Abstract
Preclinical and early clinical trials indicate synthetic oligodeoxynucleotides containing unmethylated CG dinucleotides (CpG ODN) have potent immunostimulatory effects and can enhance the anti-cancer activity of a variety of cancer treatments. Synergy between CpG ODN and monoclonal antibodies has been noted in various preclinical models. Early clinical trials indicate CpG ODN and monoclonal antibodies can be administered safely together. Preclinical models indicate CpG ODN can enhance the anti-tumor activity of both chemotherapy and radiation therapy. Thus, one possible approach to the use of CpG ODN was to use it in combination with cytotoxic chemotherapy with the goal of enhancing presentation of tumor antigen from dying cancer cells. Promising results in a randomized phase II trial in patients with non-small cell lung cancer led to initiation of two large randomized phase III trials comparing CpG ODN plus chemotherapy to chemotherapy alone. Unfortunately, interim analysis of these trials indicated CpG ODN was unlikely to enhance efficacy of chemotherapy, and they were stopped. CpG ODN also holds promise as a component of cancer vaccines including those composed of protein antigen, peptides, whole tumor cells, and antigen-pulsed dendritic cells. Finally, CpG ODN has been combined with a variety of cytokines to enhance NK activation, promote development of an active anti-tumor immune response or induce apoptosis of malignant cells that express the TLR9 receptor. Overall, both preclinical and early clinical trials suggest CpG ODN may be a valuable component of a variety of approaches to cancer therapy. However, clinical development of this recently discovered, novel class of immunostimulatory agents is just beginning, and we still have much to learn about the optimal approach to their use, and their potential.
Introduction
Over a hundred years have passed since the first anti-tumor effects of bacteria were identified by New York surgeon Dr. William Coley. His bacterial extracts were named after him and are now known as Coley’s toxin. Over the past decade we have learned that one component of these bacterial extracts, the prokaryotic bacterial DNA itself, has immunostimulatory properties due to sequence characteristics that are distinct from those found in eukaryotic DNA (1). Multiple studies have demonstrated that bacterial DNA, and synthetic oligodeoxynucleotides that contain unmethylated CG dinucleotides (CpG ODN), and a phosphorothioate or chimeric backbone that renders them nuclease resistant, have potent immunostimulatory effects. The pattern recognition receptor Toll-Like Receptor (TLR) 9 has been found to be the primary receptor for CpG ODN (2). The complex immunologic responses to CpG ODN by various immune cell subsets involve both direct and indirect effects, resulting in the activation of NK cells, T cells, B cells, monocytes, macrophages and dendritic cells (3–5).
TLR9 is a key pattern receptor that impacts on innate immunity, and on how the innate immune system interacts with adaptive immunity in both murine and the human immune systems. However, mice and humans are distinct with respect to the expression pattern of TLR9. This, and other differences between species, results in differences in the overall immune response of mice and humans to CpG ODN (6). This important observation is key to appropriate interpretation of the clinical significance of animal model results. For example, CpG ODN have impressive effects as single agents in various mouse tumor models (7–9). While clinical responses have been seen in early studies of single agent CpG ODN in human cancer, clinical results have been less impressive to date than those seen in preclinical studies. Despite this, CpG ODN can have clinical anti-tumor activity in humans and are likely to become valuable components of cancer therapy as we learn more about them.
The most extensively studied CpG ODN in the clinic is now known as PF-3512676 (also known as CpG ODN 2006, CPG 7909 and ProMune). A recently finished phase I clinical trial with this agent in 24 patients with recurrent glioblastoma demonstrated preliminary evidence of anti-tumor activity in two patients (10). We reported results from a phase I clinical trial in 23 patients with previously treated non-Hodgkin lymphoma, in which this agent was delivered as intravenous infusion. We found variable, but convincing signs of an immunologic response in some but not all subjects, and late clinical responses in two subjects (11). Anti-tumor activity in a minority of subjects has been found in Phase II studies with weekly doses of CpG ODN as a single agent in cutaneous T-cell lymphoma (12), melanoma (13, 14), and in renal cell carcinoma. Thus, CpG ODN does have modest anti-tumor activity as a single agent.
