Synopsis
Heart failure is defined as the pathological state in which the heart is unable to pump blood at a rate required by the metabolizing tissues or can do so only with an elevated filling pressure. Heart failure in adults most frequently results from the inability of the left ventricle to fill (diastolic performance) and/or eject (systolic performance) blood. The severity of heart failure and its prognosis are more closely related to the degree of diastolic filling abnormalities than the ejection fraction. This underscores the importance of understanding the mechanisms of diastolic abnormalities in heart failure.
Keywords: systole, diastole, heart failure
Introduction
The function of the heart is to supply oxygen and metabolic substrates to the peripheral tissues. Heart failure may thus be defined as the pathological state in which the heart is unable to pump blood at a rate required by the metabolizing tissues or can do so only with an elevated filling pressure. Heart failure in adults most frequently results from abnormalities of the left heart. Thus, the clinical evaluation of cardiac function predominantly concerns the performance of the left ventricle (LV).
The cardiac cycle
Mechanical events during the cardiac cycle are shown in Figure 1. Systole is defined as from the mitral valve closure to the aortic valve closure, with the rest of the cardiac cycle being defined as diastole. This definition of diastole includes portions of the cardiac cycle that may be considered to be part of systole on the basis of myocardial physiology [1]. For purposes of this discussion, systole can be divided into two phases: (1) isovolumetric contraction and (2) ejection and diastole into four phases: (1) isovolumetric relaxation, (2) early diastolic filling, (3) diastasis, and (4) atrial filling [2].
Figure 1.
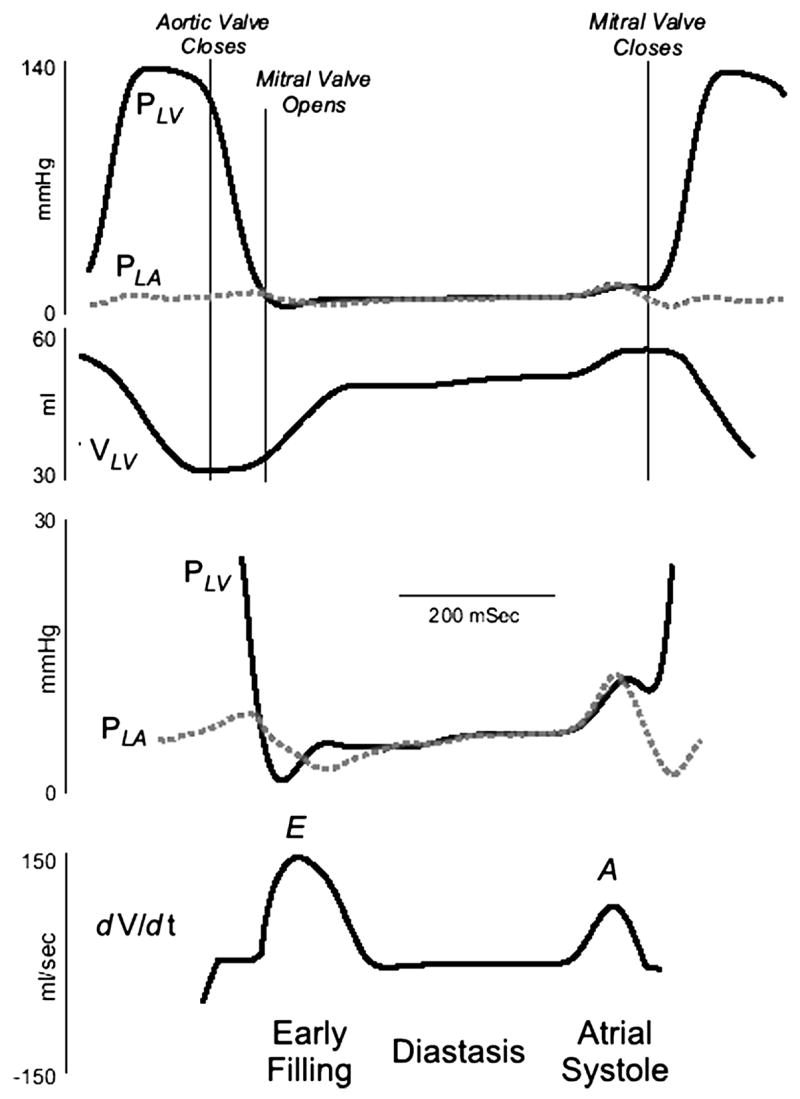
Recording of left atrial pressure (PLA), left ventricular pressure (PLV), and the rate of change of left ventricular (LV) volume (dV/dt). The early diastolic pressure gradient is generated as LV pressure falls below LA pressure and the late diastolic gradient is generated as atrial contraction increases LA pressure above LV pressure. Data from Little WC, Cheng CP. Left ventricular-arterial coupling in conscious dogs. Am J Physiol 1991;261:H70–H76.
By convention, the mechanical cycle begins at end diastole (Figure 1). The LV pressure increases without a change in volume during isovolumetric contraction. When the LV pressure exceeds the aortic pressure, the aortic valve opens. During LV ejection, LV volume falls. LV ejection terminates with closure of the aortic valve. From the aortic valve closure until mitral valve opening, the LV pressure falls without a change in volume (isovolumetric relaxation). Isovolumetric relaxation ends when the LV pressure decreases below the left atrial pressure. The atrial-to-LV pressure gradient opens the mitral valve and rapid filling of LV begins. After the filling of the LV begins, the pressure gradient from the left atrium to the LV apex decreases and then transiently reverses. The reversed mitral valve pressure gradient decelerates and then stops the rapid flow of blood into the LV early in diastole. During the mid portion of diastole (diastasis), the pressure in the left atrium and LV equilibrates, and mitral flow nearly ceases. Late in diastole, atrial contraction increases the atrial pressure, producing a second atrial-to-LV pressure gradient that again propels blood into the LV. After atrial systole, as the left atrium relaxes, its pressure decreases below the LV pressure, causing the mitral valve to begin closing. The beginning of systole produces a rapid increase in the LV pressure that seals the mitral valve and ends diastole.
