and caudate n. of rats exposed to MDMA or saline binges.
EXPERIMENTAL PROCEDURES
Subjects
Male Sprague-Dawley rats weighing 280–320 g were double-housed (lights on: 0700–1900 h) in conditions of controlled temperature (22±2° C) and humidity (45±5 %), with free access to food and water. Experiments were carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), as revised in 1996. Vivarium facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and study procedures were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP). All efforts were made to minimize the number of animals used and their suffering.
Drugs and Reagents
(±)-3,4-Methylenedioxymethamphetamine HCl (MDMA) was provided by the NIDA Drug Supply Program, Rockville MD. Sources of reagents for in vivo microdialysis, biochemical assays and radioimmunoassays (RIA) have been reported (Baumann et al., 1998, Baumann et al., 2005).
Surgical Procedures
For neuroendocrine studies, rats were anesthetized with sodium pentobarbital (60 mg/kg ip), and catheters made of Silastic tubing (Dow Corning, Midland, MI, USA) were implanted into the jugular vein (Baumann et al., 1998). Briefly, the proximal end of the catheter was inserted into the r. jugular vein and advanced to the atrium, whereas the distal end was exteriorized on the nape and plugged. Rats were single-housed post-operatively.
For microdialysis studies, rats were anesthetized with pentobarbital, and jugular catheters were implanted as above. Rats were placed into a stereotaxic apparatus, and guide cannulae (CMA 12, CMA Microdialysis, North Chelmsford, MA) were implanted into the caudate n. or n. accumbens (Baumann et al., 2005). Each rat received a single guide aimed at either caudate or accumbens. Caudate coordinates were: ML±2.4 mm and AP+1.6 mm from bregma, DV−3.4 mm from dura, whereas accumbens coordinates were: ML±1.7 mm and AP+1.6 mm from bregma, DV−6.0 mm from dura (Paxinos and Watson, 2005). Guide cannulae were secured to the skull using stainless steel screws and dental acrylic. Rats were single-housed post-operatively.
Effects of Acute MDMA on Extracellular 5-HT and Dopamine (DA)
In the first experiment, we investigated the ability of a discrimination training dose of MDMA (1.5 mg/kg, ip) to release 5-HT and DA in vivo. Rats were tested 1 week after implantation of jugular catheters and n. accumbens guide cannulae. On the night before testing, dialysis probes with 2×0.5 mm exchange surface (CMA/12, CMA Microdialysis) were inserted into guide cannulae and extension tubes were joined to catheters. Each rat was placed into a container where a flexible wire tether was connected to a plastic neck collar. Probes were perfused overnight with artificial cerebrospinal fluid (aCSF) at a flow rate of 0.6 μL/min. The next morning, dialysate samples were collected every 20 min and assayed for 5-HT and DA by HPLC with electrochemical detection (HPLC-ECD). Once three baseline samples were obtained, rats received ip injections of saline, 1.5 or 7.5 mg/kg MDMA; dialysate samples were collected for 2 h post-injection.
Dialysates (5 μL) were injected onto a microbore HPLC column linked to an amperometric detector (Model LC-4C, Bioanalytical Systems, Inc., West Lafayette IN). Mobile phase consisting of 150 mM monochloroacetic acid, 150 mM sodium hydroxide, 0.03% sodium octanesulfonic acid, 250 μM disodium EDTA, 6% methanol and 6% acetonitrile (final pH=5.3) was pumped at 60 μL/min. Data were acquired by a Millennium software system (Waters Associates, Milford, MA, USA) where peak heights of unknowns were compared to those of standards. The lower limit of assay sensitivity was 100 fg.
After dialysis perfusion, rats were decapitated and brains were stored in 10% formalin. Brains were sectioned, and placement of microdialysis probe tips within the n. accumbens was verified by visual inspection.
Hyperthermia and 5-HT Depletions Produced by MDMA Binges
In experiment 2, we assessed the effects of MDMA binge administration on acute hyperthermia and long-term 5-HT depletions. Rats were double-housed and received binges of 3 ip injections of saline or MDMA, one injection every 2 h. Injections were carried out in the vivarium where rats were returned to home cages immediately after each injection. MDMA was administered at a discrimination training dose of 1.5 mg/kg × 3 (low dose; cumulative dose of 4.5 mg/kg) or a 5-fold higher amount of 7.5 mg/kg × 3 (high dose; cumulative dose of 22.5 mg/kg). Rats were observed every h for 6 h post-injection, and core temperatures were measured via insertion of a RET-2 probe (Physitemp Instruments Inc., Clifton, NJ) into the colon.
Two weeks after binges, rats were decapitated and brain tissue was dissected to obtain the n. accumbens, caudate-putamen and mediobasal hypothalamus. These brain regions were chosen for specific reasons: the n. accumbens is a brain region involved in the rewarding effects of abused drugs (Ikemoto and Panksepp, 1999), the caudate-putamen is implicated in mediating locomotor effects of MDMA (Ball et al., 2003), and the hypothalamus regulates secretion of anterior pituitary hormones which are affected by MDMA administration (Nash et al., 1990). We wished to compare the effects of MDMA binges on these diverse brain regions. Tissue samples were weighed, homogenized in 0.1 N HClO4 and spun at 15,000 rpm for 15 min. Concentrations of 5-HT, DA and metabolites were quantified in the supernatant using HPLC-ECD (Baumann et al., 1998). Briefly, 20 μL aliquots were injected onto a HPLC column linked to a coulometric detector (Environmental Sciences Associates, Bedford, MA, USA). Mobile phase consisting of 50 mM sodium phosphate monobasic, 250 μM Na2EDTA, 0.03% sodium octanesulfonic acid and 25% methanol (final pH=2.75) was recirculated at 0.9 ml/min. Data were acquired by a Millennium software system, where peak heights of unknowns were compared to those of standards. The lower limit of assay sensitivity was 1 pg.
Neuroendocrine Responsiveness in Rats Exposed to MDMA Binges
In experiment 3, we examined neuroendocrine effects of iv MDMA challenge injections in rats that had been previously exposed to saline or MDMA binges. Rats received saline, low-dose MDMA (1.5 mg/kg, × 3) or high-dose MDMA (7.5 mg/kg × 3) as described for experiment 2. One week after binges, indwelling jugular catheters were implanted and rats were single-housed. Two weeks after binges (i.e., one week after surgery), rats received acute injections of MDMA, and blood samples were removed.
On the day of an experiment, rats were moved to the testing room in their home cages; extension tubes were connected to iv catheters and threaded outside the cages. Catheters were flushed with 0.3 ml of 48 IU/ml heparin saline, and acute injections of MDMA were administered. Rats received 1 mg/kg iv MDMA at time zero, followed by 3 mg/kg iv MDMA 60 min later. Blood samples (0.4 ml) were withdrawn immediately before and at 15, 30, 60, 75, 90 and 120 min after the first injection. The 60 min sample was drawn immediately before the 3 mg/kg MDMA injection. Blood was spun for 10 min at 1500 rpm; plasma was decanted and stored at −80°C. The occurrence of 5-HT behavioral syndrome was evaluated during the challenge test procedure. At 2, 15, and 30 min after each injection, rats were observed for 1 min and the presence of forepaw treading and flat-body posture was scored using the graded scale: 0=absent, 1=equivocal, 2=present and 3=intense (Baumann et al., 1998). Rats were given a single “syndrome” score after each dose of MDMA that consisted of the summed scores.
Plasma hormone levels were quantified using double-antibody RIA. Corticosterone levels were determined using [125I]-corticosterone RIA kits (MP Biomedicals, Irvine CA, USA). Plasma samples (10 μl) were assayed in duplicate and average intra-assay coefficient of variability was 8.8%. Prolactin levels were determined using materials provided by the National Hormone and Pituitary Program (Rockville, MD, USA). Plasma samples (50 μl) were assayed in duplicate and average intra-assay coefficient of variability was 6.9%
In Vivo Microdialysis in Rats Exposed to MDMA Binges
In the last experiment, we examined neurochemical effects of iv MDMA challenge injections in rats that had been previously exposed to saline or MDMA binges. Rats received saline, low-dose MDMA (1.5 mg/kg, × 3) or high-dose MDMA (7.5 mg/kg × 3) as described in experiment 2. One week after binges, indwelling jugular catheters and intracerebral guide cannulae were implanted. Each rat was fitted with a single guide cannula aimed at either the caudate n. or n. accumbens, and rats were single-housed. Two weeks after binges (i.e., one week after surgery), rats were subjected to in vivo microdialysis perfusion and received acute injections of MDMA.
On the night before testing, rats were prepared for microdialysis as described in experiment 1. CMA/12 dialysis probes with 2×0.5 mm exchange surface were inserted into guide cannulae, and extension tubes were attached to catheters. Probes were perfused overnight with aCSF. The next morning, dialysate samples were collected at 20-min intervals and assayed for 5-HT and DA by HPLC-ECD. Once three baseline samples were obtained, rats received 1 mg/kg iv MDMA at time zero followed by 3 mg/kg iv MDMA 60 min later. This challenge procedure was identical to the one used for examining neuroendocrine responsiveness. Dialysate samples were collected for 2 h after the 60 min MDMA injection. At the end of the dialysis perfusion, placement of the microdialysis probe tip within the caudate n. or n. accumbens was verified by visual inspection.