Animal models suggest CpG ODN may be more useful as a component of multiagent therapy for cancer rather than as a single agent. Promising combinations include CpG ODN with 1) monoclonal antibody therapy, 2) chemotherapy, 3) radiation therapy, 4) vaccination strategies and 5) cytokines including GM-CSF, IFN-alpha, IL-18 and IL-21. In this review, we focus on these five approaches to combination cancer therapy utilizing CpG ODN, and also discuss our own recent findings of the synergistic effects of CpG ODN and Interleukin 21 (IL-21) on B chronic lymphocytic leukemia cells. For a more general review about the therapeutic potential of CpG ODN see (15).
1) CpG ODN and therapeutic monoclonal antibodies
The rationale for combining CpG ODN and therapeutic monoclonal antibodies antibodies (mAb) arose from a number of observations. One of the earliest effects observed for CpG ODN administered in vivo was an increase in NK activity (16). This appears to be due to the ability of CpG ODN to induce production of IFNα by dendritic cells. Growing evidence suggests NK cells, and their ability to mediate antibody dependent cellular cytotoxicity (ADCC), are key mediators of the anti-tumor effects of mAb. Thus, activating NK cells with CpG ODN could potentiate the efficacy of mAb. We demonstrated synergy between CpG ODN and mAb several years ago in a syngeneic murine model (8) and that CpG ODN that are particularly potent at inducing IFNα are particularly good at enhancing the anti-tumor effects of mAb mediated by NK cells (17). More recent studies using human xenografts and other systems have extended these findings. MCF-7 and BT474 xenografts, and breast cancer expressing human Her2neu were found to be responsive to the anti-Her2neu antibody Herceptin when used in combination with CpG ODN (18).
A particularly promising combination is CpG ODN plus rituximab, an antibody directed against CD20, which is expressed on malignant and normal B cells. We found that CpG ODN can enhance the expression of CD20 on various types of B-cell lymphoma (19). Thus, CpG ODN may enhance efficacy of rituximab by both activating NK cells, and increasing expression of the target antigen. Friedberg et al. conducted a phase I clinical trial evaluating 4 dose levels of a CpG ODN (1018 ISS) with rituximab in 20 patients with relapsed non-Hodgkin lymphoma (NHL) (20). Patients received CpG ODN once a week for 4 weeks beginning after the second of 4 rituximab infusions. Quantitative real time PCR showed a CpG ODN dose-dependent increase of several interferon-inducible genes after CpG ODN administration including 2’5’ oligoadenylate synthetase (OAS), monocyte chemotactic proteins (MCP) 1 and 2, and IFN-γ-inducible protein 10 (IP-10). We recently completed a phase I trial using a different CpG ODN with rituximab (21) with similar results. Both studies demonstrated the combination of CpG ODN plus rituximab can be administered safely. It is still too early to assess whether clinical response from CpG ODN plus rituximab is greater than that seen with rituximab alone. This will be a difficult question to answer since rituximab is now most commonly used in combination with other agents.
2) CpG ODN and chemotherapy
When the immunostimulatory effects of CpG ODN were first described, it was assumed that these agents would not be effective when used in combination with standard chemotherapy because of the immunosuppressive effects of many cytotoxic agents. In fact, recent studies have brought surprises in terms of the positive effects immunomodulation can have on conventional therapies. Weigel et al found that the combination of chemotherapy plus CpG ODN decreased the frequency of distant metastasis in a mouse model of sarcoma (22). Pratesi et al. studied an orthotopic mouse model of a human pancreatic tumor xenograft (23). Chemotherapy with gemcitabine was followed by weekly therapy with CpG ODN. The combination delayed tumor growth and increased survival time. Tumor spread in the peritoneal cavity was reduced with the combination compared to gemcitabine alone. Van der Most et al. found potentiation of the antitumor efficacy of the novel chemotherapeutic agent coramsine by CpG ODN (24) in a murine model of malignant mesothelioma. Systemic administration of coramsine slowed tumor growth and prolonged survival time. The antitumor efficacy of coramsine was enhanced by adding CpG ODN.