Pressure-volume relations
The cardiac cycle can also be plotted in the pressure-volume plane. The relationship between LV pressure and volume in a normal ejection beat is shown in Figure 2.
Figure 2.
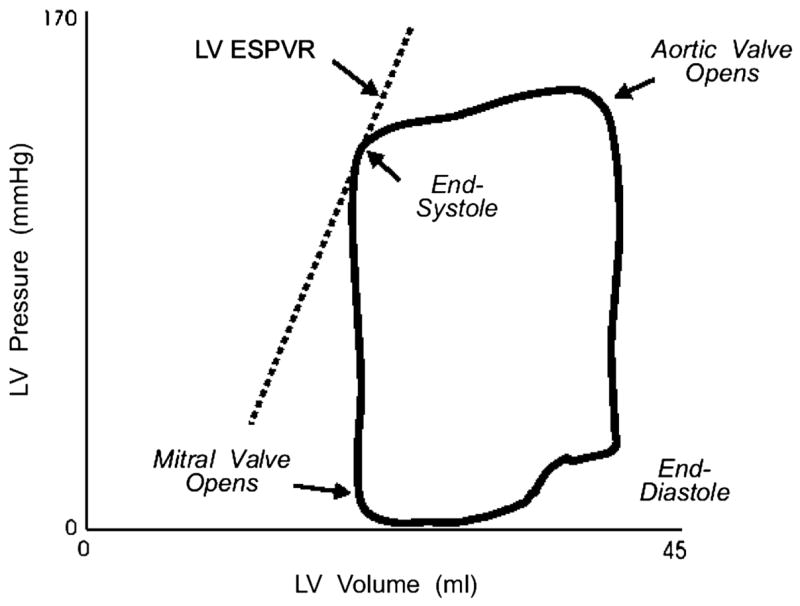
A left ventricular (LV) pressure-volume loop, describing one cardiac cycle. ESPVR indicates the end-systolic pressure-volume relation. See text for discussion. Data from Little WC, Cheng CP. Left ventricular-arterial coupling in conscious dogs. Am J Physiol 1991;261:H70–H76.
End-systolic pressure-volume relation
In the intact animal, linear end-systolic pressure-volume relations can be produced by vena cava occlusion. The slope and position of the end-systolic pressure-volume relations (ESPVR) respond to changes in myocardial contractile state; an increase in contractility increases the slope of the ESPVR, shifting the line toward the left in the physiological range, conversely, the ESPVR flattens and shifts to the right when there is depressed myocardial contractile function (Figure 3). Thus, the position and slope of the ESPVR can be used to measure contractile state.
Figure 3.
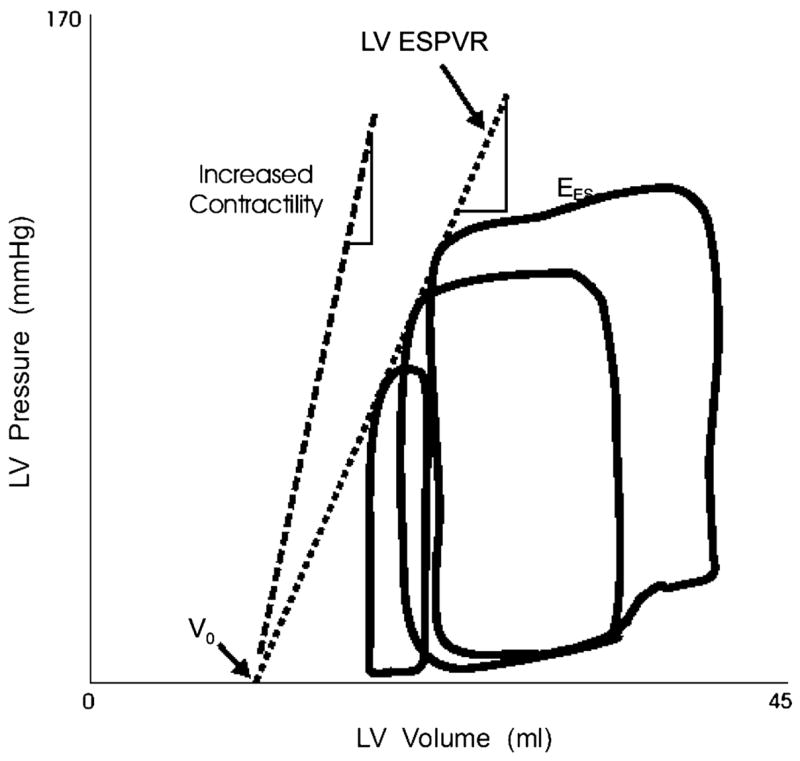
Variably loaded pressure-volume loops produced by caval occlusion are shown. End systole occurs at the upper left corner of the pressure-volume loops. The end-systolic points of variably loaded beats fall along a single relation, the left ventricular end-systolic pressure-volume relation (LV ESPVR). Within the physiological range, this relation is approximately by a straight line. The line can be described in terms of its slope (Ees) and volume axis intercept (Vo). Data from Little WC, Cheng CP, Mumma M, Igarashi Y, Vinten-Johansen J, Johnston WE. Comparison of measures of left ventricular contractile performance derived from pressure-volume loops in conscious dogs. Circulation 1989;80:1378–1387.
Assessment of LV myocardial function
The extent of myocardial shorting is determined by four factors: (1) the length of the muscle at the onset of contraction, i.e., the preload, (2) the contractility of the muscle, a fundamental property of cardiac tissue reflecting the level of activation, and the formation and cycling of the cross bridges between actin and myosin filaments, (3) the force against which myocardium contracts, i.e., afterload, and (4) the heart rate and rhythm. The factors that determine LV myocardial function (preload, afterload, and contractility) can be estimated from LV pressure and volume [2].
