Abstract
The absorption of cholesterol by the proximal small intestine represents a major pathway for the entry of cholesterol into the body pools. This cholesterol is derived primarily from the bile and the diet. In adult humans, typically several hundred milligrams of cholesterol reach the liver from the intestine daily, with the potential to impact the plasma low density lipoprotein-cholesterol (LDL-C) concentration. There are three main phases involved in cholesterol absorption. The first occurs intraluminally and culminates in micellar solubilization of unesterified cholesterol which facilitates its movement up to the brush border membrane (BBM) of the enterocyte. The second phase involves the transport of cholesterol across the BBM by Niemann-Pick C1 Like-1 (NPC1L1), while the third phase entails a series of steps within the enterocyte involving the esterification of cholesterol and its incorporation, along with other lipids and apolipoprotein B48 (apo B48), into nascent chylomicrons (CM). The discovery of the role of NPC1L1 in intestinal sterol transport occurred directly as a consequence of efforts to identify the molecular target of ezetimibe, a novel, potent, and specific inhibitor of sterol absorption that is now widely used in combination therapy with statins for the management of hypercholesterolemia in the general population. Some aspects of the role of NPC1L1 in cholesterol absorption nevertheless remain controversial and are the subject of ongoing research. For example, one report suggests that NPC1L1 is located not in the plasma membrane but intracellularly where it is thought to be involved in cytosolic trafficking of cholesterol, while another concludes that a protein other than NPC1L1 is responsible for the high affinity binding of cholesterol on intestinal BBM. However, other new studies which show that the in vivo responsiveness of different species to ezetimibe correlates with NPC1L1 binding affinity further support the widely held belief that NPC1L1 does facilitate sterol uptake by the enterocyte and is the target of ezetimibe. Added to this is the unequivocal finding that deletion of the gene for NPC1L1 in mice results in a near complete prevention of cholesterol absorption and an accelerated rate of fecal neutral sterol excretion. In summary, the development of ezetimibe and the identification of NPC1L1 as a key player in sterol absorption have taken research on the molecular control of this pathway to an exciting new level. From this it is hoped that we will now be able to determine more precisely what effect, if any, other classes of lipid lowering agents, particularly the statins, might exert on the amount of intestinal cholesterol reaching the liver.
Keywords: apolipoprotein B48, brush border membrane, cholesterol synthesis, cholesterol transport, chylomicron cholesterol, enterocyte, fractional cholesterol absorption, LDL cholesterol, phytosterol, statin monotherapy, sterol efflux, sterol uptake
1. Introduction
The body of a 70 kg non-obese individual contains about 140 grams of sterol, essentially all of which is cholesterol.1 Each day, normally about 1 to 1.5 grams of new cholesterol enters the body from synthesis by the tissues and from dietary intake. The average daily cholesterol intake for individuals consuming a typical Western diet is assumed to be around 400 mg, but clearly this value varies over a wide range. The rate at which the body synthesizes cholesterol averages about 10 mg/day/kg bw, but this can vary with changes in cholesterol intake.2, 3 Over the course of the day, an amount of cholesterol equal to what enters the body is eliminated in various forms, predominantly as bile acids and various metabolites of cholesterol in the stools. This balance between input and output thus ensures that whole body cholesterol content remains essentially constant over long periods of time. The liver plays a central role in the maintenance of whole body cholesterol balance because, not only is it the organ that receives most of the cholesterol absorbed from the small intestine, but it is also the site for the degradation and excretion of cholesterol through the bile.4 The liver also synthesizes cholesterol but the rate at which it does so varies widely depending on multiple factors, in particular, the amount of cholesterol being delivered to it from the small intestine.4 It is well documented that manipulation of the enterohepatic flux of cholesterol or of bile acids can markedly impact the intrahepatic handling of cholesterol in a way that leads to clinically significant shifts in the circulating low density lipoprotein-cholesterol (LDL-C) concentration.5–9
The transport of cholesterol from the small bowel to the liver is very substantial because it reflects the movement of not only dietary cholesterol but also of reabsorbed biliary cholesterol. In an adult human consuming a typical Western diet, the total amount of dietary and biliary cholesterol entering the lumen of the small intestine likely exceeds 1000 mg per day in most individuals.10–12 Fractional cholesterol absorption values (the proportion of the luminal pool that gets absorbed) in humans vary widely but average about 50%.13, 14 From these values, it therefore becomes apparent that a large quantity of intestinal cholesterol reaches the liver throughout the day. Such data thus explain why the cholesterol absorption pathway has long been a key target in the management of dyslipidemia. As reviewed elsewhere, attempts to develop effective and tolerable inhibitors of cholesterol absorption span almost half a century.15 Although several different classes of inhibitors were developed, often these had to be taken in gram quantities and usually only modest reductions in the plasma LDL-C level were achieved. In marked contrast, a daily dose of just 10 mg of ezetimibe inhibits cholesterol absorption by about 50% and lowers the plasma LDL-C concentration by ~ 18 to 20%.16 While ezetimibe is now widely used in combination with statins for treating hypercholesterolemia, it has also proved to be of immense value as a tool for advancing our knowledge of how cholesterol absorption is regulated at a molecular level. In particular, studies on the mechanism of action of ezetimibe led to the discovery of a major role for Niemann-Pick type C 1-Like-1 (NPC1L1) in intestinal sterol transport. The primary objective of this article then is to review our current understanding of the role of NPC1L1 and other proteins in facilitating the uptake and intracellular handling of cholesterol by the enterocyte. It also discusses how our new knowledge in this aspect of intestinal sterol metabolism will potentially be of value in determining, at a mechanistic level, how other classes of lipid lowering drugs, particularly statins, might directly or indirectly have an impact on the amount of chylomicron cholesterol (CM-C) reaching the liver.