In 2003, Coley Pharmaceutical Group initiated a randomized, open-label human phase II clinical study of CpG ODN (PF-3512676) plus taxane/platinum chemotherapy versus chemotherapy alone in 112 patients with advanced, stage IIIb or IV non-small-cell lung cancer (NSCLC) who were not previously treated (25). Patients were randomized 2:1 and received 4 – 6 three-week cycles of a standard taxane and platinum plus PF-3512676 on weeks 2 and 3 of each cycle or chemotherapy alone. Each dose of PF-3512676 was given s.c. at 0.20 mg/kg. The overall response rate was 40% in the presence of PF-3512676 versus 23% with chemotherapy alone. The Kaplan-Meyer curves showed a trend to improved progression-free survival and overall survival (12.2 months vs. 6.8 months). The most common adverse events directly attributable to PF-3512676 were mild to moderate local injection site reactions, and mild flu-like symptoms. Objective interpretation of this study requires consideration of the limitations of randomized phase II studies. Nevertheless, these results were encouraging, and led to design of two more rigorous phase III trials initiated in late 2005 by Pfizer designed to compare standard of care chemotherapy (paclitaxel/carboplatin or gemcitabine/cisplatin) to chemotherapy plus PF-3512676 as first-line treatment in patients with advanced non-small cell lung cancer. Each of the two Phase III clinical trials enrolled approximately 800 adult patients with Stage IIIb or IV disease who have not received prior chemotherapy or immunotherapy treatment. The primary endpoint for these Phase III clinical trials was overall survival, with secondary endpoints of overall confirmed objective response rate, duration of response, progression-free survival and time to tumor progression. A scheduled interim analysis of one of the Phase III clinical trials showed no evidence that PF-3512676 produced additional clinical efficacy over that achieved with the standard cytotoxic chemotherapy regimen alone and the trial was discontinued (June 20, 2007 press release, Coley Pharmaceutical Group). The negative result from this highly ambitious trial suggests combining CpG ODN with chemotherapy of TLR9-negative, relatively chemotherapy resistant malignancies may not be the best approach to the therapeutic use of this potent new class of agents.
In retrospect, non-small cell lung cancer, a malignancy that is known to be relatively refractory to cytotoxic chemotherapy, may not have been the best choice as a disease site for testing such a novel approach to therapy. B cell malignancies would seem to be more attractive as targets for combination CpG ODN and chemotherapy as they express TLR9 and respond to CpG ODN by undergoing apoptosis, and also respond more consistently to cytotoxicity chemotherapy.
3) CpG ODN and radiation
The sensitizing effect of CpG ODN is not limited to chemotherapeutic agents but can also be observed with radiation. Experiments in murine models suggest CpG ODN can enhance the response to radiation treatment for both immunogenic (26) and non-immunogenic tumors (27). The combination of CpG ODN with radiation and chemotherapy can also be effective. For example, recent studies indicate treatment with CpG ODN enhances the response and improves the cure rate of murine tumors treated with the combination of radiation and docetaxel (28). Treatment with either CpG ODN or radiotherapy alone induced complete tumor remissions in one-third of rats inoculated with the 9L glioma. When both treatments were combined, complete tumor remission was achieved in two-thirds of the animals (29).
Wei and colleagues recently reported preliminary results of a phase I/II study exploring the injection of PF-3512676 into irradiated lymph nodes of patients with low grade lymphoma. (30) We are conducting a phase I clinical trial of the combination of PF-3512676 with radiolabeled anti-CD20 mAb in subjects with B cell lymphoma. It is still too early to know about the efficacy of approaches that combine radiation and CpG ODN, but preliminary results suggest this approach is worth pursuing further.