Data Analysis and Statistics
Data are expressed as mean±SEM. Microdialysis, temperature, neuroendocrine and 5-HT syndrome data were evaluated using two-factor analysis of variance (ANOVA). When significant F values were obtained, a one-factor (Dose) ANOVA was run at each time point followed by Newman-Keul’s post-hoc test to detect differences between group means. Post-mortem neurochemical data were analyzed by one-factor ANOVA followed by Newman-Keul’s test. Relationships between acute hyperthermic effects of MDMA and subsequent monoamine depletions were carried out using Pearson correlation analyses. P<0.05 was the minimum criterion for significance.
RESULTS
Effects of Acute MDMA on Extracellular 5-HT and DA
Fig. 1 illustrates the effects of ip MDMA administration on levels of 5-HT (top panel) and DA (bottom panel) in dialysate samples from rat n. accumbens. There were main effects of Dose (F2,135=354.87, P<0.0001) and Time (F8,135=41.89, P<0.001) on extracellular 5-HT, with significant increases in 5-HT after both doses of MDMA. Specifically, 1.5 and 7.5 mg/kg MDMA produced elevations in 5-HT that peaked at 5-fold and 30-fold above saline control levels 40 min post-injection. There were main effects of Dose (F2,135=135.20, P<0.0001) and Time (F8,135=14.47, P<0.001) on extracellular DA as well, but only 7.5 mg/kg MDMA significantly elevated DA, to about 5-fold above control. It is noteworthy that 1.5 mg/kg MDMA did not cause overt behavioral effects, while 7.5 mg/kg produced motor stimulation and elements of the 5-HT behavioral syndrome including forepaw treading and flat-body posture (data not shown).
Figure 1.
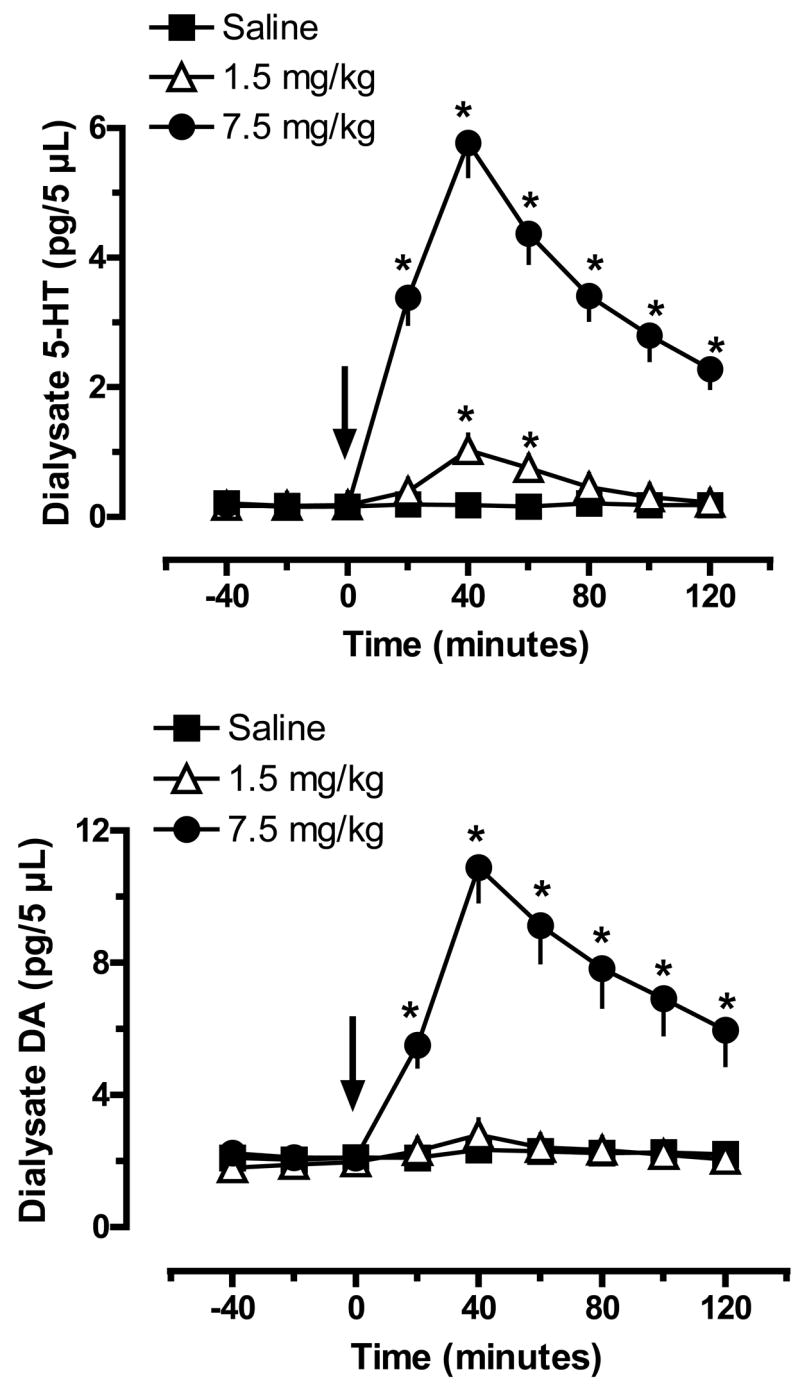
Effects of MDMA administration on extracellular levels of 5-HT (top panel) and DA (bottom panel) in rat n. accumbens. Male rats undergoing in vivo microdialysis received single ip injections of saline, 1.5 mg/kg or 7.5 mg/kg MDMA at time zero. Dialysate samples were collected at 20 min intervals and assayed for 5-HT and DA by HPLC-ECD. Data are pg/5 μL sample expressed as mean±SEM for N=6 rats/group. * = P < 0.05 compared to saline control at a given time point.
Hyperthermia and 5-HT Depletions Produced by MDMA Binges
Given the increases in extracellular 5-HT and DA produced by 1.5 and 7.5 mg/kg MDMA shown in Fig. 1, we sought to examine acute and long-term pharmacological effects of these same doses when administered as repeated injection binges. The data in Fig. 2 show acute effects of MDMA binges on core body temperature, where ip injections of saline or MDMA were given a time zero, 2 h and 4 h. There were main effects of MDMA Dose (F2,105=218.11, P<0.0001) and Time (F6,105=23.72, P<0.001) on body temperature, with 7.5 mg/kg MDMA producing marked and sustained hyperthermia. Core temperatures were significantly increased after the first injection of 7.5 mg/kg MDMA; temperatures continued to rise with subsequent injections, peaking at 40°C, or 3°C above saline control levels, 2 h after the third injection. By contrast, repeated injections of 1.5 mg/kg MDMA did not significantly increase core body temperature above saline control levels at any time point.
Figure 2.
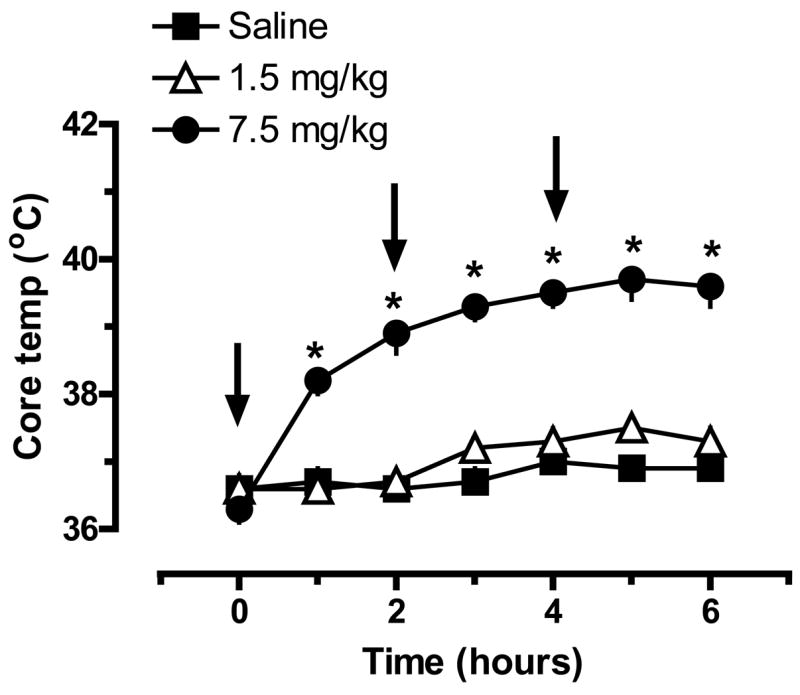
Effects of MDMA binge administration on body temperature in rats. Male rats received 3 ip injections of saline, 1.5 mg/kg (low dose) or 7.5 mg/kg MDMA (high dose); injections were given at time zero, 2h and 4h. Core body temperature was monitored hourly by insertion of a temperature probe into the colon. Data are degrees Celsius expressed as mean±SEM for N=6 rats/group. * = P < 0.05 compared to saline control at a given time point.
Two weeks after the binges, rats were killed by decapitation, and tissue from the n. accumbens, caudate-putamen and mediobasal hypothalamus was dissected. Fig. 3 shows post-mortem tissue concentrations of 5-HT (top panel) and DA (bottom panel) in brain regions from rats exposed to MDMA binges. The neurochemical data in Fig. 3 are derived from the same rats whose temperature data are depicted in Fig. 2. There was a main effect of MDMA Dose on tissue 5-HT concentrations in the n. accumbens (F2,17=50.52, P<0.0001), caudate-putamen (F2,17=25.49, P<0.0001) and hypothalamus (F2,17=14.31, P<0.001), with 7.5 mg/kg causing significant 5-HT depletions. The extent of 5-HT loss was about 50% of saline-treated control values in all three areas examined. It should be noted that repeated injections of 1.5 mg/kg MDMA did not alter tissue 5-HT concentrations in any region, and MDMA binge administration produced no changes in post-mortem tissue concentrations of DA.