2. Atherogenic potential of intestinally-derived cholesterol
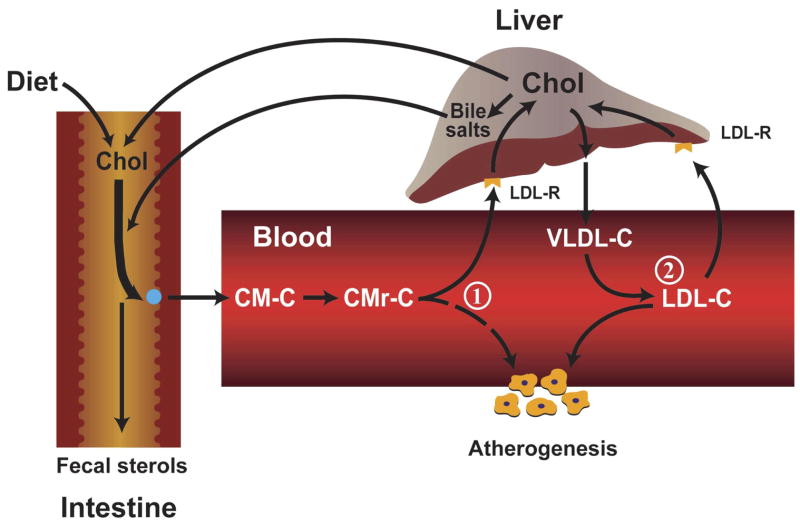
As depicted in the schematic shown in Figure 1, the delivery of intestinal cholesterol into the circulation can potentially impact events at the vessel wall in at least two ways. One (1) is through an effect on the plasma LDL-C concentration. The liver is the primary regulator of circulating LDL-C levels, not only because it is the site of formation of very low density lipoproteins (VLDL), which can be converted to LDL, but also because the bulk of receptor mediated clearance of LDL normally takes place in the liver.17 The sustained delivery of excess intestinal cholesterol to the liver can potentially increase hepatic cholesterol content. This, in turn, can lead to an accelerated rate of VLDL-cholesterol (VLDL-C) secretion into the plasma, as well as a downregulation of hepatic LDL receptor (LDL-R) activity.17 The extent to which these events take place is dictated not only by the amount of chylomicron cholesterol (CM-C) reaching the liver, but also by the quantity and type of fatty acid that accompany the cholesterol.18
Figure 1.
Atherogenic potential of intestinally-derived lipoproteins. This schematic illustrates two mechanisms through which the entry of chylomicron cholesterol (CM-C) into the circulation can potentially play a role in atherosclerotic plaque formation. (1) The uptake of CM-C by the liver can lead to a shift in intrahepatic cholesterol metabolism that in turn causes either a downregulation of low density lipoprotein receptor (LDL-R) activity, or/and an increase in hepatic very low density lipoprotein-cholesterol (VLDL-C) secretion. Either of these events can lead to a rise in the steady-state plasma low density lipoprotein-cholesterol (LDL-C) concentration. (2) Upon entering the circulation, CM’s lose much of their triacylglycerol content, resulting in the formation of a much smaller, cholesterol rich remnant particle (CMr) that can permeate the vessel wall. Ordinarily, CMr particles are rapidly cleared from the circulation by the liver but in Type 2 diabetes and other disorders this clearance rate can be markedly delayed.
A second (2) way in which intestinal cholesterol can potentially contribute to atherosclerotic plaque formation is through chylomicron remnant (CMr) particles which are normally rapidly cleared from the circulation by the liver primarily through the LDL-R and LDL receptor-related protein (LRP) pathways.19 In diabetes and various other disorders, the clearance of these CMr’s can be markedly delayed.19, 20 These particles, which carry a marker protein, apolipoprotein B48 (apo B48), are small enough to penetrate the vessel wall. Apo B48 has been found in human atherosclerotic plaque.21 The relative contribution of LDL and CMr’s (and other lipoproteins such as VLDL remnants) to the cholesterol contained in atherosclerotic plaque is unknown. Nevertheless, the underlying objective in using inhibitors of cholesterol absorption is to diminish the amount of CM-C entering the circulation that may potentially contribute to atherogenesis through one mechanism or another.
3. Intestinal cholesterol absorption
In a recent classic review of this subject,22 the author defines intestinal cholesterol absorption as “the transfer of intraluminal cholesterol into intestinal or thoracic duct lymph,” and intestinal uptake of cholesterol as “its entry from the lumen into intestinal absorptive cells.” This point is emphasized here because of a common misconception that “uptake” constitutes “absorption.” As will be discussed in a subsequent section, the uptake step in the absorption pathway has been intensely studied as a consequence of research into the molecular target of ezetimibe. Before reviewing all the recent discoveries and current controversies regarding the proteins involved in sterol uptake and intracellular trafficking by the enterocyte, a number of general points about cholesterol absorption warrant comment.
(a) Sources of sterols entering lumen
Although the main focus is on cholesterol from the diet and bile, depending on the dietary habits of an individual, there may also be substantial quantities of phytosterols and various other non-cholesterol sterols like those found in certain types of shellfish entering the intestinal sterol pool. The identities and sources of these sterols are described elsewhere.23, 24 When the inflow of biliary cholesterol is combined with cholesterol and non-cholesterol sterols from the diet, the total amount of sterol entering the lumen of the small intestine is probably in the range of at least 1000 to 2000 mg for most individuals and can reach several grams per day in those consuming products that are enriched with large quantities of plant stanol or sterol esters. When taken in gram quantities, these stanols/sterols can have LDL-C lowering benefits.25
(b) Specificity of sterol absorption
Despite the presence of significant quantities of phytosterols and other non-cholesterol sterols in the diet of most individuals, only trace amounts of such sterols are normally found in the circulation, even though these sterols appear to be transported into the enterocyte. As discussed in detail elsewhere,22 this “selectivity” in sterol absorption is articulated through the actions of two sterol efflux proteins, adenosine triphosphate binding cassette transporters G5 and G8 (ABCG5/G8). These proteins are not direct targets of absorption inhibitors like ezetimibe, but the relative amounts of mRNA for ABCG5/G8 in the enterocyte do reflect drug- and diet-induced changes in the amount of cholesterol and other sterols transported into the cell.22, 26 The actions of ABCG5/G8 are not totally effective in blocking all non-cholesterol sterol absorption. As discussed later, the plasma concentration of certain phytosterols like sitosterol and campesterol relative to cholesterol has proved to be a useful marker for relative rates of cholesterol absorption in some settings.