Preload
LV preload can be assessed from the LV filling pressure, the LV end-diastolic volume, or LV end-diastolic stress. The pressure distending the ventricle immediately prior to contraction is the end-diastolic pressure. This is equivalent to the pressure in the left atrium at this time in the absence of the disease of the mitral valve. Clinically, LV preload is assessed by measurements of the pulmonary capillary wedge pressure.
Afterload
In the intact heart, LV afterload may be defined as the tension or stress developed in the LV wall during ejection. LV afterload is determined by the arterial pressure as well as the volume and thickness of the LV according to Laplace’s law. Thus, at similar arterial pressure levels, a larger ventricle will have greater wall tension than a smaller ventricle. The arterial pressure, in turn, is determined by the peripheral vascular resistance, the physical characteristics of the arterial tree, and the volume of blood the LV ejects. Clinically, the level of LV afterload can be estimated by the systolic arterial pressure in the absence of aortic stenosis.
Contractility
As discussed above, the slope and position of the ESPVR in the LV pressure-volume plane provide useful information on the contractility of the LV. The invasive nature of the procedures for obtaining the ESPVR, however, limits its clinical use for assessment of the contractility of the LV. In the absence of abnormal afterload or valvular diseases, the LV ejection fraction, a measure of LV systolic performance (see later), reflects the contractility of the LV.
Responses of the LV to changes in preload and afterload and contractility
The effects of altering preload, afterload, and contractility on LV performance are readily described in the LV pressure-volume plane (Figure 4). An acute increase in afterload results in a greater proportion of the contractile energy being utilized to develop pressure so there is less myocardial shortening. As a consequence, emptying is impaired, causing reduced stroke volume. Thus, increased afterload can decrease LV systolic emptying without any depression of myocardial contractility. An increase in preload (increased end-diastolic volume), if it occurs without a change in end-systolic pressure, results in a larger stroke volume as the ventricle ejects to a similar end-systolic volume. A primary increase in myocardial contractility results in a steeper ESPVR. If preload and afterload remain constant, this brings about an increase in stroke volume. It is important to recognize that in the intact circulation preload and afterload change together and not in isolation.
Figure 4.
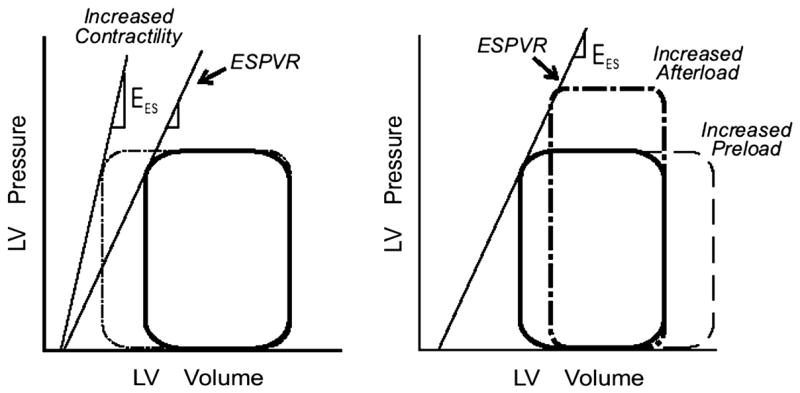
The responses of the left ventricle (LV) to increases in afterload and preload and contractility in the pressure-volume plane. LV ESPVR, the left ventricular end-systolic pressure-volume relation; Ees, the slope of the end-systolic pressure-volume relation. See text for discussion. Adapted from Little WC. Assessment of normal and abnormal cardiac function. In: Braunwald, Zipes, Libby, eds. Heart disease: A textbook of cardiovascular medicine. 6 ed. Philadelphia: W.B. Saunders Company; 2001:479–502.
Transformation of LV myocardial function to pump function
Analysis of the LV as a pump is a useful method to understand how the LV receives and ejects the blood necessary to maintain the normal circulation.
LV pump performance
The left heart can be analyzed as a pump with an input (the pulmonary venous or mean left atrial pressure) and an output (the cardiac output). The relationship between the input and output is the ventricular curve or the Frank-Starling relationship (Figure 5) [2]. In this relationship, the output can be considered to be the stroke volume, cardiac output, or the stroke work. A family of the Frank-Starling curves reflects the response of the pump performance of the ventricle to a spectrum of contractile states. The position of a given curve provides a description of ventricular pump performance. In contrast, movement along a single curve represents the operation of the Frank-Starling principle, which indicates that stroke volume, cardiac output, or stroke work varies with preload.
Figure 5.
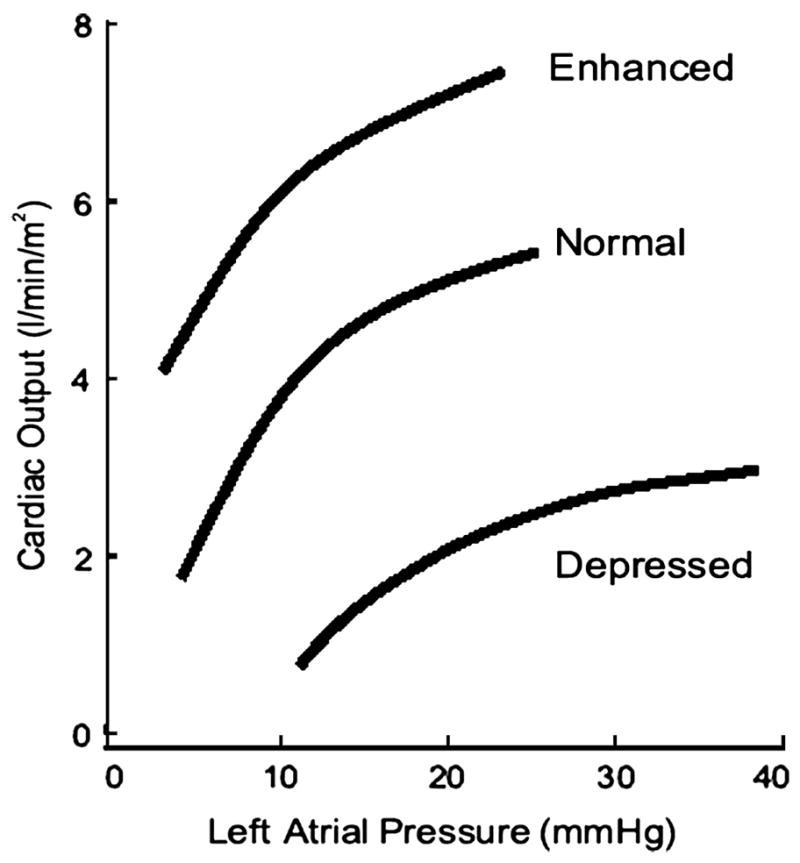
The Starling relationship. With increasing left ventricular filling pressure measured by the pulmonary capillary wedge pressure, there is an increase in cardiac output. The positions of the curves are influenced by the contractile state of the left ventricle. Adapted from Little WC. Assessment of normal and abnormal cardiac function. In: Braunwald, Zipes, Libby, eds. Heart disease: A textbook of cardiovascular medicine. 6 ed. Philadelphia: W.B. Saunders Company; 2001:479–502.