The hypothesis that is the basis for exploring combinations of CpG ODN with cytotoxic therapies is that dendritic cells (DC) acquire antigens released from the tumor cells after cytotoxic therapy. These DCs then migrate to the regional lymph nodes where they encounter and activate tumor-specific cytotoxic T cells. In this system, the primary effect of CpG ODN is to enhance activation, antigen uptake and maturation of the DC. This concept is supported by a recent report from Den Brok et al. who showed that in situ cryoablation of different murine tumors and CpG ODN treatment synergize in inducing dendritic cell maturation and efficient cross-presentation in tumor-bearing mice, leading to superior DC function in vivo (31). The combination of cryoablation plus CpG ODN was more effective in the eradication of local and systemic tumors than either treatment modality alone. The authors favor the concept that in situ tumor destruction in combination with CpG ODN administration creates a unique "in situ DC vaccine" that would be readily applicable in the clinic.
4) CpG ODN and vaccination strategies
The effect of CpG ODN on antigen presenting cells suggests this class of agents may be useful in combination with vaccination strategies. Indeed, animal studies as well as clinical trials have demonstrated that CpG ODN can enhance both the humoral and cellular immune response to anti-viral vaccines (32, 33). In 1997 we evaluated the effect of CpG ODN in a murine model using the lymphoma idiotype as a tumor specific antigen in immunocompetent mice. CpG ODN was able to enhance the immune and therapeutic response in this prophylactic tumor vaccination model (34). Prophylactic cancer vaccines are most likely to be useful for virally induced cancers. Recently, Gendron et al evaluated the effect of CpG ODN on tumors induced by human papilloma-virus (HPV). Mice were vaccinated with HPV 16 E7 peptide with and without CpG ODN (35). The combination was more effective not only in a prophylactic setting but also in tumor-bearing mice. Tetramer analysis demonstrated increased numbers of activated, E7-specific lymphocytes in the spleens and tumors of animals treated with the combination vaccine when compared with controls.
An additional challenge with any cancer vaccine is the ability to break tolerance. Mukherjee et al. were able to induce an immunologic and tumor response against MUC1-expressing colon cancer by immunizing transgenic MUC1-positive mice with a combination of class I and class II restricted MUC1 peptides, GM-CSF and CpG ODN (36). Combinations of immunostimulatory agents may be required to induce the most robust immune response. Preliminary preclinical studies of combinations suggest CpG ODN can contribute to enhancing immune responses more effectively than immune adjuvants currently being developed for clinical use (37).
An alternative to peptide-based vaccination for tumor therapy may represent whole tumor cell vaccination. Advantages would be the broader antigenic repertoire and no need for isolation of certain peptides. Oshashi et al found that the combination of CpG ODN with tumor cells that secrete GM-CSF was quite effective at curing large tumors when combined with tumor excision. (38).
Immunization with antigen-pulsed dendritic cells (DC) is being explored extensively as a form of cancer immunotherapy. DCs are highly responsive to CpG ODN, and a number of preclinical and clinical studies with this combination have been reported. Pilon-Thomas et al. evaluated the prophylactic and therapeutic efficacy of CpG ODN in combination with DC-based immunotherapy in a murine melanoma model (39). In the prophylactic model, long-term protection was only achieved in mice vaccinated with both CpG ODN and antigen-pulsed DC. This protection correlated with an enhanced antigen-specific T cell immune response. In a therapeutic model of established subcutaneous B16 melanoma, mice treated intratumorally with CpG ODN and B16 lysate-pulsed DC or DC pulsed with the melanoma peptide TRP-2 demonstrated a reduced tumor burden and prolonged survival. Du et al. investigated a DC-based lung cancer vaccine in combination with CpG ODN (40). Lewis lung cancer cells were fused or co-cultured with immature DC and then either matured with CpG ODN or not. The results showed that CpG ODN-matured DC fused with tumor cells consistently induced the highest and most effective anti-tumor immune responses in vivo.