(c) Individual variability in fractional cholesterol absorption values
Several studies have shown that fractional cholesterol absorption values in humans vary over a wide range.13, 16 While such data suggest that the average subject absorbs about half of the cholesterol entering the luminal pool, there appear to be subsets of individuals who either hypo- or hyper-absorb cholesterol. As discussed in a later section, fractional cholesterol absorption data must be interpreted carefully because they do not necessarily faithfully reflect the absolute amount of cholesterol reaching the liver. In any cohort of experimental subjects, there will likely be wide individual differences in the size of the intraluminal sterol pool. When a marker of absorption is introduced into that pool, the fractional absorption value obtained will be determined by multiple factors, one of which is the degree to which the marker is diluted in the luminal sterol pool. Despite this limitation of fractional absorption data, studies by Miettinen and Kesaniemi27 have nevertheless shown that there are wide individual differences in the absolute rates of cholesterol absorption, and furthermore, that the rate of whole body cholesterol synthesis varies inversely with the amount of cholesterol absorbed. The molecular basis for individual variability in cholesterol absorption rates remains to be elucidated.
(d) Phases of cholesterol absorption
As discussed in detail elsewhere, the cholesterol absorption pathway as a whole is essentially a triphasic process.28, 29 The initial or intraluminal phase involves a constellation of physicochemial events that culminate in the delivery of sterols contained in mixed micelles up to the brush border membrane (BBM) of the enterocyte. The ensuing second, or BBM phase, entails the uptake of cholesterol and other sterols across the BBM via NPC1L1 and possibly other transporters (discussed later). The third, or intracellular phase, encompasses multiple events including the re-esterification of much of the cholesterol after it is taken up into the enterocyte, and the incorporation of cholesterol and other lipids, along with apo B48, into nascent CM particles. The main reason to outline the phases of the absorption process here is to emphasize the point that, historically, the development of different classes of absorption inhibitors has broadly paralleled advances in our knowledge of the events that take place in each phase.
(e) Sites of action of different classes of inhibitors
Several detailed reviews on sterol absorption inhibitors are available.8, 15, 25 The steps involved in the intraluminal phase of absorption were, for the most part, delineated more than two decades ago. Hence, it was this stage of absorption that agents like phytosterols and surfomer (alpha-olefin maleic acid) (AOMA) were aimed at disrupting.15 The multiple events that take place in the intracellular phase were the next to be delineated. This, in turn, prompted the development of agents for either inhibiting cholesterol esterification (acyl CoA:cholesterol acyltransferase (ACAT)), or disrupting CM assembly (microsomal triglyceride transfer protein (MTP)). The most recent major breakthroughs in our knowledge of how the sterol absorption pathway is regulated center around the BBM phase and involve key proteins like NPC1L1 and ABCG5/G8.22 The discovery and development of ezetimibe changed the course of research into the regulation of intestinal sterol homeostasis. It has taught us much about the role of NPC1L1 in intestinal sterol transport and has also stimulated the search for other proteins that may be involved in the uptake and intracellular trafficking of sterols within the enterocyte.
4. Molecular control of cholesterol absorption
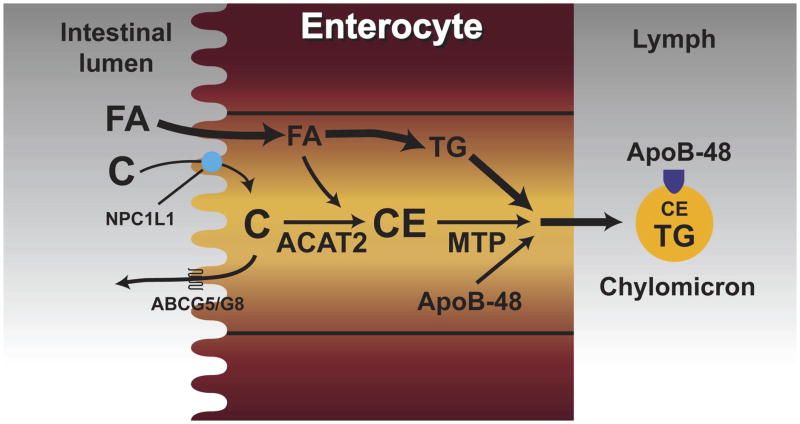
Although most of the major proteins that are involved in the intraluminal and intracellular phases of cholesterol absorption were identified many years ago, those known (or thought) to be involved in sterol uptake across the BBM, as well as those that facilitate sterol efflux from the enterocyte, have been discovered much more recently. The schematic shown in Figure 2 summarizes the main steps involved in the cholesterol absorption pathway and the locations where specific proteins act. The role of NPC1L1 and other putative sterol transporters is discussed below. Currently, the major questions that remain to be answered are, first, whether NPC1L1 acts alone in facilitating sterol uptake and, second, what the mechanisms are for moving newly internalized sterol to other sites within the enterocyte.
Figure 2.