The pump performance of the LV depends on its ability to fill (diastolic performance) and to empty (systolic performance). The stroke volume is the quantity of blood ejected with each beat and is equal to the difference between end-diastolic and end-systolic volumes. Thus, the generation of the stroke volume depends on the conversion of the filling pressure to end-diastolic volume (diastolic performance) and on the ability to eject blood (systolic performance)
Systolic performance
LV chamber systolic performance is the ability of the LV to empty. Although myocardial contractility is an important determinant of the LV systolic performance, systolic performance is influenced also by load and ventricular configuration. Thus, systolic performance and contractility are not interchangeable [2]. For example, it is possible to have abnormal systolic performance despite normal contractility when LV afterload is excessive. Alternatively, LV systolic performance may be normal despite decreased myocardial contractility when LV afterload is low, as occurs in patients with mitral regurgitation.
The ability of the LV to empty can be quantified as LV emptying fraction or an ejection fraction (EF; a ratio of stroke volume to end-diastolic volume). Thus, LV systolic dysfunction is defined as a decreased EF. An operational definition of systolic dysfunction is an EF of less than 0.50 [3]. The EF can be obtained by determining the LV volume by use of two-dimensional echocardiography with or without contrast, radionuclide ventriculography or magnetic resonance imaging.
Diastolic performance
For the LV to function effectively as a pump, it must not only be able to eject but also fill blood (diastolic function). Diastolic function has conventionally been assessed on the basis of the LV end-diastolic pressure-volume relation. A shift of the curve upward and to the left has been considered to be the hallmark of diastolic dysfunction (Figure 6, Curve A) [4]. In this situation, each LV end-diastolic volume is associated with a high end-diastolic pressure, and thus, the LV is less distensible. Furthermore, impaired relaxation may result in persistent pressure generation at end-diastole and may thus contribute to reduced LV distensibility. The invasive nature of the procedures for obtaining the LV end-diastolic pressure-volume relation, however, limits its clinical use for assessment of diastolic function. Furthermore, there is more to adequate diastolic performance of the LV than is apparent by examining the diastolic pressure-volume relation. For example, patients with dilated cardiomyopathy have a LV end-diastolic pressure-volume relationship that is shifted to the right. Although this dilated LV is more distensible, its diastolic function is not supernormal. Instead, these patients have slow relaxation, elevated left atrial pressure, and impaired diastolic suction (see below).
Figure 6.
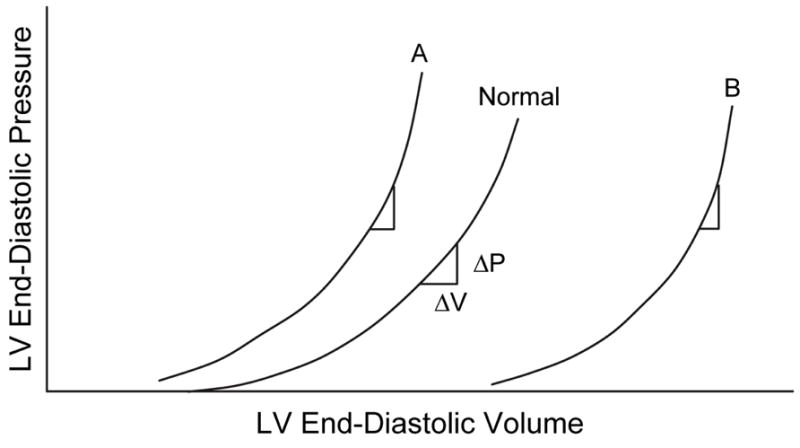
A shift of the curve to A indicates that a higher left ventricular (LV) pressure will be required to distend the LV to a similar volume, indicating that the ventricle is less distensible. The slope of the LV end-diastolic pressure-volume relation indicates the passive chamber stiffness. Since the relation is exponential in shape, the slope (ΔP/ΔV) increases as the end-diastolic pressure increases. From Little WC. Diastolic dysfunction beyond distensibility: adverse effects of ventricular dilatation. Circulation 2005;112:2888–2890.
Diastolic function has also been assessed based on LV filling patterns by use of Doppler echocardiography [5]. The LV fills in diastole in response to the pressure gradient from the left atrium to the LV (Figure 1). This occurs two times during the cardiac cycle: early in diastole after mitral valve opening and late in diastole during atrial systole. Most of LV filling occurs early in diastole, and less than 25% of the LV stroke volume enters the LV during atrial systole. The rate of early LV filling pressure is determined by two factors: the rate of LV relaxation and left atrial pressure at the time of mitral valve opening.
In the absence of mitral stenosis, three patterns of LV filling (Figure 7) indicate progressive impairment of diastolic function: (1) reduced early diastolic filling with a compensatory increase in importance of atrial filling (impaired relaxation); (2) most filling early in diastole, but with rapid deceleration of mitral flow (pseudonormalization); and (3) almost all filling of the LV occurring very early in diastole in association with very rapid deceleration of mitral flow (restrictive filling) [6].