5) CpG ODN and cytokines
A large number of cytokines have been tested in combination with CpG ODN for their potential to enhance tumor-specific immune responses. Indeed, there is rationale to evaluate combinations of CpG ODN with the growing list of cytokines that impact on NK cells or DCs. Brown et al. evaluated peripheral blood mononuclear cells treated with CpG ODN, IFNα, or both agents for their in vitro effects against human melanoma cells (41) and in murine models of melanoma. The combination of CpG ODN and IFNα resulted in enhanced cytotoxicity and activation of natural killer cells as well as enhanced antitumor activity in the murine model of B16 melanoma. Another interesting recent example is IL-18 which has stimulating effects on murine natural killer dendritic cells (NKDC), a recently identified DC population in mice with natural killer cell properties (42). The combination of IL-18 and CpG ODN resulted in a synergistic increase of IFNγ production both in vitro and in vivo. Treatment with IL-18 and CpG ODN reduced the number of B16F10 melanoma lung metastases in tumor-bearing mice.
A recent finding of our own laboratory is the synergistic relationship between CpG ODN and another member of the IL-2 family of cytokines, interleukin 21 (IL-21). We found that CpG ODN plus IL-21 are able to turn malignant B cells from patients with chronic lymphocytic leukemia (B-CLL) into Granzyme B-secreting cytotoxic cells (43). These cells not only underwent apoptosis but were also able to induce apoptosis of untreated autologous bystander B-CLL cells in a granzyme B-dependent manner. Our data also suggest the ability to induce production of functional granzyme B by B cells after CpG ODN/IL-21 treatment could open new approaches to the therapy of B-CLL and other B cell disorders.
In summary, we have learned much about the immunologic effects of immunostimulatory CpG ODN in the 12 years since this novel class of agents was first described. CpG ODN can enhance tumor antigen presentation and T cell response when administered as an adjuvant with cancer vaccines or following antigen release that results from standard cytotoxic treatments such as chemotherapy or radiation. It can induce production from dendritic cells of cytokines such as IFN alpha which lead NK cell activation. These activated NK cells can mediate enhanced antibody dependent cellular cytotoxicity or direct killing of NK sensitive cancers. CpG ODN can also lead directly to activation-induced cell death of TLR9 positive B cell malignancies (Figure 1). Early clinical trials indicate CpG ODN can have anti-tumor effects as well. While a highly ambitious clinical trial exploring the combination of CpG ODN and chemotherapy as a treatment for non-small cell lung cancer failed to show CpG ODN added clinical benefit, strategies combining CpG ODN with other anti-cancer treatments remain attractive. We still have much to learn about CpG ODN, and which of the very large number of possible approaches to their use is likely to be most effective clinically. Continued preclinical studies, and clinical trials with rigorous laboratory correlates, will be required if we are to determine whether the promising preclinical anti-cancer effects of CpG ODN can be translated into a clinical reality.
Figure 1. Mechanisms by which CpG ODN may contribute to cancer immunotherapy.
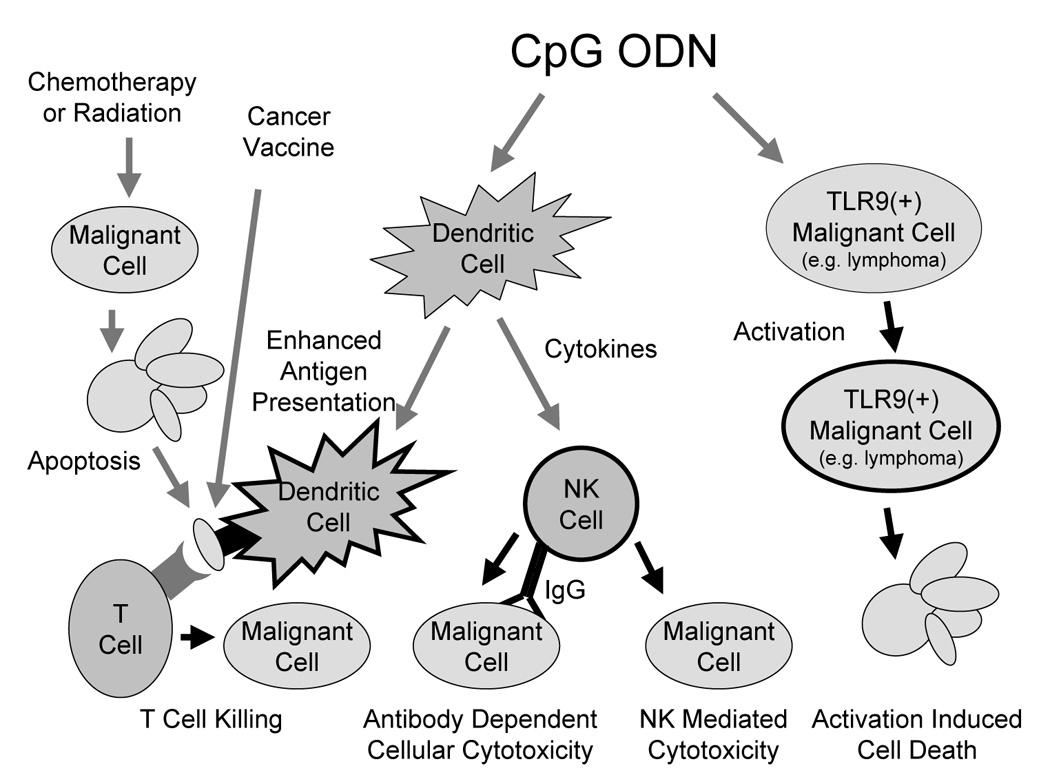