Transport of intestinal cholesterol. This illustration summarizes the main steps involved in the uptake of cholesterol (C) from the lumen into the enterocyte, and the intracellular events that lead to the eventual incorporation of the cholesterol into nascent chylomicron (CM) particles. Here, Niemann-Pick C1 Like1 (NPC1L1) is depicted as the brush border membrane sterol transporter that facilitates the uptake of cholesterol and non-cholesterol sterols into the enterocyte. As discussed in the text, some studies suggest that other proteins might perform this function, and that NPC1L1 might instead be located at intracellular sites. Fatty acids (FA), which enter the cell through other mechanisms, are either incorporated into triacylglycerols (TG), or used to form cholesteryl esters (CE) through the action of acyl CoA: cholesterol acyltransferase 2 (ACAT2). Subsequently, the CE, as well as some unesterified cholesterol, along with TG and apolipoprotein B48 (apo B48), are packaged into nascent CM through the action of microsomal triglyceride transfer protein (MTP).
(a) Niemann-Pick C1-Like1 (NPC1L1)
Ezetimibe is a potent and selective inhibitor of cholesterol absorption in numerous species including various non-human primates and humans.16, 30–32 At the time it was approved for clinical use its molecular target was not known, although earlier studies with an ezetimibe analog, SCH58053, demonstrated that this class of absorption inhibitor did not directly regulate the intestinal expression of key known sterol transporters such as ABCA1 or ABCG5/G8.26 In a seminal paper published early in 2004, Altmann et al. identified NPC1L1 as a critical protein for the absorption of dietary and biliary cholesterol.33 These studies showed that deletion of NPC1L1 in mice resulted in a dramatic reduction in cholesterol absorption. Later in 2004, the same laboratory further demonstrated that NPC1L1 transported phytosterols, as well as cholesterol, and was a key modulator of whole body cholesterol homeostasis.34 The following year, Garcia-Calvo et al. published evidence clearly demonstrating that NPC1L1 is a target of ezetimibe.35 In more recent studies, Hawes et al. have shown that the in vivo responsiveness to ezetimibe correlates directly with NPC1L1 binding affinity.36 These new data explain why the potency of ezetimibe in inhibiting sterol absorption varies so widely amongst different species. Other areas of research have started to focus on genetic variation in NPC1L1 expression in human populations and how these can be associated with individual variations in plasma LDL-C levels and responsiveness to ezetimibe treatment.37, 38 Such studies exemplify the major clinical relevance of research on the molecular control of cholesterol transport into and within the enterocyte.
(b) Other proteins thought to be involved in intestinal sterol uptake and intracellular trafficking
Although several lines of evidence firmly anchor NPC1L1 as a fundamentally important protein in the regulation of intestinal cholesterol absorption, not all investigators in the field currently accept that NPC1L1 is located in the brush border membrane and serves in facilitating the uptake of cholesterol and non-cholesterol sterols into the enterocyte. Several publications conclude that other proteins, not NPC1L1, facilitate sterol uptake, and that NPC1L1 might instead function in the movement of cholesterol intracellularly. For example, one study purports to show that scavenger receptor class B type 1 (SR-B1) might play such a role,39 although it is known that in mice lacking SR-B1 there is no change in fractional cholesterol absorption values, irrespective of the dietary cholesterol intake of the mice.40 Another study using the CaCo-2 cell line reported that targeted disruption of NPC1L1 in this model resulted in diminished mRNA expression for SR-BI, but not for other transporters like ABCG5/G8 and fatty acid translocase (also called CD36).41 The role of SR-BI in the intestinal handling of cholesterol continues to be evaluated. This is also the case with CD36, a membrane protein that is believed to facilitate fatty acid uptake. Recently published data showed that in enterocytes from the proximal small intestine of CD36 knockout mice the uptake of cholesterol, as well as of fatty acids, was markedly reduced.42 In 2004, when evidence for a key role of NPC1L1 in intestinal transport was published,33, 34 another laboratory reported that an annexin 2-caveolin 1 complex regulates intestinal cholesterol transport, and was responsive to ezetimibe treatment.43 A subsequent study, however, showed that cholesterol absorption was unchanged in caveolin-1 knockout mice.44 Another laboratory further reported that the annexin 2-caveolin 1 complex, documented earlier in zebrafish and mouse small intestine cytosol, was not present in cytosol and brush border membrane vesicles from rabbit small intestine.45 This same laboratory, in a subsequent publication, reported that aminopeptidase N (CD13) is a molecular target of ezetimibe.46 The effect of deleting the gene for CD13 on cholesterol absorption has yet to be determined. These authors also speculate that several proteins, rather than a single cholesterol transporter, are involved in intestinal sterol uptake and trafficking.46 This concept is supported by even more recent studies from two other groups of investigators.47, 48
In evaluating the divergent findings of all these studies, one must take into account the wide differences in the techniques and model systems that were utilized. It is important that putative intestinal sterol transport proteins, identified under in vitro conditions, are demonstrated to perform the same functions in vivo. We have already seen several examples of proteins that in vitro appeared to play critical roles in intestinal sterol transport, but when the genes for these proteins were deleted in mice, there was little or no impact on cholesterol absorption. In summary, while many proteins are now purported to be involved in intestinal cholesterol uptake and trafficking, the balance of evidence at this time still strongly favors NPC1L1 as being of central importance in dictating the amount of intestinal cholesterol that ultimately reaches the liver.