Figure 7.
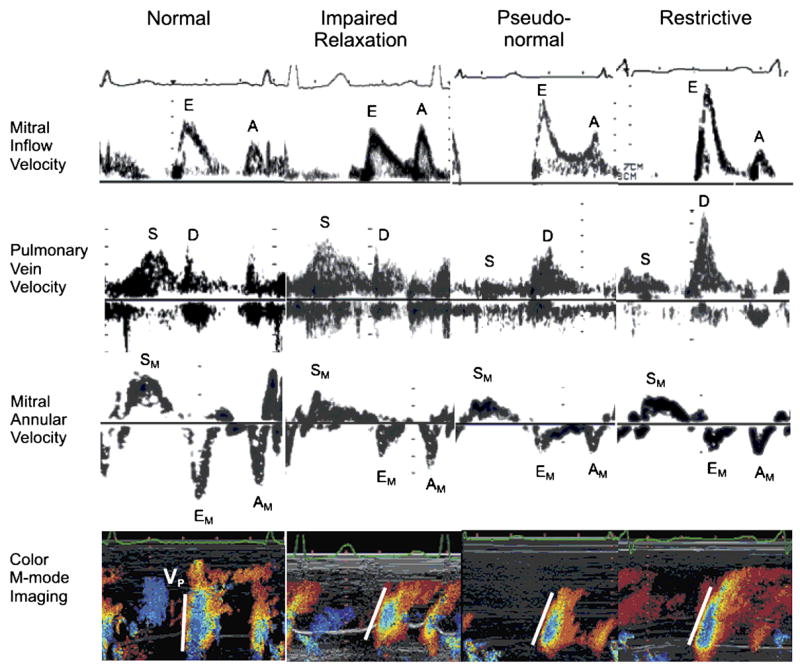
Transmitral inflow velocity, pulmonary vein flow velocity, mitral annular velocity, and color M-mode imaging in stages of diastolic dysfunction. E indicates early diastolic mitral inflow velocity; A, late diastolic mitral inflow velocity; S, systolic pulmonary vein velocity; D, diastolic pulmonary vein velocity; SM, systolic mitral annular velocity; EM, early diastolic mitral annular velocity; AM, late diastolic mitral annular velocity; and Vp, velocity of propagation of mitral inflow to the apex. Reprinted with permission. From Fukuta H, Little WC. Contribution of systolic and diastolic abnormalities to heart failure with a normal and a reduced ejection fraction. Prog Cardiovasc Dis 2007;49:229–240.
These Doppler LV filling patterns, however, are influenced not only by LV diastolic properties alone but also by left atrial pressure. In contrast, tissue Doppler measurement of mitral annular velocity and color M-mode measurement of the velocity of propagation of mitral inflow to the apex are much less load sensitive. The peak early diastolic mitral annular velocity (EM) provides a relatively load insensitive measure of LV relaxation [7]. EM progressively decreases with increasing severity of diastolic dysfunction [8;9]. Similarly, the velocity of propagation of mitral inflow to the apex (Vp) is reduced in conditions with impaired LV relaxation [9]. Thus, reduced EM and Vp can be used for differentiating pseudonormalized filling from normal filling pattern.
Furthermore, analysis of pulmonary venous flow pattern provides useful information on LV compliance and left atrial pressure [10]. With increase in the LV end-diastolic pressure, the pulmonary venous atrial flow reversal velocity increases, and duration also increases longer than duration of mitral late diastolic velocity. With decrease in LV compliance and increase in the mean left atrial pressure, the systolic component of pulmonary venous flow decreases and the diastolic component of pulmonary venous flow increases. Figure 7 shows stages of diastolic dysfunction incorporating pulmonary venous flow and tissue Doppler and color M-mode imaging.
Finally, Doppler echocardiography noninvasively provides an accurate estimate of LV filling pressure. Since early diastolic mitral inflow velocity (E) becomes higher and relaxation-related parameters (EM and Vp) remain reduced as filling pressure increases, the E/EM and E/Vp can estimate LV filling pressure with a reasonable accuracy over a wide range of an EF [11;12]. Thus, if these Doppler parameters are interpreted with other two-dimensional echocardiographic parameters including LV size and motion, the position of the LV end-diastolic pressure-volume relation (left upward or right downward shift) can noninvasively be estimated [13].
Integrated cardiovascular performance: the response to exercise
The integrated pumping function of the cardiovascular system results in the cardiac output. The cardiac output can be measured by use of thermodilution technique or the Fick principle (cardiac output = oxygen consumption/arteriovenous oxygen difference).
The cardiovascular system must function under a wide variety of demand. For example, during exercise, the body’s oxygen consumption increases dramatically. This is normally accomplished by a combination of an increase in cardiac output and widening of the arteriovenous oxygen difference. During strenuous exercise, the oxygen uptake increases up to 18-fold. This is accomplished by a 6-fold increase in cardiac output and a 3-fold increase in the arteriovenous oxygen difference, with the mixed venous oxygen saturation decreasing from 75% to 25% [2].
Heart failure
Definition of heart failure
Heart failure is defined as the pathological state in which the heart is unable to pump blood at a rate required by the metabolizing tissues or can do so only with an elevated filling pressure. Inability of the heart to pump blood sufficiently to meet the needs of the body’s tissues may be due to the inability of the LV to fill (diastolic performance) and/or eject (systolic performance). When the heart failure is associated with a reduced EF, the pathological state may be called systolic heart failure. In contrast, when the heart failure is associated with diastolic dysfunction in the absence of a reduced EF, the pathological state may be called diastolic heart failure.
Systolic and diastolic heart failure have several similarities in LV structural and functional characteristics, including increased LV mass and increased LV end-diastolic pressure. The clearest difference between the two forms of heart failure is the difference in LV geometry and LV function; systolic heart failure is characterized by LV dilatation, eccentric LV hypertrophy, and abnormal systolic and diastolic function, whereas diastolic heart failure is characterized by concentric LV hypertrophy, a normal EF and abnormal diastolic function.