Figure 3.
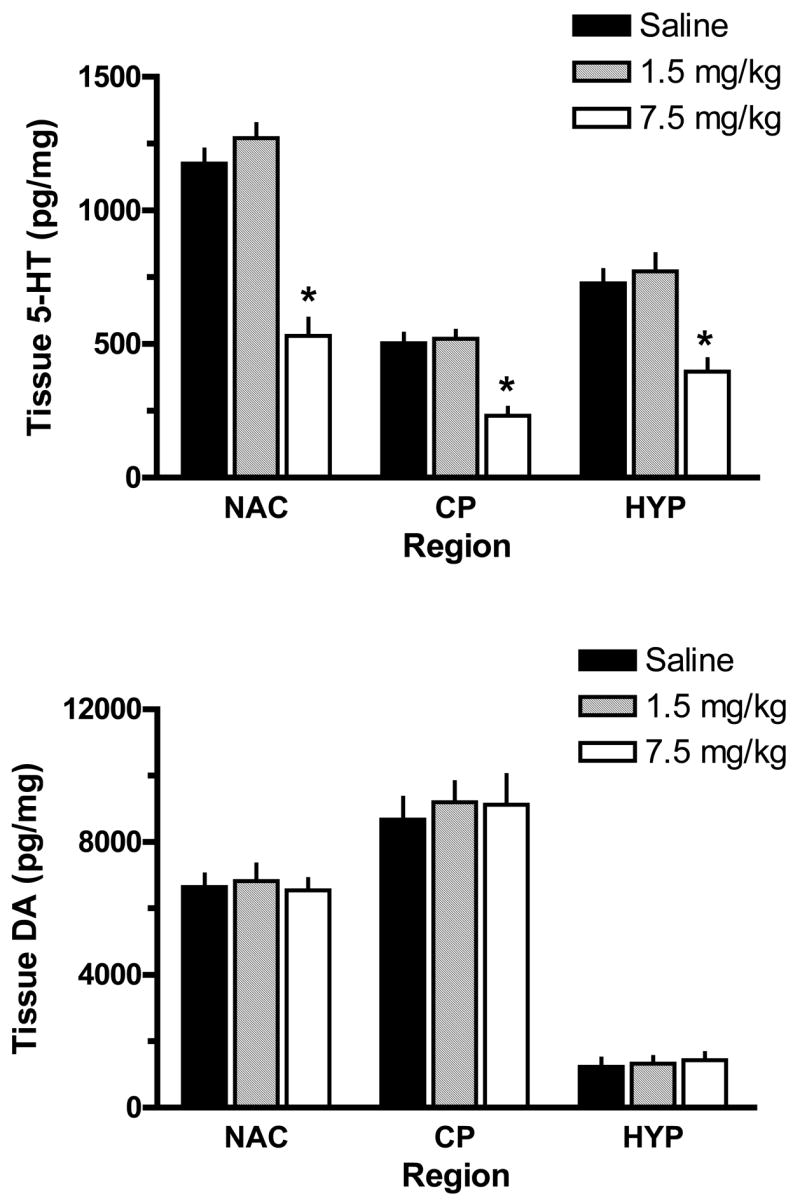
Effects of MDMA binge administration on tissue levels of 5-HT (top panel) and DA (bottom panel) in microdissected rat brain regions. Male rats received 3 ip injections of saline, 1.5 mg/kg (low dose) or 7.5 mg/kg MDMA (high dose). Rats were euthanized 2 weeks later, and tissue was dissected from n. accumbens (NAC), caudate-putamen (CP) and mediobasal hypothalalmus (HYP). Concentrations of 5-HT and DA were quantified by HPLC-ECD. Data are pg/mg wet weight expressed as mean±SEM for N= 6 rats/group. * = P < 0.05 compared to saline control for a specified brain region.
Given that high-dose MDMA caused acute hyperthermia and persistent 5-HT depletions, we examined the relationship between body temperature and post-mortem tissue monoamines using Pearson correlation analysis. Body temperature was negatively correlated with tissue 5-HT in the n. accumbens (r=−0.8630, P<0.0001), caudate-putamen. (r=−0.8089, P<0.0001) and hypothalamus (r=−0.6910, P<0.001). In contrast, there were no significant correlations between body temperature and tissue DA. Figure 4 depicts representative data from the n. accumbens, where MDMA-induced elevations in core temperature were significantly correlated with depletion of tissue 5-HT (top panel) but not DA (bottom panel).
Figure 4.
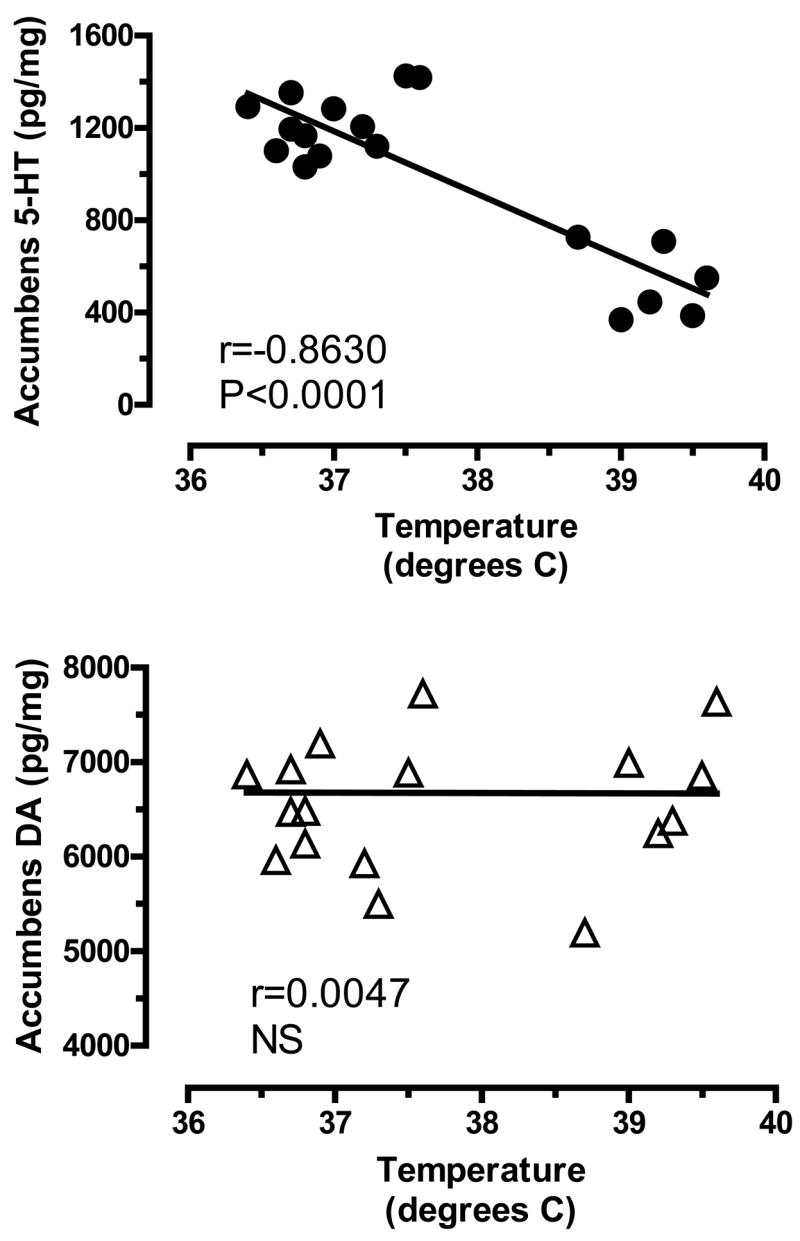
Correlations between acute body temperature and post-mortem tissue levels of 5-HT (top panel) and DA (bottom panel) in rat n. accumbens measured two weeks later. Body temperatures represent average values obtained from 1–6 h after MDMA or saline injections, as shown in Fig. 2. Post-mortem tissue levels of 5-HT and DA were measured two weeks later by HPLC-ECD, as shown in Fig. 3. Data points represent average temperature vs. tissue amine concentration from individual rats (N=18 rats), and Pearson correlation coefficients (r) are given.
Neuroendocrine Responsiveness in Rats Exposed to MDMA Binges
Given the long-term 5-HT depletions produced by high-dose MDMA binges shown in Fig. 3, we wished to examine functional consequences of such depletions using a neuroendocrine challenge paradigm similar to that employed in clinical investigations (Gerra et al., 1998, Gerra et al., 2000). The data in Fig. 5 illustrate the effects of acute iv injections of MDMA on plasma prolactin (top panel) and corticosterone (bottom panel) in rats pretreated with MDMA binges. MDMA binges did not alter baseline (time zero) prolactin levels measured two weeks later; values were 2.4±0.6, 1.9±0.5 and 1.8±0.5 ng/ml for saline, 1.5 and 7.5 mg/kg groups, respectively. Acute iv administration of MDMA produced significant dose-related increases in plasma prolactin in all groups, but there were main effects of MDMA Pretreatment Dose (F2,147=21.03, P<0.0001) and Time (F6,147=35.12, P<0.0001) on this response. In particular, rats pretreated with 7.5 mg/kg had blunted prolactin responses to acute MDMA challenge injections, such that peak prolactin levels evoked by 1 and 3 mg/kg MDMA were reduced to 40% and 60% of corresponding levels measured in saline-pretreated controls. By contrast, rats pretreated with 1.5 mg/kg MDMA displayed prolactin responses similar to those of rats pretreated with saline.