5. Cholesterol absorption values and their interpretation
Intestinal cholesterol absorption is frequently measured as a fractional or percentage value based on the appearance in the plasma or stools of markers labeled with stable or radioisotopes that were delivered intragastrically several days beforehand.49, 50 A popular alternative technique involves the measurement in plasma of the ratio of the concentration of non-cholesterol sterols such as campesterol, sitosterol, or cholestanol to that of cholesterol.51–54 These methods, while useful in many settings, provide only relative levels of cholesterol absorption and do not necessarily reflect changes in the absolute amount of cholesterol moving from the small intestine to the liver. This can be determined only if both the fractional absorption value and the pool of absorbable sterol within the intestinal lumen are known, as defined by the following expression:
While it is relatively easy to measure the fractional value, it is technically very difficult to obtain even an approximate value for the pool size, especially in humans, although this has been done.55 The pool size is determined largely by input of cholesterol from the diet and from the bile. If it is known with certainty that a particular treatment has no effect on the amount of biliary cholesterol entering the lumen over the course of the day, then changes in the fractional absorption value can be taken as a reliable measure of the degree of change in the amount of CM-C reaching the liver, providing the daily cholesterol intake is known.
There are numerous examples of published studies in both animal models and humans that can be used to illustrate these points. One study in ACAT2+/+ mice fed graded increases in dietary cholesterol showed that the fractional cholesterol absorption value decreased as the dietary cholesterol intake was raised, and yet hepatic cholesterol concentration in the same mice increased in proportion to cholesterol intake.56 However, when these concentration values were compared directly to the absolute amounts of dietary cholesterol that were absorbed, there was a strong positive correlation. Clearly then, in this situation the radiolabeled markers used to measure the fractional absorption value became increasingly diluted within the lumen as the amount of exogenous cholesterol entering the pool increased. Exactly the same effect would occur if the treatment being tested caused a significant increase in the amount of biliary cholesterol entering the lumen.
In other situations where dietary cholesterol intake is kept low and constant in the face of treatment with variable doses of an agent like ezetimibe, the dose related reduction in fractional cholesterol absorption values is paralleled by compensatory increases in hepatic and intestinal cholesterol synthesis that are directly proportional to the magnitude of change in the fractional absorption value.31 Thus in this instance, such values can be interpreted to mean that there is a true net reduction in the absolute amount of cholesterol delivered from the small intestine to the liver because it is known that one of the primary determinants of the rate at which the liver synthesizes cholesterol is the amount of cholesterol it gets from the small bowel. The marked reductions in fractional absorption values seen in ezetimibe treated animal models clearly do represent diminished CM-C delivery to the liver because in most cases hepatic cholesterol concentrations remain essentially normal in the face of pronounced increases in dietary cholesterol intake.31, 32, 57
The expanding use of the absolute or relative concentrations in plasma of sterols such as campesterol, sitosterol, cholestanol as surrogate measures of cholesterol absorption efficiency, especially in studies with human subjects, can potentially yield misleading information about whether specific agents do change the amount of cholesterol absorbed from the small bowel. This is because the extent to which any such marker appears in the plasma will be determined in part by events within the lumen relating to the micellar solubilization of sterols and their delivery up to the BBM of the enterocyte for possible uptake into the cell. In the lumen, phytosterols and other non-cholesterol markers must compete with large amount of biliary and dietary cholesterol for incorporation into these micelles. Clearly then, the probability of any of these markers ultimately appearing in the circulation may change if there are sustained alterations in the amount of biliary or dietary cholesterol entering the luminal pool.52 There are numerous studies where the use of such surrogate markers of cholesterol absorption have provided reliable information about the direction and magnitude of change in cholesterol absorption in response to specific treatments. Generally, however, such data should be interpreted with caution, particularly if they are not accompanied by other molecular or metabolic measurements that more fully reflect sterol homeostasis within the enterocyte and hepatocyte.
6. Statin monotherapy and intestinal cholesterol absorption
The discovery of ezetimibe and the ensuing expansion of our knowledge about the molecular control of the cholesterol absorption pathway have stimulated interest in determining whether other classes of lipid-lowering drugs might in some way affect the amount of cholesterol we absorb. There is a particular interest in whether statins, which act primarily by reducing whole body cholesterol synthesis, might over time cause a compensatory increase in cholesterol absorption. It is well established that when cholesterol absorption is inhibited, there is a compensatory increase in cholesterol synthesis in the liver, and to some extent in the small intestine as well.31, 58 Hence, the question arises as to whether, in the converse situation, the inhibition of cholesterol synthesis by statins might cause adaptive changes in cholesterol homeostasis within the enterocyte that would involve more cholesterol being absorbed.
Several studies have reported an increase in either fractional cholesterol absorption values or the plasma concentration of specific phytosterols relative to that of cholesterol during treatment with various statins.51–54, 59 While not every study has found this to be the case,55 there appears nevertheless to be a growing belief in the lipid research community that statins cause a compensatory increase in cholesterol absorption.60 It is important to emphasize that at this time there are no published data unequivocally demonstrating that there is, or is not, such an effect. We do know, however, that the modest number of articles purporting to show an increase in absorption with statins are based on methods that will likely overestimate the level of cholesterol absorption during statin treatment. This is because statins significantly reduce the amount of biliary cholesterol reaching the lumen of the small bowel.10, 55, 59, 61 For example, one of these studies showed that after 5 to 6 weeks of treatment with lovastatin (40 mg/day), biliary cholesterol secretion fell 33% from 55.3 to 37.2 mg/hr.10 Presumably, such reductions result in a significant contraction of the pool size of cholesterol in the lumen. Thus, cholesterol absorption markers that mix with this smaller pool are likely to be absorbed at a higher rate, thereby yielding increased values for fractional absorption or plasma phytosterol:cholesterol ratios. Unfortunately, such data are often taken to mean that more cholesterol was absorbed. In a study with 63 male subjects, Miettinen and Kesäniemi found a positive correlation between the rates of biliary cholesterol secretion (measured indirectly) and whole body cholesterol synthesis.27 It is therefore not surprising that statins reduce biliary cholesterol output. Given that the reabsorption of biliary cholesterol is an important determinant of how much CM-C reaches the liver, the impact of statins on biliary cholesterol secretion must be taken into account when interpreting cholesterol absorption data from patients on statin monotherapy.