Mechanisms of abnormal diastolic filling in systolic heart failure
Although the pathophysiology of systolic heart failure is predominantly dependent on systolic dysfunction, systolic heart failure is accompanied by abnormal LV filling dynamics and impaired relaxation despite the LV dilatation [3;14]. Furthermore, the severity of heart failure and its prognosis are more closely related to the degree of diastolic filling abnormalities than EF [14–20]. This underscores the importance of understanding the mechanisms of abnormal diastolic filling in systolic heart failure.
Patients with systolic heart failure require a large end-diastolic volume to produce an adequate stroke volume and cardiac output. This is represented as the rightward shift of the LV end-diastolic pressure-volume relation (Figure 6, Curve B). In this situation, each volume is associated with a lower pressure. This could be interpreted as indicating that the LV is more distensible. Nevertheless, patients with systolic heart failure have abnormal LV diastolic filling and markedly elevated left atrial pressure. Although this could merely reflect overfilling of the LV that has displaced the operating point to a portion of the pressure-volume relation at which chamber stiffness (ΔP/ΔV) is high, there appear to be other mechanisms [4].
First, reduced elastic recoil due to reduced LV contractility contributes to abnormal LV filling in systolic heart failure. During LV ejection, energy is stored as myocytes are compressed, and the elastic elements in the myocardial wall are compressed and twisted. Relaxation of myocardial contraction allows this energy to be released as the elastic elements recoil. Normally, this causes LV pressure to rapidly fall during isovolumetric relaxation, producing an early diastolic pressure gradient from the left atrium to the apex. Rapid untwisting also plays an important role in producing the pressure gradient [21;22]. The pressure gradient accelerates blood out of the left atrium and produces rapid early diastolic flow that quickly propagates to the apex. Since the diastolic intraventricular pressure gradient pulls blood to the apex, it can be considered a measure of LV suction. In the hypocontractile LV, however, less energy is stored during systole and released during diastole, which in turn results in impaired LV suction [23]. In this situation, the LV filling entirely depends on the elevated left atrial pressure.
Second, LV dilatation itself may contribute to abnormal LV filling in systolic heart failure. Yotti et al investigated the mechanisms of reduced intraventricular diastolic pressure gradient in dilated cardiomyopathy using color M-mode Doppler echocardiography and then applying the Euler equation [24]. The Euler equation relates the pressure gradient to inertial acceleration and convective deceleration [25]. Inertial acceleration is the change in velocity with respect to time; whereas, convective deceleration is proportional to the reduction in velocity with respect to distance. Convective deceleration would be expected to be increased in dilated ventricle because of divergence of the blood flow away from the longitudinal axis of the ventricle, forming vortices (Figure 8). Such an increase in convective deceleration would decrease the intraventricular pressure gradient. In fact, they found that the intraventricular diastolic pressure gradient was reduced in dilated cardiomyopathy because of both the impaired inertial acceleration and enhanced convective deceleration. They also found that the amount of convective deceleration was proportional to the ventricular-annular disproportion; the more dilated ventricle, the greater convective deceleration. The reduced inertial acceleration was presumably the result of impaired elastic recoil. Thus, the dilated hypocontractile ventricle produces a lower intraventricular pressure gradient in early diastole as the result of both reduced elastic recoil and dilated ventricle itself.
Figure 8.
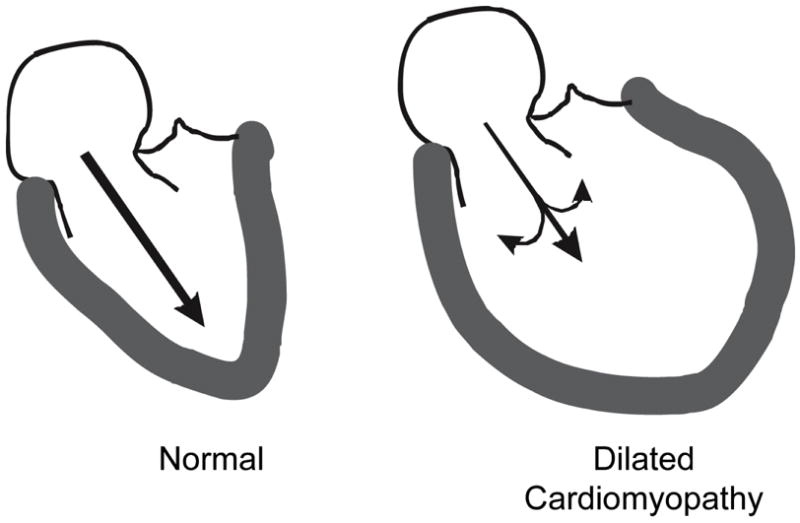
Diagram of the early diastolic left ventricular (LV) filling. In the normal heart, recoil of elastic elements produces a progressive pressure gradient from the left atrium to the apex of the left ventricle. This results in acceleration of blood from the left atrium to the LV apex, resulting in rapid diastolic filling. In a patient with a dilated cardiomyopathy, there is less elastic recoil. The acceleration of blood to the apex is further reduced by convective deceleration which is the tendency for blood to diverge from the longitudinal axis forming vortices in the dilated ventricle. Reprinted with permission. From Little WC. Diastolic dysfunction beyond distensibility: adverse effects of ventricular dilatation. Circulation 2005;112:2888–2890.
Thus, eccentric ventricular remodeling impairs not only LV systolic function but also LV diastolic filling. In patients with dilated cardiomyopathy, left atrial pressure is elevated, and LV filling is impaired because of slow relaxation, reduced elastic recoil, displacement onto a stiff portion to LV end-diastolic pressure-volume relation, and impairment of filling from the ventricular dilatation itself. Thus, despite the enhanced passive distensibility, the dilated LV has substantial diastolic abnormalities.