Figure 5.
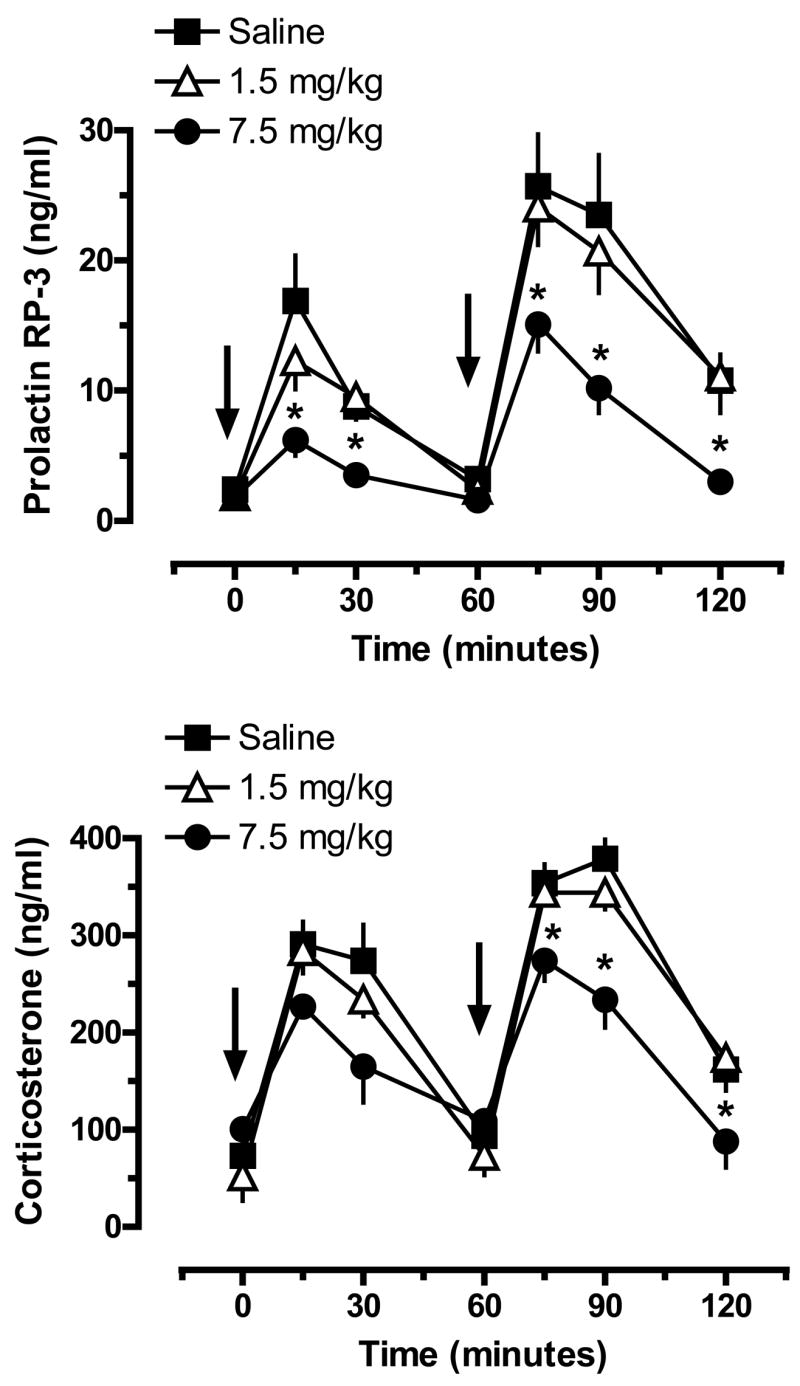
Plasma concentrations of prolactin (top panel) and corticosterone (bottom panel) in rats pretreated with saline or MDMA binges. Male rats received 3 ip injections of saline, 1.5 mg/kg (low dose) or 7.5 mg/kg MDMA (high dose). Two weeks later, rats were given iv challenge injections of 1 mg/kg MDMA at time zero, followed by 3 mg/kg MDMA at 60 min. Blood samples were withdrawn immediately before and at 15, 30, 60, 75, 90 and 120 min after the first iv injection. Plasma hormones were assayed by double-antibody RIA methods. Data are ng/ml expressed as mean±SEM for N=8 rats/group. * = P < 0.05 compared to saline-pretreated control at a given time point.
MDMA binges did not alter baseline levels of plasma corticosterone measured two weeks later; values were 73±18, 52±14 and 98±25 ng/ml for the saline, 1.5 and 7.5 mg/kg groups. Acute MDMA injections produced significant increases in plasma corticosterone, and there were main effects of MDMA Pretreatment Dose (F2,147=12.20, P<0.0001) and Time (F6,147=56.68, P<0.0001) on this response. Post-hoc tests revealed that rats pretreated with 7.5 mg/kg binges had reduced corticosterone responses to a 3 mg/kg MDMA challenge, with peak corticosterone levels significantly less than those of saline-pretreated controls. Rats pretreated with 1.5 mg/kg MDMA binges exhibited corticosterone responses similar to those of rats pretreated with saline.
Fig. 6 shows the effects of acute MDMA injections on 5-HT syndrome scores in rats previously exposed to MDMA binges. Behavioral data in Fig. 6 are derived from the same rats whose hormone data are depicted in Fig. 5. Acute iv administration of MDMA produced robust 5-HT syndrome, and there was a main effect of MDMA Pretreatment Dose (F2,42=8.15, P<0.001) on this response. Specifically, rats pretreated with 7.5 mg/kg binges displayed lower syndrome scores after 3 mg/kg iv MDMA when compared to corresponding scores in saline-pretreated rats.
Figure 6.
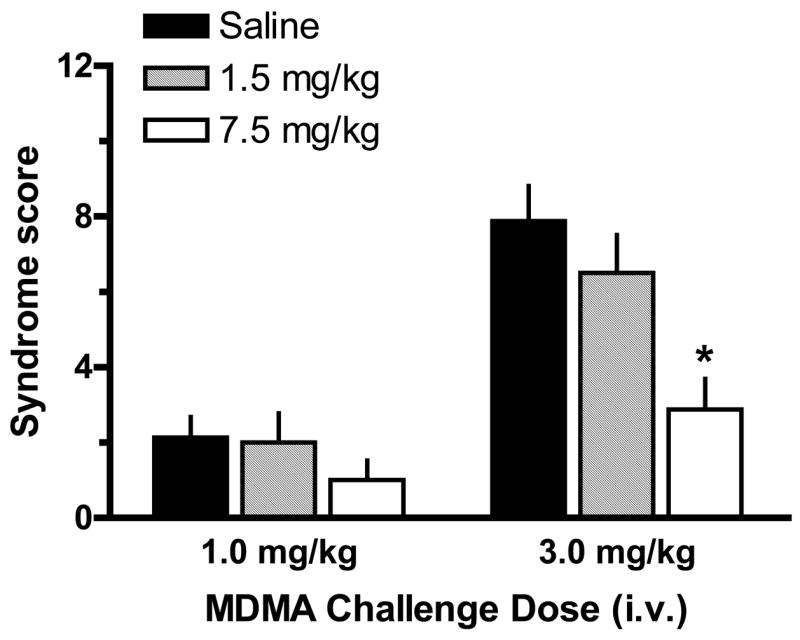
5-HT behavioral syndrome in rats pretreated with saline or MDMA binges. Male rats received 3 ip injections of saline, 1.5 mg/kg (low dose) or 7.5 mg/kg MDMA (high dose). Two weeks later, rats were given iv challenge injections of 1 mg/kg MDMA at time zero, followed by 3 mg/kg MDMA at 60 min. The occurrence of flat-body posture and forepaw treading were scored after each dose of acute iv MDMA. Data are syndrome scores expressed as mean±SEM for N=8 rats/group. * = P < 0.05 compared to saline-pretreated control at the corresponding challenge dose of MDMA.
In Vivo Microdialysis in Rats Exposed to MDMA Binges
Based on the neuroendocrine findings shown in Fig. 5, we evaluated whether the blunted hormone responses after 7.5 mg/kg MDMA might be reflective of neurochemical changes in the brain. Fig. 7 shows the effects of acute MDMA injections on dialysate 5-HT (top panel) and DA (bottom panel) in n. accumbens of rats previously exposed to MDMA binges. MDMA binges did not alter baseline dialysate levels of 5-HT measured two weeks later; values were 0.23±0.05, 0.22±0.04 and 0.20±0.04 pg/5 μL for the saline, 1.5 and 7.5 mg/kg groups, respectively. Acute iv MDMA evoked dose-related elevations in dialysate 5-HT in all groups, but there were main effects of MDMA Pretreatment Dose (F2,161=22.95, P<0.0001) and Time (F8,161=67.93, P<0.0001) on this response. Specifically, rats pretreated with 7.5 mg/kg MDMA had blunted 5-HT release in the n. accumbens in response to 1 and 3 mg/kg MDMA challenge. The magnitude of 5-HT responses in the 7.5 mg/kg rats was about 50% of the corresponding responses in saline-pretreated rats at both challenge doses of MDMA. Rats pretreated with 1.5 mg/kg MDMA binges exhibited 5-HT responses that were similar to saline-pretreated responses.
Figure 7.