CpG ODN can enhance tumor antigen presentation and T cell response when administered as an adjuvant with cancer vaccines or following antigen release induced by standard cytotoxic treatments such as chemotherapy or radiation. CpG ODN can induce production from dendritic cells of cytokines such as IFN alpha which activate NK cells. The activated NK cells then mediate enhanced antibody dependent cellular cytotoxicity or direct killing of NK sensitive cancers. CpG ODN can also lead directly to activation-induced cell death of TLR9 positive B cell malignancies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 2.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 3.Ashkar AA, Rosenthal KL. Toll-like receptor 9, CpG DNA and innate immunity. Curr Mol Med. 2002 Sep;2(6):545–556. doi: 10.2174/1566524023362159. [DOI] [PubMed] [Google Scholar]
- 4.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 5.McCluskie MJ, Weeratna RD. Novel adjuvant systems. Curr Drug Targets Infect Disord. 2001;1(3):263–271. doi: 10.2174/1568005014605991. [DOI] [PubMed] [Google Scholar]
- 6.Jahrsdorfer B, Weiner GJ. Immunostimulatory CpG oligodeoxynucleotides and antibody therapy of cancer. Semin Oncol. 2003;30(4):476–482. doi: 10.1016/s0093-7754(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 7.Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdorfer B, Krug A, Rothenfusser S, Endres S, Hartmann G. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169(7):3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 8.Wooldridge JE, Ballas Z, Krieg AM, Weiner GJ. Immunostimulatory oligodeoxynucleotides containing CpG motifs enhance the efficacy of monoclonal antibody therapy of lymphoma. Blood. 1997;89(8):2994–2998. [PubMed] [Google Scholar]
- 9.Warren TL, Dahle CE, Weiner GJ. CpG Oligodeoxynucleotides Enhance Monoclonal Antibody Therapy of a Murine Lymphoma. Clinical Lymphoma. 2000;1(1):57–61. doi: 10.3816/clm.2000.n.005. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier A, Laigle-Donadey F, Zohar S, Capelle L, Behin A, Tibi A, Martin-Duverneuil N, Sanson M, Lacomblez L, Taillibert S, Puybasset L, Van Effenterre R, Delattre JY, Carpentier AF. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro oncol. 2006;8(1):60–66. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, Rasmussen WL, Krieg AM, Weiner GJ. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29(5):558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 12.Wysocka M, Benoit BM, Newton S, Azzoni L, Montaner LJ, Rook AH. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood. 2004;104(13):4142–4149. doi: 10.1182/blood-2004-03-1190. [DOI] [PubMed] [Google Scholar]
- 13.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115(3):739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pashenkov M, Goess G, Wagner C, Hormann M, Jandl T, Moser A, Britten CM, Smolle J, Koller S, Mauch C, Tantcheva-Poor I, Grabbe S, Loquai C, Esser S, Franckson T, Schneeberger A, Haarmann C, Krieg AM, Stingl G, Wagner SN. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24(36):5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 15.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 16.Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. Journal of Immunology. 1996;157(5):1840–1845. [PubMed] [Google Scholar]