Contribution of diastolic abnormalities to exercise intolerance in heart failure
Limited exercise tolerance due to fatigue and dyspnea is a major symptom and a cause of disability in heart failure and results from many factors, including abnormal central hemodynamics, alterations in the skeletal muscles and vasculature, and deconditioning. There is much evidence that systolic function is not or only weakly related to exercise capacity in patients with systolic heart failure [26;27]. Instead, several lines of evidence suggest that an increase in left atrial pressure may be the most important hemodynamic determinant of exercise capacity in these patients [28]. Thus, diastolic abnormalities may play a role in the mechanisms of exercise intolerance in systolic and diastolic heart failure.
During exercise, the LV must increase cardiac output to meet an increase in the body’s oxygen consumption. This is normally accomplished by increased heart rate and maintained or augmented LV stroke volume. An increase in heart rate during exercise, however, decreases the duration of diastole and thus shortens the time for diastolic filling. Thus, LV filling rate must increase to maintain or augment the LV stroke volume. This is accomplished by enhancement of LV relaxation and lower early diastolic LV pressure (Figure 9) [29]. The mechanisms underlying the enhanced LV relaxation and lower early diastolic LV pressure during exercise result from the combined effects of sympathetic stimulation, tachycardia, enhanced elastic recoil due to contraction to lower volume, and/or rapid untwisting [21;22;29].
Figure 9.
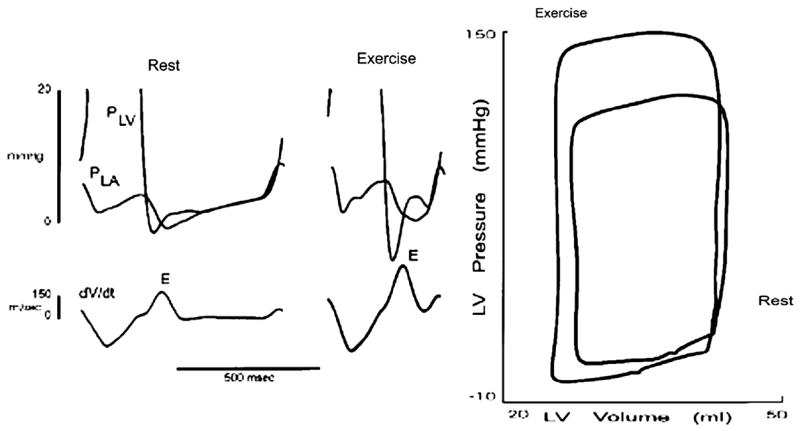
Recording of left atrial pressure (PLA), left ventricular pressure (PLV), and the rate of change of left ventricular (LV) volume (dV/dt) in a conscious animal at rest and during exercise. The data are also shown in the pressure-volume plane. See text for discussion. Data from Cheng CP, Igarashi Y, Little WC. Mechanism of augmented rate of left ventricle filling during exercise. Circ Res 1992;70:9–19.
This normal response to exercise is lost during the development of heart failure. In an animal model of systolic heart failure produced by several weeks of pacing, during exercise after heart failure, the stroke volume, the peak early LV filling rate, and the peak early diastolic pressure gradient increased as much as during normal exercise [30]. The cause of the increased early LV filling during exercise after heart failure, however, was different (Figure 10) [30]. Instead of the normal response of rapid LV relaxation and lower early diastolic LV pressure, during exercise after heart failure, LV relaxation which was slower at rest, was further reduced and the early diastolic LV pressure increased. Thus, the increased early LV filling rate and mitral valve pressure gradient during exercise after heart failure resulted from an abnormal increase in left atrial pressure. Similar observations are reported in patients with dilated cardiomyopathy [31] and diastolic heart failure [32].
Figure 10.
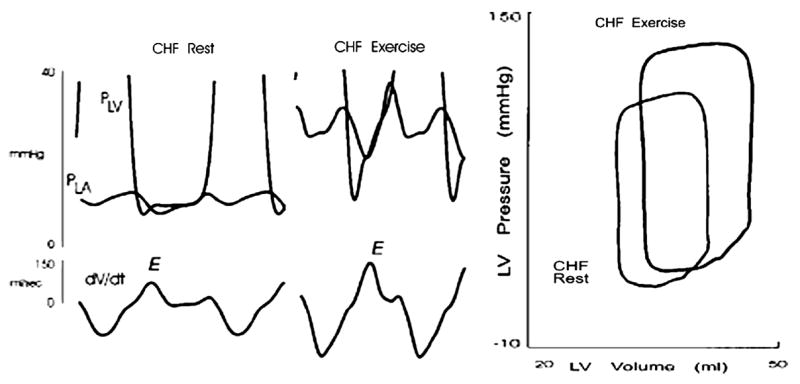
Recording from the same animal as in Figure 9 after the induction of pacing-induced heart failure (CHF). The data are also shown in the pressure-volume plane. See text for discussion. Data from Cheng CP, Noda T, Nozawa T, Little WC. Effect of heart failure on the mechanism of exercise-induced augmentation of mitral valve flow. Circ Res 1993;72:795–806.