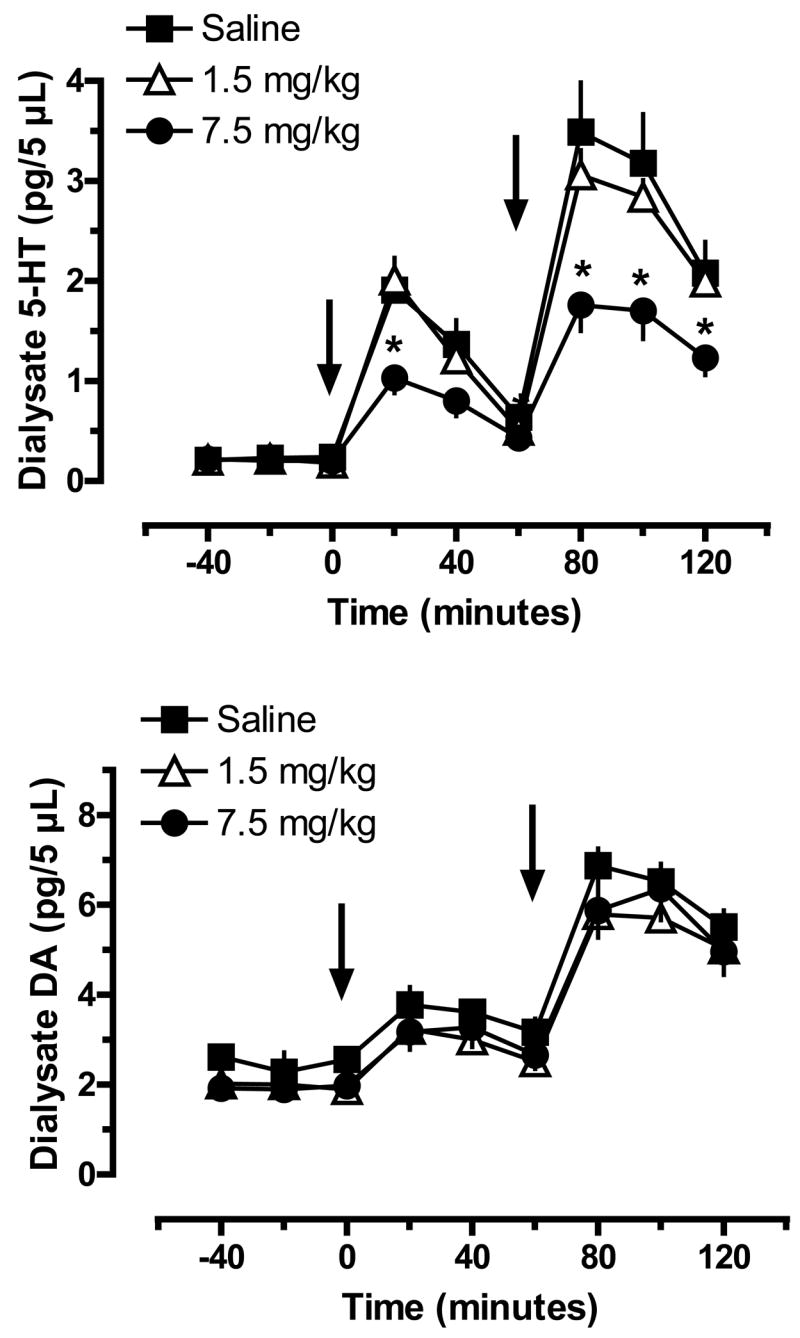
Extracellular levels of 5-HT (top panel) and DA (bottom panel) in n. accumbens of rats pretreated with saline or MDMA binges. Male rats received 3 ip injections of saline, 1.5 mg/kg (low dose) or 7.5 mg/kg MDMA (high dose). Two weeks later, rats undergoing in vivo microdialysis were given iv challenge injections of 1 mg/kg MDMA at time zero, followed by 3 mg/kg MDMA at 60 min. Dialysate samples collected at 20 min intervals were assayed for 5-HT and DA by microbore HPLC-ECD. Data are pg/5 μL expressed as mean±SEM for N=8 rats/group. * = P < 0.05 compared to saline-pretreated control at a given time point.
MDMA binges did not affect baseline dialysate DA in n. accumbens; values were 2.28±0.44, 2.02±0.38 and 1.90±0.28 pg/5 μL for the saline, 1.5 and 7.5 groups. Acute MDMA evoked dose-related increases in dialysate DA, and there was no significant effect of MDMA Pretreatment Dose on this transmitter response. Rats from all pretreatment groups displayed similar DA release in response to MDMA challenge.
The data in Fig. 8 illustrate the effects of acute MDMA injections on dialysate 5-HT (top panel) and DA (bottom panel) in caudate n. of rats previously exposed to MDMA binges. MDMA binges did not alter baseline dialysate levels of 5-HT measured two weeks later; specific 5-HT values were 0.32±0.05, 0.31±0.07, 0.25±0.05 pg/5 μL for the saline, 1.5 and 7.5 mg/kg groups, respectively. Acute iv MDMA evoked dose-related elevations in dialysate 5-HT in all groups, but there were main effects of MDMA Pretreatment Dose (F2,162=37.97, P<0.0001) and Time (F8,162=61.50, P<0.0001) on this transmitter response. Similar to the effects noted in the n. accumbens, rats pretreated with 7.5 mg/kg MDMA displayed blunted 5-HT levels in response to 1 and 3 mg/kg MDMA challenge. The magnitude of 5-HT release in the 7.5 mg/kg rats was about 60% of the corresponding responses in saline-pretreated rats at both challenge doses of MDMA. Rats pretreated with 1.5 mg/kg MDMA exhibited 5-HT responses that were nearly identical to those of saline-pretreated rats.
Figure 8.
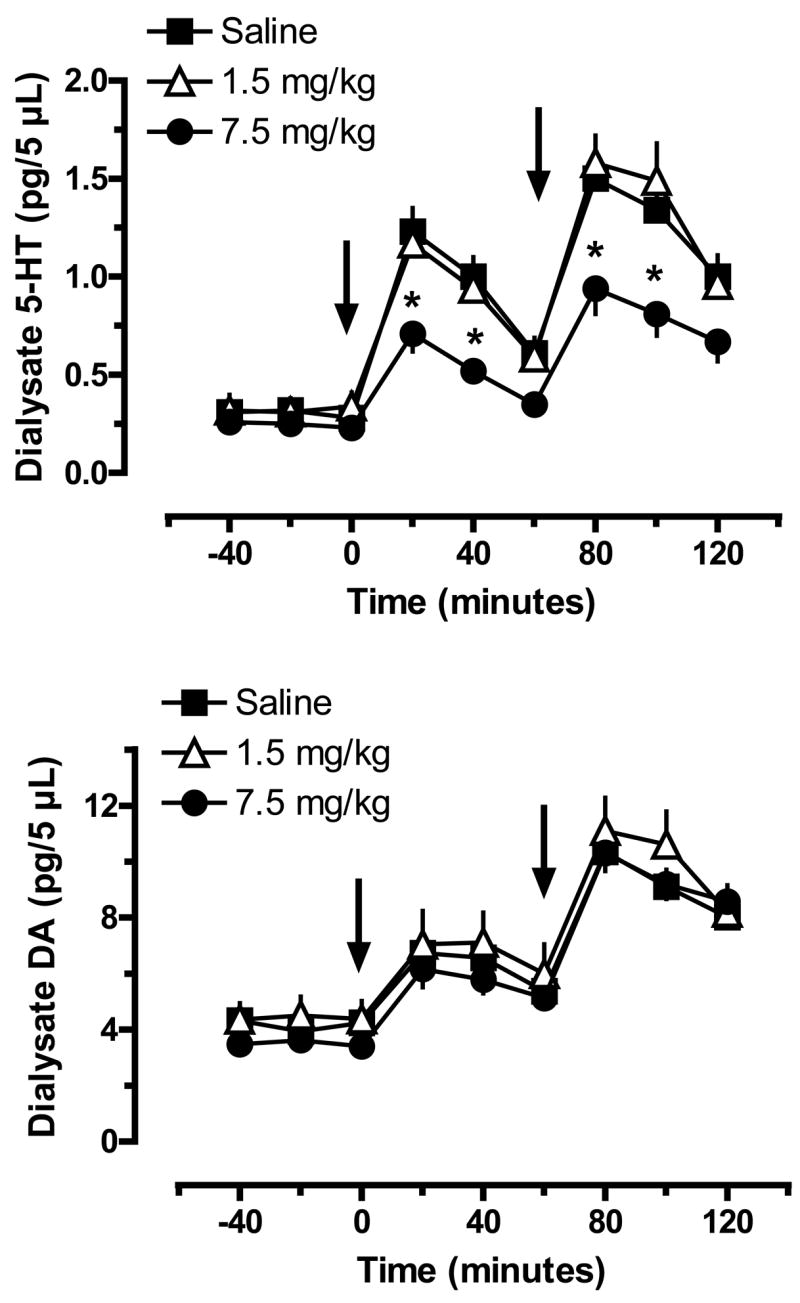
Extracellular levels of 5-HT (top panel) and DA (bottom panel) in caudate n. of rats pretreated with saline or MDMA binges. Male rats received 3 ip injections of saline, 1.5 mg/kg (low dose) or 7.5 mg/kg MDMA (high dose). Two weeks later, rats undergoing in vivo microdialysis were given iv challenge injections of 1 mg/kg MDMA at time zero, followed by 3 mg/kg MDMA at 60 min. Dialysate samples collected at 20 min intervals were assayed for 5-HT and DA by microbore HPLC-ECD. Data are pg/5 μL expressed as mean±SEM for N=8 rats/group. * = P < 0.05 compared to saline-pretreated control at a given time point.