- 17.Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, Weiner GJ. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol. 2001;167(9):4878–4886. doi: 10.4049/jimmunol.167.9.4878. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Rayburn ER, Wang W, Kandimalla ER, Agrawal S, Zhang R. Immunomodulatory oligonucleotides as novel therapy for breast cancer: pharmacokinetics, in vitro and in vivo anticancer activity, and potentiation of antibody therapy. Mol Cancer Ther. 2006;5(8):2106–2114. doi: 10.1158/1535-7163.MCT-06-0158. [DOI] [PubMed] [Google Scholar]
- 19.Jahrsdorfer B, Muhlenhoff L, Blackwell SE, Wagner M, Poeck H, Hartmann E, Jox R, Giese T, Emmerich B, Endres S, Weiner GJ, Hartmann G. B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clin Cancer Res. 2005;11(4):1490–1499. doi: 10.1158/1078-0432.CCR-04-1890. [DOI] [PubMed] [Google Scholar]
- 20.Friedberg JW, Kim H, McCauley M, Hessel EM, Sims P, Fisher DC, Nadler LM, Coffman RL, Freedman AS. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood. 2005 Jan 15;105(2):489–495. doi: 10.1182/blood-2004-06-2156. [DOI] [PubMed] [Google Scholar]
- 21.Leonard JP, Link BK, Emmanouilides C, Gregory SA, Weisdorf D, Andrey J, Hainsworth J, Sparano JA, Tsai DE, Horning S, Krieg AM, Weiner GJ. Phase I Trial of Toll-Like Receptor 9 Agonist PF-3512676 with and Following Rituximab in Patients with Recurrent Indolent and Aggressive Non Hodgkin's Lymphoma. Clin Cancer Res. 2007 Oct 15;13(20):6168–6174. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- 22.Weigel BJ, Rodeberg DA, Krieg AM, Blazar BR. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res. 2003 Aug 1;9(8):3105–3114. [PubMed] [Google Scholar]
- 23.Pratesi G, Petrangolini G, Tortoreto M, Addis A, Belluco S, Rossini A, Selleri S, Rumio C, Menard S, Balsari A. Therapeutic synergism of gemcitabine and CpG-oligodeoxynucleotides in an orthotopic human pancreatic carcinoma xenograft. Cancer Res. 2005;65(14):6388–6393. doi: 10.1158/0008-5472.CAN-05-0602. [DOI] [PubMed] [Google Scholar]
- 24.van der Most RG, Himbeck R, Aarons S, Carter SJ, Larma I, Robinson C, Currie A, Lake RA. Antitumor efficacy of the novel chemotherapeutic agent coramsine is potentiated by cotreatment with CpG-containing oligodeoxynucleotides. J Immunother. 2006;29(2):134–142. doi: 10.1097/01.cji.0000187958.38179.a9. [DOI] [PubMed] [Google Scholar]
- 25.Leichmann G, Gravenor D, Woytowitz D, Mezger J, Albert G, Schmalbach T, Al-Adhami M, Manegold C. CPG 7909, a TLR9 Agonist, Added to First Line Taxane/Platinum for Advanced Non-Small Cell Lung Cancer, A Randomized, Controlled Phase II Study; Journal of Clinical Oncology, 2005 ASCO Annual Meeting Proceedings; 2005. (16S, Part I of II (June 1 Supplement)): Abstract # 7039. [Google Scholar]
- 26.Milas L, Mason KA, Ariga H, Hunter N, Neal R, Valdecanas D, Krieg AM, Whisnant JK. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64(15):5074–5077. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 27.Mason KA, Ariga H, Neal R, Valdecanas D, Hunter N, Krieg AM, Whisnant JK, Milas L. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin Cancer Res. 2005 Jan 1;11(1):361–369. [PubMed] [Google Scholar]
- 28.Mason KA, Neal R, Hunter N, Ariga H, Ang K, Milas L. CpG oligodeoxynucleotides are potent enhancers of radio- and chemoresponses of murine tumors. Radiother Oncol. 2006;80(2):192–198. doi: 10.1016/j.radonc.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Meng Y, Carpentier AF, Chen L, Boisserie G, Simon JM, Mazeron JJ, Delattre JY. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005;116(6):992–997. doi: 10.1002/ijc.21131. [DOI] [PubMed] [Google Scholar]