Another key point to be addressed here is whether statins inhibit cholesterol synthesis within the intestinal mucosa, and if so, whether this would necessarily be a trigger for absorptive cells to compensate by increasing the uptake of sterols from the lumen. While statins unquestionably slow down the rate of sterol synthesis by the body,2, 55, 59 there are no published data detailing whether this reduction is primarily in the liver, the small intestine, or the other extrahepatic organs. In a primate model that manifests a rate of whole body cholesterol synthesis comparable to that reported for humans, about 13% of total synthesis occurs in the small intestine.62 If this were also the case in humans, then the reduction in whole body cholesterol synthesis seen during statin therapy might partly reflect a lower rate of intestinal synthesis. While there are no in vivo data to support this contention, one study demonstrated in vitro that the incubation of human intestinal epithelial cells with lovastatin resulted in a marked acute inhibition of sterol synthesis.63 Even if statins do cause a deficit of newly synthesized cholesterol within the enterocyte, it is possible that such a shortfall could be balanced by increased uptake of cholesterol from other sources such as LDL.17 Recent studies in a miniature pig model showed a marked increase in the relative mRNA level for the LDL-R in the small intestine when cholesterol absorption was inhibited with ezetimibe.64
Taken together then, these various findings make a compelling case for a major research effort to now be undertaken on statin monotherapy and intestinal sterol homeostasis. This is currently one of the most challenging and clinically important questions in the field of lipidology. By combining established techniques for measuring biliary cholesterol secretion rates, fractional cholesterol absorption values, and most importantly, the expression and activities of all the proteins now known or thought to be involved in intestinal sterol homeostasis, we can be confident of advancing our knowledge of how statins and other classes of lipid lowering drugs might potentially impact the amount of cholesterol we absorb.
Acknowledgments
The author thanks John M. Dietschy, MD and Joyce J. Repa, PhD for insightful discussions on the subject of this article, Sean Campbell for assistance with preparation of the figures, and Kerry Foreman for preparation of the manuscript. Supported in large part by a grant from the U.S. Public Health Service (R01 HL09610).
Footnotes
Disclosures
Speaker’s Bureau: Merck & Co., Schering-Plough, Merck/Schering-Plough
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cook RP. Distribution of Sterols in Organisms and in Tissues. In: Cook RP, editor. Cholesterol Chemistry, Biochemistry, and Pathology. New York: Academic Press Inc; 1958. [Google Scholar]
- 2.Duane WC. Serum lathosterol levels in human subjects reflect changes in whole body cholesterol synthesis induced by lovastatin but not dietary cholesterol. J Lipid Res. 1995;36:343–348. [PubMed] [Google Scholar]
- 3.McMurry MP, Connor WE, Lin DS, Cerqueira MT, Connor SL. The absorption of cholesterol and the sterol balance in the Tarahumara Indians of Mexico fed cholesterol-free and high cholesterol diets. Am J Clin Nutr. 1985;41:1289–1298. doi: 10.1093/ajcn/41.6.1289. [DOI] [PubMed] [Google Scholar]
- 4.Turley SD, Dietschy JM. The Metabolism and Excretion of Cholesterol by the Liver. In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA, editors. The Liver: Biology and Pathobiology. New York: Raven Press; 1988. [Google Scholar]
- 5.Zema MJ. Colesevelam HCl and ezetimibe combination therapy provides effective lipid-lowering in difficult-to-treat patients with hypercholesterolemia. Am J Ther. 2005;12:306–310. doi: 10.1097/01.mjt.0000155109.69831.a3. [DOI] [PubMed] [Google Scholar]
- 6.Davis HR, Jr, Veltri EP. Zetia: inhibition of Niemann-Pick C1 like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscler Thromb. 2007;14:99–108. doi: 10.5551/jat.14.99. [DOI] [PubMed] [Google Scholar]
- 7.Insull W., Jr Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. 2006;99:257–273. doi: 10.1097/01.smj.0000208120.73327.db. [DOI] [PubMed] [Google Scholar]
- 8.Sudhop T, von Bergmann K. Cholesterol absorption inhibitors for the treatment of hypercholesterolaemia. Drugs. 2002;62:2333–2347. doi: 10.2165/00003495-200262160-00002. [DOI] [PubMed] [Google Scholar]
- 9.Xydakis AM, Guyton JR, Chiou P, Stein JL, Jones PH, Ballantyne CM. Effectiveness and tolerability of ezetimibe add-on therapy to a bile acid resin-based regimen for hypercholesterolemia. Am J Cardiol. 2004;94:795–797. doi: 10.1016/j.amjcard.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell JC, Logan GM, Stone BG, Duane WC. Effects of lovastatin on biliary lipid secretion and bile acid metabolism in humans. J Lipid Res. 1991;32:71–78. [PubMed] [Google Scholar]
- 11.Grundy SM, Metzger AL. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology. 1972;62:1200–1217. [PubMed] [Google Scholar]
- 12.Grundy SM. Absorption and metabolism of dietary cholesterol. Ann Rev Nutr. 1983;3:71–96. doi: 10.1146/annurev.nu.03.070183.000443. [DOI] [PubMed] [Google Scholar]
- 13.Bosner MS, Lange LG, Stenson WF, Ostlund RE., Jr Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res. 1999;40:302–308. [PubMed] [Google Scholar]