MDMA binges did not affect baseline dialysate DA in caudate; specific values were 3.93±0.56, 4.50±0.68 and 3.62±0.38 pg/5 μL for the saline, 1.5 and 7.5 groups. Acute MDMA evoked dose-related increases in dialysate DA, and while the main effect of MDMA Pretreatment Dose did not reach statistical significance (F2,162=2.85, P<0.06), there was a significant effect of Time (F8,162=32.30, P<0.0001). In general, rats from all pretreatment groups displayed similar DA release profiles in response to iv MDMA challenge.
DISCUSSION
It is well established that MDMA administration to laboratory animals causes deficits in 5-HT neurons (e.g., depletion of brain tissue 5-HT), but the functional significance and clinical relevance of such changes are less clear (Easton and Marsden, 2006, Baumann et al., 2007). A major aim of the present study was to examine in vivo functional correlates of 5-HT deficits produced by MDMA in rats. We found that high-dose MDMA binges (7.5 mg/kg, ip, 3 injections) caused acute hyperthermia and long-term loss of brain 5-HT, whereas low-dose binges (1.5 mg/kg, ip, 3 injections) did not. High-dose MDMA binges were associated with blunted prolactin and corticosterone responses to MDMA challenge injections given two weeks later, consistent with the development of tolerance. Interestingly, human MDMA users are reported to display attenuated hormone responses to the 5-HT releaser d-fenfluramine, and this reduced sensitivity can last for months after abstinence (Gerra et al., 1998, Gerra et al., 2000, Verkes et al., 2001). In vivo microdialysis in the n. accumbens and caudate n. revealed that rats exposed to high-dose MDMA binges had blunted 5-HT release, but unaltered DA release, when challenged with acute MDMA injections. Collectively, the data suggest that tolerance to neuroendocrine effects of MDMA is due to impairments in SERT-mediated release of 5-HT from nerve terminals in the brain. Our findings could have implications for human MDMA users who often report the development of tolerance to subjective effects after prolonged use (Verheyden et al., 2003, Parrott, 2005).
One problem with extrapolating MDMA data from animals to humans is that laboratory animals receive non-contingent doses of drug which are much higher than those taken recreationally by humans (Easton and Marsden, 2006, Baumann et al., 2007). We sought to minimize this obstacle by designing an MDMA dosing regimen in rats based on threshold pharmacological doses. To this end, a typical dose of MDMA used for discrimination training in rats is 1.5 mg/kg ip (Schechter, 1988, Glennon and Higgs, 1992). Interestingly, human subjects discriminate this same dose administered orally in an experimental setting (Johanson et al., 2006), and recreational doses are in the range of 1–3 mg/kg (Cole et al., 2002, Schifano et al., 2006). When 1.5 mg/kg MDMA was given to rats undergoing microdialysis, extracellular levels of 5-HT in n. accumbens were elevated 5-fold above baseline while DA levels were not affected (Fig. 1). Our study represents the first to demonstrate in vivo neurochemical effects of an ip administered discrimination training dose of MDMA. The ability of low-dose MDMA to preferentially increase dialysate 5-HT supports the predominant role of serotonergic mechanisms in mediating stimulus properties of MDMA (Schechter, 1988, Goodwin et al., 2003). When the dose of MDMA was increased to 7.5 mg/kg ip, extracellular levels of 5-HT and DA increased to 30-fold and 5-fold above baseline, respectively. Many investigators have demonstrated that high-dose MDMA increases dialysate levels of 5-HT and DA in diverse brain regions (Gudelsky and Nash, 1996, Kankaanpaa et al., 1998, Gough et al., 2002). The in vivo microdialysis data are consistent with the established molecular mechanism of MDMA which involves transporter-mediated release of monoamines from neurons (White et al., 1996, Rothman and Baumann, 2002, Green et al., 2003).
Based on the neurochemical actions of 1.5 and 7.5 mg/kg MDMA, we examined acute and long-term effects produced by 3-injection binges of these same doses. Low-dose MDMA binges failed to affect body temperature, while high-dose binges caused hyperthermia (Fig. 2) analogous to previous reports (Nash et al., 1988, Dafters, 1995, Mechan et al., 2002). Since 1.5 mg/kg MDMA preferentially increases 5-HT release, it seems that 5-HT mechanisms alone are not sufficient to evoke hyperthermia. In contrast, a dose of 7.5 mg/kg MDMA which evokes simultaneous release of 5-HT and DA significantly elevates core body temperature, and D1 DA receptors have been implicated in MDMA-induced hyperthermia (Mechan et al., 2002). The same rats that exhibited hyperthermia in our study had substantial loss of brain tissue 5-HT when measured two weeks later (Fig. 3), suggesting a crucial link between hyperthermia and 5-HT depletions. Indeed, we found significant negative correlations between acute effects of MDMA on body temperature and subsequent measures of tissue 5-HT in the n. accumbens, caudate-putamen, and mediobasal hypothalamus (Fig. 4). These findings support the work of others who showed acute hyperthermic effects of MDMA can exacerbate the extent of long-term 5-HT depletions (Broening et al., 1995, O’Shea et al., 1998). We observed that high-dose MDMA binges produce about 50% loss of tissue 5-HT in the caudate, accumbens and hypothalamus; this degree of depletion is similar to that reported by our laboratory (Wang et al., 2005, Wang et al., 2007) and others who administered high-dose MDMA injection regimens to rats (Battaglia et al., 1987, Commins et al., 1987, Stone et al., 1987).
We chose to employ a neuroendocrine challenge paradigm for examining in vivo correlates of MDMA-induced 5-HT depletions. This strategy allows direct comparison of data from rats and humans, as both species show similar hormone responses to 5-HT releasers like MDMA and fenfluramine (Levy et al., 1994, Baumann et al., 1998). In particular, MDMA administration increases prolactin and adrenocorticotropin (ACTH) secretion from the anterior pituitary, and glucocorticoid secretion from the adrenals, in rats (Nash et al., 1988, Baumann et al., 2007) and humans (Mas et al., 1999, Harris et al., 2002, Farre et al., 2004). These specific MDMA-induced hormone responses involve the release of 5-HT from nerve terminals in the hypothalamus, followed by activation of 5-HT receptors (Nash et al., 1990). We showed that sequential iv injections of MDMA evoke dose-related elevations in plasma prolactin and corticosterone (Fig. 5), but MDMA pretreatment influenced hormonal responsiveness. Specifically, rats exposed to high-dose MDMA binges had significantly reduced hormone responses to MDMA challenge, with prolactin secretion being more severely affected. The blunted endocrine sensitivity to MDMA reported here confirms prior preliminary findings (Baumann et al., 2007) and suggests the development of tolerance. In comparable experiments, rats exposed to high-dose fenfluramine injections have reduced prolactin and corticosterone responses to fenfluramine challenge (Baumann et al., 1998). Moreover, as mentioned earlier, human MDMA users display suppression of prolactin and cortisol responses to d-fenfluramine which lasts for months after abstinence (Gerra et al., 1998, Gerra et al., 2000, Verkes et al., 2001). Not all studies report blunted neuroendocrine responses after pretreatment with MDMA. For example, Poland and coworkers (Poland et al., 1997) demonstrated that rats pretreated with high-dose MDMA exhibit decreases in fenfluramine-induced ACTH and corticosterone secretion, but increases in prolactin secretion. While we can not explain the discrepancies between our prolactin data and those of Poland et al., most evidence indicates that at least some hormone responses evoked by 5-HT-releasing agents are reduced in rats and humans exposed to MDMA.
MDMA administration produces a complex pattern of motor activity in rats that is characterized by forward locomotion and elements of the 5-HT behavioral syndrome, including flat-body posture and forepaw treading (Gold et al., 1988, Slikker et al., 1989, Spanos and Yamamoto, 1989). MDMA-induced motor activity depends upon monoamine release from neurons in the brain, followed by subsequent activation of multiple 5-HT and DA receptor subtypes (Callaway et al., 1991, Bankson and Cunningham, 2001). We monitored 5-HT syndrome during neuroendocrine challenge tests in MDMA-pretreated rats. As expected, we found that iv injections of MDMA caused dose-related increases in flat-body posture and forepaw treading (Fig. 6). Similar to the neuroendocrine findings, the ability of MDMA to elicit 5-HT syndrome was significantly reduced in rats pretreated with high-dose MDMA binges. These behavioral data are consistent with investigations showing reductions in evoked 5-HT syndrome after repeated injections of high-dose MDMA (Slikker et al., 1989, Marston et al., 1999, Brennan and Schenk, 2006). Not all studies have found evidence for such behavioral tolerance after repeated MDMA treatments. In fact, several reports show reverse tolerance, or sensitization, to locomotor effects of MDMA in rats pretreated with the drug (Spanos and Yamamoto, 1989, Kalivas et al., 1998, McCreary et al., 1999). Possible reasons for inconsistencies in the behavioral findings include differences in MDMA dosing regimens, time intervals between pretreatment and behavioral testing, and specific end-points examined. In general, investigators that have employed repeated high-dose MDMA regimens (i.e., single or multiple doses of 10 mg/kg, ip or greater) have found evidence for tolerance, whereas those using lower doses have found sensitization.