- 30.Ai WYZ, Kim Y, Hoppe RT, Shah S, Horning SJ, Tibshirani R, Levy R. Preliminary Report on a Phase I/II Study of Intratumoral Injection of PF-3512676 (CpG 7909), a TLR9 Agonist, Combined with Radiation in Recurrent Low-Grade Lymphomas. Blood. 2006;108(11) Abstract #2716. [Google Scholar]
- 31.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Toonen LW, Figdor CG, Ruers TJ, Adema GJ. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66(14):7285–7292. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 32.Klinman DM, Xie H, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the licensed anthrax vaccine. Ann N Y Acad Sci. 2006:137–150. doi: 10.1196/annals.1348.030. [DOI] [PubMed] [Google Scholar]
- 33.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 34.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory Oligodeoxynucleotides Containing the CpG Motif are Effective As Immune Adjuvants In Tumor Antigen Immunization. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gendron KB, Rodriguez A, Sewell DA. Vaccination with human papillomavirus type 16 E7 peptide with CpG oligonucleotides for prevention of tumor growth in mice. Arch Otolaryngol Head Neck Surg. 2006;132(3):327–332. doi: 10.1001/archotol.132.3.327. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee P, Pathangey LB, Bradley JB, Tinder TL, Basu GD, Akporiaye ET, Gendler SJ. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25(9):1607–1618. doi: 10.1016/j.vaccine.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SK, Ragupathi G, Cappello S, Kagan E, Livingston PO. Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine. 2000 Oct 15;19(4–5):530–537. doi: 10.1016/s0264-410x(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 38.Ohashi K, Kobayashi G, Fang S, Zhu X, Antonia SJ, Krieg AM, Sandler AD. Surgical excision combined with autologous whole tumor cell vaccination is an effective therapy for murine neuroblastoma. J Pediatr Surg. 2006;41(8):1361–1368. doi: 10.1016/j.jpedsurg.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 39.Pilon-Thomas S, Li W, Briggs JJ, Djeu J, Mule JJ, Riker AI. Immunostimulatory effects of CpG-ODN upon dendritic cell-based immunotherapy in a murine melanoma model. J Immunother. 2006;29(4):381–387. doi: 10.1097/01.cji.0000199199.20717.67. [DOI] [PubMed] [Google Scholar]
- 40.Du YC, Lin P, Zhang J, Lu YR, Ning QZ, Wang Q. Fusion of CpG-ODN-stimulating dendritic cells with Lewis lung cancer cells can enhance anti-tumor immune responses. Tissue Antigens. 2006;67(5):368–376. doi: 10.1111/j.1399-0039.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 41.Brown L, Roda J, Terrell C, Chaudhury AR, Crespin T, Carson WE, Lesinski GB. Interferon alpha and CPG oligodeoxynucleotides elicit additive immunostimulatory and antitumor effects. Surgery. 2006;140(2):297–306. doi: 10.1016/j.surg.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhry UI, Kingham TP, Plitas G, Katz SC, Raab JR, DeMatteo RP. Combined stimulation with interleukin-18 and CpG induces murine natural killer dendritic cells to produce IFN-gamma and inhibit tumor growth. Cancer Res. 2006;66(21):10497–10504. doi: 10.1158/0008-5472.CAN-06-1908. [DOI] [PubMed] [Google Scholar]
- 43.Jahrsdorfer B, Blackwell SE, Wooldridge JE, Huang J, Andreski MW, Jacobus LS, Taylor CM, Weiner GJ. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108(8):2712–2719. doi: 10.1182/blood-2006-03-014001. [DOI] [PMC free article] [PubMed] [Google Scholar]