- 14.Clarenbach JJ, Reber M, Lütjohann D, von Bergmann K, Sudhop T. The lipid-lowering effect of ezetimibe in pure vegetarians. J Lipid Res. 2006;47:2820–2824. doi: 10.1194/jlr.P600009-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Homan R, Krause BR. Established and emerging strategies for inhibition of cholesterol absorption. Curr Pharm Des. 1997;3:29–44. [Google Scholar]
- 16.Sudhop T, Lütjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 17.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 18.Xie C, Woollett LA, Turley SD, Dietschy JM. Fatty acids differentially regulate hepatic cholesteryl ester formation and incorporation into lipoproteins in the liver of the mouse. J Lipid Res. 2002;43:1508–1519. doi: 10.1194/jlr.m200146-jlr200. [DOI] [PubMed] [Google Scholar]
- 19.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–2192. [PubMed] [Google Scholar]
- 20.De Man FHAF, Cabezas MC, Van Barlingen HHJJ, Erkelens DW, De Bruin TWA. Triglyceride-rich lipoproteins in non-insulin-dependent diabetes mellitus: post-prandial metabolism and relation to premature atherosclerosis. Eur J Clin Invest. 1996;26:89–108. doi: 10.1046/j.1365-2362.1996.114256.x. [DOI] [PubMed] [Google Scholar]
- 21.Pal S, Semorine K, Watts GF, Mamo J. Identification of lipoproteins of intestinal origin in human atherosclerotic plaque. Clin Chem Lab Med. 2003;41:792–795. doi: 10.1515/CCLM.2003.120. [DOI] [PubMed] [Google Scholar]
- 22.Wang DQ-H. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 23.Ostlund RE., Jr Phytosterols in human nutrition. Annu Rev Nutr. 2002;22:533–549. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- 24.Connor WE, Lin DS. The effect of shellfish in the diet upon the plasma lipid levels in humans. Metabolism. 1982;31:1046–1051. doi: 10.1016/0026-0495(82)90150-0. [DOI] [PubMed] [Google Scholar]
- 25.Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- 26.Repa JJ, Dietschy JM, Turley SD. Inhibition of cholesterol absorption by SCH 58053 in the mouse is not mediated via changes in the expression of mRNA for ABCA1, ABCG5 or ABCG8 in the enterocyte. J Lipid Res. 2002;43:1864–1874. doi: 10.1194/jlr.m200144-jlr200. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen TA, Kesäniemi YA. Cholesterol absorption: regulation of cholesterol synthesis and elimination and within-population variations of serum cholesterol levels. Am J Clin Nutr. 1989;49:629–635. doi: 10.1093/ajcn/49.4.629. [DOI] [PubMed] [Google Scholar]
- 28.Stange EF, Dietschy JM. Cholesterol Absorption and Metabolism by the Intestinal Epithelium. In: Danielsson H, Sjövall J, editors. Sterols and Bile Acids. New York: Elsevier Science Publishers; 1985. [Google Scholar]
- 29.Turley SD, Dietschy JM. Sterol absorption by the small intestine. Curr Opin Lipidol. 2003;14:233–240. doi: 10.1097/00041433-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 30.van Heek M, Compton DS, Davis HR., Jr The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur J Pharmacol. 2001;415:79–84. doi: 10.1016/s0014-2999(01)00825-1. [DOI] [PubMed] [Google Scholar]
- 31.Repa JJ, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J Lipid Res. 2005;46:779–789. doi: 10.1194/jlr.M400475-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.van Heek M, Austin TM, Farley C, Cook JA, Tetzloff GG, Davis HR., Jr Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes. 2001;50:1330–1335. doi: 10.2337/diabetes.50.6.1330. [DOI] [PubMed] [Google Scholar]
- 33.Altmann SW, Davis HR, Jr, Zhu L-j, Yao X, Hoos LM, Tetzloff G, Iyer SPN, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 34.Davis HR, Jr, Zhu L-j, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SPN, Lam M-H, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, MacIntyre DE, Ogawa A, O’Neill KA, Iyer SPN, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-like 1 (NPC1L1) Proc Natl Acad Sci USA. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawes BE, O’Neill KA, Yao X, Crona JH, Davis HR, Jr, Graziano MP, Altmann SW. In vivo responsiveness to ezetimibe correlates with Niemann-Pick C1 like-1 (NPC1L1) binding affinity: comparison of multiple species NPC1L1 orthologs. Mol Pharmacol. 2007;71:19–29. doi: 10.1124/mol.106.027896. [DOI] [PubMed] [Google Scholar]
- 37.Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, Hobbs HH. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci USA. 2006;103:1810–1815. doi: 10.1073/pnas.0508483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon JS, Karnoub MC, Devlin DJ, Arreaza MG, Qiu P, Monks SA, Severino ME, Deutsch P, Palmisano J, Sachs AB, Bayne ML, Plump AS, Schadt EE. Sequence variation in NPC1L1 and association with improved LDL-cholesterol lowering in response to ezetimibe treatment. Genomics. 2005;86:648–656. doi: 10.1016/j.ygeno.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Labonté ED, Howles PN, Granholm NA, Rojas JC, Davies JP, Ioannou YA, Hui DY. Class B type I scavenger receptor is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochim Biophys Acta. 2007;1771:1132–1139. doi: 10.1016/j.bbalip.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mardones P, Quiñones V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen HE, Trigatti B, Krieger M, VanPatten S, Cohen DE, Rigotti A. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type 1-deficient mice. J Lipid Res. 2001;42:170–180. [PubMed] [Google Scholar]