In our final experiments, we wished to address possible mechanisms underlying diminished sensitivity to MDMA administration in MDMA-pretreated rats. Because high-dose MDMA binges depleted brain 5-HT and engendered tolerance, we speculated that blunted responsiveness to MDMA might be mediated by impairments in presynaptic 5-HT release. To test this hypothesis directly, in vivo microdialysis was used to monitor extracellular levels of 5-HT and DA in the caudate n. and n. accumbens of rats pretreated with binges of saline or MDMA. We demonstrated that sequential iv doses of MDMA increase extracellular levels of 5-HT and DA in the n. accumbens (Fig. 7) and caudate n. (Fig. 8), but MDMA pretreatment modified neurochemical responses. Specifically, rats pretreated with high-dose MDMA displayed blunted 5-HT release, but unaltered DA release, when challenged with MDMA. Our study is the first to provide evidence that MDMA causes impairments in SERT-mediated 5-HT release in rat n. accumbens, a region implicated in the drug addiction (Ikemoto and Panksepp, 1999). The magnitude of reduction in evoked 5-HT release was comparable in the accumbens and caudate. Our microdialysis data agree with previous reports where rats pretreated with high-dose MDMA had impaired 5-HT release in response to pharmacological challenge (Series et al., 1994, Shankaran and Gudelsky, 1999) or physiological provocation (Gartside et al., 1996, Matuszewich et al., 2002). In a study analogous to ours, Shankaran and Gudelsky (Shankaran and Gudelsky, 1999) found reductions in striatal 5-HT release, 5-HT syndrome, and hyperthermia produced by MDMA, in rats pretreated with doses that deplete brain 5-HT. In contrast to our results, Kalivas et al. (1998) reported that MDMA-induced DA release is enhanced in rats exposed to high-dose MDMA, and this heightened DA response was correlated with behavioral sensitization. We have no simple explanation for the discrepancies between our findings and those of Kalivas et al., but there are a number of methodological differences between the two studies as noted above. Interestingly, high-dose MDMA binges did not alter baseline levels of dialysate 5-HT or DA in our experiments, despite 50% loss of brain tissue 5-HT under similar dosing conditions. Others have shown that depletions of brain 5-HT produced by substituted amphetamines, or the neurotoxin 5,7-dihydroxytryptamine, do not change baseline levels of dialysate 5-HT (Series et al., 1994, Kirby et al., 1995, Hall et al., 1999, Shankaran and Gudelsky, 1999). These observations suggest that compensatory mechanisms (e.g., less 5-HT reuptake) maintain normal concentrations of synaptic 5-HT even when intracellular transmitter stores are severely depleted. Taken together, our data indicate that neuroendocrine and behavioral tolerance in MDMA-pretreated rats could be related to specific impairments in SERT-mediated release of 5-HT from nerve terminals in the brain.
Tolerance can be defined as the diminished responsiveness to a drug after repeated exposure – and we have shown that rats are rendered less sensitive to pharmacological effects of MDMA after single high-dose binges. The blunted responsiveness to MDMA shown here agrees with previous reports of tolerance to diverse effects of MDMA in rats, including anorexia (Zacny et al., 1990), discriminative stimulus properties (Schechter, 1991, Virden and Baker, 1999), hyperthermia (Shankaran and Gudelsky, 1999, Piper et al., 2006) and locomotor activity (Callaway and Geyer, 1992, Brennan and Schenk, 2006). Tolerance to behavioral actions of MDMA is also well documented in non-human primates (Frederick et al., 1995, Frederick and Paule, 1997, Fantegrossi et al., 2004, Fantegrossi, 2007). On the other hand, a number of studies in rodents provide evidence for sensitized responsiveness after repeated MDMA exposure (Poland et al., 1997, Kalivas et al., 1998, Giorgi et al., 2005), suggesting tolerance development is not a universal outcome. In humans, the emergence of tolerance to subjective effects of MDMA is a chief complaint which often leads to dangerous dose escalation in an attempt to recapture the “magic” of the initial Ecstasy experience (Verheyden et al., 2003, Parrott, 2005). The mechanisms underlying tolerance in humans are not well characterized, and obvious ethical constraints have limited the investigation of repeated MDMA dosing in people. In one clinical study, Farre et al. (2004) examined the pharmacological effects produced by two successive doses of MDMA (100 mg, po) given 24 h apart in a controlled experimental setting. These investigators showed that certain effects of MDMA (e.g. prolactin secretion) were smaller than expected after a second dose, despite higher plasma levels of MDMA. Acute tolerance of this type could be related to any number of mechanisms, including altered 5-HT release or desensitization of post-synaptic receptor sites. In the present study, rats pretreated with high-dose MDMA binges displayed a distinct triad of symptoms: 1) central 5-HT depletions; 2) blunted pharmacological responsiveness; and 3) impaired SERT-mediated 5-HT release. Our data suggest the possibility that humans who use repeated high doses of MDMA may develop tolerance due to deficits in brain 5-HT function (Verheyden et al., 2003, Parrott, 2005). To this end, recent experiments in rats reveal that administration of the 5-HT precursor, L-5-hydroxytryptophan, can restore MDMA-induced 5-HT depletions (Wang et al., 2007), indicating a potential therapeutic approach for abstinent MDMA users who require treatment. Clearly, more investigations are needed to determine the molecular underpinnings of MDMA tolerance in animals and humans.
Acknowledgments
This research was generously supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, USA. Portions of the data reported here were presented at the 2005 Annual Meeting of the College on Problems of Drug Dependence (CPDD) held in Orlando, FL, USA.
Abbreviations
- ANOVA
analysis of variance
- ACTH
adrenocorticotropin
- DA
dopamine
- HPLC-ECD
high-performance liquid chromatography with electrochemical detection
- 5-HT
5-hydroxytryptamine or serotonin
- MDMA
(±)-3,4-methylenedioxymethamphetamine
- MDA
(±)-3,4,-methylenedioxyamphetamine
- RIA
radioimmunoassay
- SERT
serotonin transporter
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ball KT, Budreau D, Rebec GV. Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Res. 2003;994:203–215. doi: 10.1016/j.brainres.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Rothman RB. Functional consequences of central serotonin depletion produced by repeated fenfluramine administration in rats. J Neurosci. 1998;18:9069–9077. doi: 10.1523/JNEUROSCI.18-21-09069.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB. N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or ‘Ecstasy’) Neuropsychopharmacology. 2005;30:550–560. doi: 10.1038/sj.npp.1300585. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KA, Schenk S. Initial deficit and recovery of function after MDMA preexposure in rats. Psychopharmacology (Berl) 2006;184:239–246. doi: 10.1007/s00213-005-0278-y. [DOI] [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (+/−)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine1B agonist. J Pharmacol Exp Ther. 1992;263:318–326. [PubMed] [Google Scholar]
- Callaway CW, Johnson MP, Gold LH, Nichols DE, Geyer MA. Amphetamine derivatives induce locomotor hyperactivity by acting as indirect serotonin agonists. Psychopharmacology (Berl) 1991;104:293–301. doi: 10.1007/BF02246026. [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M, Mas M, Roset PN, Poudevida S, Mas A, San L, de la Torre R. Human pharmacology of 3,4-methylenedioxymethamphetamine (“ecstasy”): psychomotor performance and subjective effects. J Clin Psychopharmacol. 2000;20:455–466. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- Cole JC, Bailey M, Sumnall HR, Wagstaff GF, King LA. The content of ecstasy tablets: implications for the study of their long-term effects. Addiction. 2002;97:1531–1536. doi: 10.1046/j.1360-0443.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS. Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther. 1987;241:338–345. [PubMed] [Google Scholar]
- Dafters RI. Hyperthermia following MDMA administration in rats: effects of ambient temperature, water consumption, and chronic dosing. Physiol Behav. 1995;58:877–882. doi: 10.1016/0031-9384(95)00136-7. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M. Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci. 2004;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Easton N, Marsden CA. Ecstasy: are animal data consistent between species and can they translate to humans? J Psychopharmacol. 2006;20:194–210. doi: 10.1177/0269881106061153. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE. Reinforcing effects of methylenedioxy amphetamine congeners in rhesus monkeys: are intravenous self-administration experiments relevant to MDMA neurotoxicity? Psychopharmacology (Berl) 2007;189:471–482. doi: 10.1007/s00213-006-0320-8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Mathuna BO, Roset PN, Peiro AM, Torrens M, Ortuno J, Pujadas M, Cami J. Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology (Berl) 2004;173:364–375. doi: 10.1007/s00213-004-1789-7. [DOI] [PubMed] [Google Scholar]
- Frederick DL, Ali SF, Slikker W, Jr, Gillam MP, Allen RR, Paule MG. Behavioral and neurochemical effects of chronic methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neurotoxicol Teratol. 1995;17:531–543. doi: 10.1016/0892-0362(95)00013-h. [DOI] [PubMed] [Google Scholar]
- Frederick DL, Paule MG. Effects of MDMA on complex brain function in laboratory animals. Neurosci Biobehav Rev. 1997;21:67–78. doi: 10.1016/0149-7634(95)00064-x. [DOI] [PubMed] [Google Scholar]