These observations suggest that the enhanced impairment of LV relaxation during exercise leads to an increase in left atrial pressure, thereby contributing to the dyspnea that limits exercise tolerance in systolic and diastolic heart failure. Thus, diastolic abnormalities appear to play an important role in the mechanisms of exercise intolerance in heart failure. It should be recognized that other central mechanisms including exercise-induced functional mitral regurgitation [33] and peripheral mechanisms [27] may also contribute to exercise intolerance in patients with systolic heart failure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brutsaert DL, De Keulenaer GW. Diastolic heart failure: a myth. Curr Opin Cardiol. 2006;21:240–248. doi: 10.1097/01.hco.0000221587.02114.da. [DOI] [PubMed] [Google Scholar]
- 2.Little WC. Assessment of normal and abnormal cardiac function. In: Braunwald, Zipes, Libby, editors. Heart disease: A text book of cardiovascular medicine. 6. Philadelphia: W.B. Saunders Company; 2001. pp. 479–502. [Google Scholar]
- 3.Little WC, Applegate RJ. Congestive heart failure: systolic and diastolic function. J Cardiothorac Vasc Anesth. 1993;7:2–5. doi: 10.1016/1053-0770(93)90091-x. [DOI] [PubMed] [Google Scholar]
- 4.Little WC. Diastolic dysfunction beyond distensibility: adverse effects of ventricular dilatation. Circulation. 2005;112:2888–2890. doi: 10.1161/CIRCULATIONAHA.105.578161. [DOI] [PubMed] [Google Scholar]
- 5.Little WC, Warner JG, Jr, Rankin KM, et al. Evaluation of left ventricular diastolic function from the pattern of left ventricular filling. Clin Cardiol. 1998;21:5–9. doi: 10.1002/clc.4960210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuta H, Little WC. Contribution of systolic and diastolic abnormalities to heart failure with a normal and a reduced ejection fraction. Prog Cardiovasc Dis. 2007;49:229–240. doi: 10.1016/j.pcad.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa H, Little WC, Ohno M, et al. Diastolic mitral annular velocity during the development of heart failure. J Am Coll Cardiol. 2003;41:1590–1597. doi: 10.1016/s0735-1097(03)00260-2. [DOI] [PubMed] [Google Scholar]
- 8.Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 9.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–875. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]
- 10.Oh JK, Appleton CP, Hatle LK, et al. The noninvasive assessment of left ventricular diastolic function with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 1997;10:246–270. doi: 10.1016/s0894-7317(97)70062-2. [DOI] [PubMed] [Google Scholar]
- 11.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 13.Oh JK, Hatle L, Tajik AJ, et al. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–506. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Brucks S, Little WC, Chao T, et al. Contribution of left ventricular diastolic dysfunction to heart failure regardless of ejection fraction. Am J Cardiol. 2005;95:603–606. doi: 10.1016/j.amjcard.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Rihal CS, Nishimura RA, Hatle LK, et al. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation. 1994;90:2772–2779. doi: 10.1161/01.cir.90.6.2772. [DOI] [PubMed] [Google Scholar]
- 16.Vanoverschelde JL, Raphael DA, Robert AR, et al. Left ventricular filling in dilated cardiomyopathy: relation to functional class and hemodynamics. J Am Coll Cardiol. 1990;15:1288–1295. doi: 10.1016/s0735-1097(10)80016-6. [DOI] [PubMed] [Google Scholar]
- 17.Shen WF, Tribouilloy C, Rey JL, et al. Prognostic significance of Doppler-derived left ventricular diastolic filling variables in dilated cardiomyopathy. Am Heart J. 1992;124:1524–1533. doi: 10.1016/0002-8703(92)90067-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Yip G, Yu CM, et al. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. 2005;45:272–277. doi: 10.1016/j.jacc.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 19.Dokainish H, Zoghbi WA, Lakkis NM, et al. Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol. 2005;45:1223–1226. doi: 10.1016/j.jacc.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Troughton RW, Prior DL, Frampton CM, et al. Usefulness of tissue doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005;96:257–262. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 21.Notomi Y, Martin-Miklovic MG, Oryszak SJ, et al. Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation. 2006;113:2524–2533. doi: 10.1161/CIRCULATIONAHA.105.596502. [DOI] [PubMed] [Google Scholar]
- 22.Foster E, Lease KE. New untwist on diastole: what goes around comes back. Circulation. 2006;113:2477–2479. doi: 10.1161/CIRCULATIONAHA.106.626697. [DOI] [PubMed] [Google Scholar]
- 23.Firstenberg MS, Smedira NG, Greenberg NL, et al. Relationship between early diastolic intraventricular pressure gradients, an index of elastic recoil, and improvements in systolic and diastolic function. Circulation. 2001;104:I330–I335. doi: 10.1161/hc37t1.094834. [DOI] [PubMed] [Google Scholar]
- 24.Yotti R, Bermejo J, Antoranz JC, et al. A noninvasive method for assessing impaired diastolic suction in patients with dilated cardiomyopathy. Circulation. 2005;112:2921–2929. doi: 10.1161/CIRCULATIONAHA.105.561340. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg NL, Vandervoort PM, Firstenberg MS, et al. Estimation of diastolic intraventricular pressure gradients by Doppler M-mode echocardiography. Am J Physiol Heart Circ Physiol. 2001;280:H2507–H2515. doi: 10.1152/ajpheart.2001.280.6.H2507. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis. 1995;38:1–22. doi: 10.1016/s0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 27.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 28.Packer M. Abnormalities of diastolic function as a potential cause of exercise intolerance in chronic heart failure. Circulation. 1990;81:III78–III86. [PubMed] [Google Scholar]
- 29.Cheng CP, Igarashi Y, Little WC. Mechanism of augmented rate of left ventricular filling during exercise. Circ Res. 1992;70:9–19. doi: 10.1161/01.res.70.1.9. [DOI] [PubMed] [Google Scholar]
- 30.Cheng CP, Noda T, Nozawa T, et al. Effect of heart failure on the mechanism of exercise-induced augmentation of mitral valve flow. Circ Res. 1993;72:795–806. doi: 10.1161/01.res.72.4.795. [DOI] [PubMed] [Google Scholar]
- 31.Sato H, Hori M, Ozaki H, et al. Exercise-induced upward shift of diastolic left ventricular pressure-volume relation in patients with dilated cardiomyopathy. Effects of beta-adrenoceptor blockade. Circulation. 1993;88:2215–2223. doi: 10.1161/01.cir.88.5.2215. [DOI] [PubMed] [Google Scholar]
- 32.Kitzman DW, Higginbotham MB, Cobb FR, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 33.Lapu-Bula R, Robert A, Van CD, et al. Contribution of exercise-induced mitral regurgitation to exercise stroke volume and exercise capacity in patients with left ventricular systolic dysfunction. Circulation. 2002;106:1342–1348. doi: 10.1161/01.cir.0000028812.98083.d9. [DOI] [PubMed] [Google Scholar]