- 41.Sané AT, Sinnett D, Delvin E, Bendayan M, Marcil V, Ménard D, Beaulieu J-F, Levy E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J Lipid Res. 2006;47:2112–2120. doi: 10.1194/jlr.M600174-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;27:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 43.Smart EJ, De Rose RA, Farber SA. Annexin 2-caveolin 1 complex is a target of ezetimibe and regulates intestinal cholesterol transport. Proc Natl Acad Sci USA. 2004;101:3450–3455. doi: 10.1073/pnas.0400441101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Valasek MA, Weng J, Shaul PW, Anderson RGW, Repa JJ. Caveolin-1 is not required for murine intestinal cholesterol transport. J Biol Chem. 2005;280:28103–28109. doi: 10.1074/jbc.M504609200. [DOI] [PubMed] [Google Scholar]
- 45.Kramer W, Corsiero D, Girbig F, Jähne G. Rabbit small intestine does not contain an annexin II/caveolin 1 complex as a target for 2-azetidinone cholesterol absorption inhibitors. Biochim Biophys Acta. 2006;1758:45–54. doi: 10.1016/j.bbamem.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, Jähne G, Rhein M, Wendler W, Lottspeich F, Hochleitner EO, Orsó E, Schmitz G. Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. J Biol Chem. 2005;280:1306–1320. doi: 10.1074/jbc.M406309200. [DOI] [PubMed] [Google Scholar]
- 47.Knöpfel M, Davies JP, Duong PT, Kværnø L, Carreira EM, Phillips MC, Ioannou YA, Hauser H. Multiple plasma membrane receptors but not NPC1L1 mediate high-affinity, ezetimibe-sensitive cholesterol uptake into the intestinal brush border membrane. Biochim Biophys Acta. 2007;1771:1140–1147. doi: 10.1016/j.bbalip.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Field FJ, Watt K, Mathur SN. Ezetimibe interferes with cholesterol trafficking from the plasma membrane to the endoplasmic reticulum in CaCo-2 cells. J Lipid Res. 2007;48:1735–1745. doi: 10.1194/jlr.M700029-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Gibson JC. Lipid Res Methodol. New York: Alan R. Liss, Inc; 1984. Clinical and Experimental Methods for the Determination of Cholesterol Absorption. [PubMed] [Google Scholar]
- 50.Wang DQ-H, Carey MC. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct versus indirect methodologies. J Lipid Res. 2003;44:1042–1059. doi: 10.1194/jlr.D200041-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Lamon-Fava S, Diffenderfer MR, Barrett PHR, Buchsbaum A, Matthan NR, Lichtenstein AH, Dolnikowski GG, Horvath K, Asztalos BF, Zago V, Schaefer EJ. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J Lipid Res. 2007;48:1746–1753. doi: 10.1194/jlr.M700067-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Miettinen TA, Gylling H, Lindbohm N, Miettinen TE, Rajaratnam RA, Relas H. Serum noncholesterol sterols during inhibition of cholesterol synthesis by statins. J Lab Clin Med. 2003;141:131–137. doi: 10.1067/mlc.2003.9. [DOI] [PubMed] [Google Scholar]
- 53.Watts GF, Chan DC, Barrett PHR, O’Neill FH, Thompson GR. Effect of a statin on hepatic apolipoprotein B-100 secretion and plasma campesterol levels in the metabolic syndrome. Int J Obes. 2003;27:862–865. doi: 10.1038/sj.ijo.0802287. [DOI] [PubMed] [Google Scholar]
- 54.Matthan NR, Giovanni A, Schaefer EJ, Brown BG, Lichtenstein AH. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J Lipid Res. 2003;44:800–806. doi: 10.1194/jlr.M200439-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Vanhanen H, Kesaniemi YA, Miettinen TA. Pravastatin lowers serum cholesterol, cholesterol-precursor sterols, fecal steroids, and cholesterol absorption in man. Metabolism. 1992;41:588–595. doi: 10.1016/0026-0495(92)90050-k. [DOI] [PubMed] [Google Scholar]
- 56.Repa JJ, Buhman KK, Farese RV, Jr, Dietschy JM, Turley SD. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 2004;40:1088–1097. doi: 10.1002/hep.20439. [DOI] [PubMed] [Google Scholar]
- 57.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in apoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–2038. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 58.Turley SD, Dietschy JM. Effects of clofibrate, cholestyramine, zanchol, probucol, and AOMA feeding on hepatic and intestinal cholesterol metabolism and on biliary lipid secretion in the rat. J Cardiovasc Pharmacol. 1980;2:281–297. doi: 10.1097/00005344-198005000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Miettinen TA, Gylling H. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur J Clin Invest. 2003;33:976–982. doi: 10.1046/j.1365-2362.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 60.Santosa S, Varady KA, AbuMweis S, Jones PJH. Physiological and therapeutic factors affecting cholesterol metabolism: Does a reciprocal relationship between cholesterol absorption and synthesis really exist? Life Sci. 2007;80:505–514. doi: 10.1016/j.lfs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Smith JL, Roach PD, Wittenberg LN, Riottot M, Pillay SP, Nestel PJ, Nathanson LK. Effects of simvastatin on hepatic cholesterol metabolism, bile lithogenicity and bile acid hydrophobicity in patients with gallstones. J Gastroenterol Hepatol. 2000;15:871–879. doi: 10.1046/j.1440-1746.2000.02231.x. [DOI] [PubMed] [Google Scholar]
- 62.Turley SD, Spady DK, Dietschy JM. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J Lipid Res. 1995;36:67–79. [PubMed] [Google Scholar]
- 63.Sviridov DD, Pavlov MY, Safonova IG, Repin VS, Smirnov VN. Inhibition of cholesterol synthesis and esterification regulates high density lipoprotein interaction with isolated epithelial cells of human small intestine. J Lipid Res. 1990;31:1821–1830. [PubMed] [Google Scholar]
- 64.Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PHR, Huff MW. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J Lipid Res. 2007;48:699–708. doi: 10.1194/jlr.M600439-JLR200. [DOI] [PubMed] [Google Scholar]