- Gartside SE, McQuade R, Sharp T. Effects of repeated administration of 3,4-methylenedioxymethamphetamine on 5-hydroxytryptamine neuronal activity and release in the rat brain in vivo. J Pharmacol Exp Ther. 1996;279:277–283. doi: 10.1163/2211730x96x00153. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Ferri M, Zambelli U, Timpano M, Neri E, Marzocchi GF, Delsignore R, Brambilla F. Long-lasting effects of (+/−)3,4-methylenedioxymethamphetamine (ecstasy) on serotonin system function in humans. Biol Psychiatry. 2000;47:127–136. doi: 10.1016/s0006-3223(99)00180-8. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Giucastro G, Maestri D, Monica C, Sartori R, Caccavari R, Delsignore R. Serotonergic function after (+/−)3,4-methylene-dioxymethamphetamine (‘Ecstasy’) in humans. Int Clin Psychopharmacol. 1998;13:1–9. doi: 10.1097/00004850-199801000-00001. [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Pizzanelli C, Ferrucci M, Lazzeri G, Faetti M, Giusiani M, Pontarelli F, Busceti CL, Murri L, Fornai F. Previous exposure to (+/−) 3,4-methylenedioxymethamphetamine produces long-lasting alteration in limbic brain excitability measured by electroencephalogram spectrum analysis, brain metabolism and seizure susceptibility. Neuroscience. 2005;136:43–53. doi: 10.1016/j.neuroscience.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Higgs R. Investigation of MDMA-related agents in rats trained to discriminate MDMA from saline. Pharmacol Biochem Behav. 1992;43:759–763. doi: 10.1016/0091-3057(92)90405-5. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- Goodwin AK, Pynnonen DM, Baker LE. Serotonergic-dopaminergic mediation of MDMA’s discriminative stimulus effects in a three-choice discrimination. Pharmacol Biochem Behav. 2003;74:987–995. doi: 10.1016/s0091-3057(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Gough B, Imam SZ, Blough B, Slikker W, Jr, Ali SF. Comparative effects of substituted amphetamines (PMA, MDMA, and METH) on monoamines in rat caudate: a microdialysis study. Ann N Y Acad Sci. 2002;965:410–420. doi: 10.1111/j.1749-6632.2002.tb04182.x. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Hall FS, Devries AC, Fong GW, Huang S, Pert A. Effects of 5,7-dihydroxytryptamine depletion of tissue serotonin levels on extracellular serotonin in the striatum assessed with in vivo microdialysis: relationship to behavior. Synapse. 1999;33:16–25. doi: 10.1002/(SICI)1098-2396(199907)33:1<16::AID-SYN2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Kilbey M, Gatchalian K, Tancer M. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among d-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend. 2006;81:27–36. doi: 10.1016/j.drugalcdep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, White SR. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology. 1998;18:469–479. doi: 10.1016/S0893-133X(97)00195-4. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Lillsunde P, Seppala T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Kreiss DS, Singh A, Lucki I. Effect of destruction of serotonin neurons on basal and fenfluramine-induced serotonin release in striatum. Synapse. 1995;20:99–105. doi: 10.1002/syn.890200202. [DOI] [PubMed] [Google Scholar]
- Levy AD, Baumann MH, Van de Kar LD. Monoaminergic regulation of neuroendocrine function and its modification by cocaine. Front Neuroendocrinol. 1994;15:85–156. doi: 10.1006/frne.1994.1006. [DOI] [PubMed] [Google Scholar]
- Lin JH. Applications and limitations of interspecies scaling and in vitro extrapolation in pharmacokinetics. Drug Metab Dispos. 1998;26:1202–1212. [PubMed] [Google Scholar]
- Marston HM, Reid ME, Lawrence JA, Olverman HJ, Butcher SP. Behavioural analysis of the acute and chronic effects of MDMA treatment in the rat. Psychopharmacology (Berl) 1999;144:67–76. doi: 10.1007/s002130050978. [DOI] [PubMed] [Google Scholar]
- Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, Cami J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- Matuszewich L, Filon ME, Finn DA, Yamamoto BK. Altered forebrain neurotransmitter responses to immobilization stress following 3,4-methylenedioxymethamphetamine. Neuroscience. 2002;110:41–48. doi: 10.1016/s0306-4522(01)00539-5. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Bankson MG, Cunningham KA. Pharmacological studies of the acute and chronic effects of (+)-3, 4-methylenedioxymethamphetamine on locomotor activity: role of 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B/1D) receptors. J Pharmacol Exp Ther. 1999;290:965–973. [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O’Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br J Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JF, Jr, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;245:873–879. [PubMed] [Google Scholar]
- Nash JF, Jr, Meltzer HY, Gudelsky GA. Neuroendocrinological effects of MDMA in the rat. In: Peroutka SJ, editor. Ecstasy: The Clinical, Pharmacological and Neurotoxicological Effects of the Drug MDMA. Kluwer Academic; Boston: 1990. pp. 225–239. [Google Scholar]
- O’Shea E, Granados R, Esteban B, Colado MI, Green AR. The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy’) Neuropharmacology. 1998;37:919–926. doi: 10.1016/s0028-3908(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or Ecstasy. J Psychopharmacol. 2005;19:71–83. doi: 10.1177/0269881105048900. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Vu HL, Safain MG, Oliver AJ, Meyer JS. Repeated adolescent 3,4-methylenedioxymethamphetamine (MDMA) exposure in rats attenuates the effects of a subsequent challenge with MDMA or a 5-hydroxytryptamine(1A) receptor agonist. J Pharmacol Exp Ther. 2006;317:838–849. doi: 10.1124/jpet.105.095760. [DOI] [PubMed] [Google Scholar]
- Poland RE, Lutchmansingh P, McCracken JT, Zhao JP, Brammer GL, Grob CS, Boone KB, Pechnick RN. Abnormal ACTH and prolactin responses to fenfluramine in rats exposed to single and multiple doses of MDMA. Psychopharmacology (Berl) 1997;131:411–419. doi: 10.1007/s002130050311. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Yuan J, McCann UD. (+/−)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology. 2000;42:5–10. doi: 10.1159/000026664. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther. 2002;95:73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Serotonergic-dopaminergic mediation of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Biochem Behav. 1988;31:817–824. doi: 10.1016/0091-3057(88)90390-5. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Effect of MDMA neurotoxicity upon its conditioned place preference and discrimination. Pharmacol Biochem Behav. 1991;38:539–544. doi: 10.1016/0091-3057(91)90010-y. [DOI] [PubMed] [Google Scholar]
- Schifano F, Corkery J, Deluca P, Oyefeso A, Ghodse AH. Ecstasy (MDMA, MDA, MDEA, MBDB) consumption, seizures, related offences, prices, dosage levels and deaths in the UK (1994–2003) J Psychopharmacol. 2006;20:456–463. doi: 10.1177/0269881106060147. [DOI] [PubMed] [Google Scholar]
- Series HG, Cowen PJ, Sharp T. p-Chloroamphetamine (PCA), 3,4-methylenedioxy-methamphetamine (MDMA) and d-fenfluramine pretreatment attenuates d-fenfluramine-evoked release of 5-HT in vivo. Psychopharmacology (Berl) 1994;116:508–514. doi: 10.1007/BF02247485. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Gudelsky GA. A neurotoxic regimen of MDMA suppresses behavioral, thermal and neurochemical responses to subsequent MDMA administration. Psychopharmacology (Berl) 1999;147:66–72. doi: 10.1007/s002130051143. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Holson RR, Ali SF, Kolta MG, Paule MG, Scallet AC, McMillan DE, Bailey JR, Hong JS, Scalzo FM. Behavioral and neurochemical effects of orally administered MDMA in the rodent and nonhuman primate. Neurotoxicology. 1989;10:529–542. [PubMed] [Google Scholar]
- Spanos LJ, Yamamoto BK. Acute and subchronic effects of methylenedioxymethamphetamine [(+/−)MDMA] on locomotion and serotonin syndrome behavior in the rat. Pharmacol Biochem Behav. 1989;32:835–840. doi: 10.1016/0091-3057(89)90044-0. [DOI] [PubMed] [Google Scholar]
- Stone DM, Merchant KM, Hanson GR, Gibb JW. Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in brain of rat. Neuropharmacology. 1987;26:1677–1683. doi: 10.1016/0028-3908(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Verheyden SL, Henry JA, Curran HV. Acute, sub-acute and long-term subjective consequences of ‘ecstasy’ (MDMA) consumption in 430 regular users. Hum Psychopharmacol. 2003;18:507–517. doi: 10.1002/hup.529. [DOI] [PubMed] [Google Scholar]
- Verkes RJ, Gijsman HJ, Pieters MS, Schoemaker RC, de Visser S, Kuijpers M, Pennings EJ, de Bruin D, Van de Wijngaart G, Van Gerven JM, Cohen AF. Cognitive performance and serotonergic function in users of ecstasy. Psychopharmacology (Berl) 2001;153:196–202. doi: 10.1007/s002130000563. [DOI] [PubMed] [Google Scholar]
- Virden TB, Baker LE. Disruption of the discriminative stimulus effects of S(+)-3,4-methylenedioxymethamphetamine (MDMA) by (+/−)-MDMA neurotoxicity: protection by fluoxetine. Behav Pharmacol. 1999;10:195–204. doi: 10.1097/00008877-199903000-00008. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Rothman RB. Restoration of 3,4-methylenedioxymethamphetamine-induced 5-HT depletion by the administration of L-5-hydroxytryptophan. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.05.024. in press. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Morales M, Rothman RB. (+/−)-3,4-Methylenedioxymethamphetamine administration to rats does not decrease levels of the serotonin transporter protein or alter its distribution between endosomes and the plasma membrane. J Pharmacol Exp Ther. 2005;314:1002–1012. doi: 10.1124/jpet.105.088476. [DOI] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, “Ecstasy”) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Virus RM, Woolverton WL. Tolerance and cross-tolerance to 3,4-methylenedioxymethamphetamine (MDMA), methamphetamine and methylenedioxyamphetamine. Pharmacol Biochem Behav. 1990;35:637–642. doi: 10.1016/0091-3057(90)90301-w. [DOI] [PubMed] [Google Scholar]


