Abstract
Inflammation plays a critical role in promoting smooth muscle migration and proliferation during vascular diseases such as post-angioplasty restenosis and atherosclerosis. Another common feature of many vascular diseases is the contribution of reactive oxygen (ROS) and nitrogen (RNS) species to vascular injury. Primary sources of ROS and RNS in smooth muscle are several isoforms of NADPH oxidase (Nox) and the cytokine-regulated inducible nitric oxide (NO) synthase (iNOS). One important example of the interaction between NO and ROS is the reaction of NO with superoxide to yield peroxynitrite, which may contribute to the pathogenesis of hypertension. In this review, we discuss the literature that supports an alternate possibility: Nox-derived ROS modulate NO bioavailability by altering the expression of iNOS. We highlight data showing co-expression of iNOS and Nox in vascular smooth muscle and demonstrating the functional consequences of iNOS and Nox during vascular injury. We describe the relevant literature demonstrating that the mitogen activated protein kinases (MAP kinases) are important modulators of pro-inflammatory cytokine-dependent expression of iNOS. A central hypothesis discussed is that ROS-dependent regulation of the serine/threonine kinase protein kinase Cδ (PKCδ) is essential to understanding how Nox may regulate signaling pathways leading to iNOS expression. Overall, the integration of non-phagocytic NADPHoxidase with cytokine signaling in general and in vascular smooth muscle in particular is poorly understood and merit further investigation.
Keywords: smooth muscle, nitric oxide, iNOS, superoxide, NADPHoxidase, protein kinase C, MAPKinase, NF-κB
Introduction
Many vascular diseases such as atherosclerosis, post-angioplasty restenosis, in stent restenosis, and post-transplant coronary arteriopathy are characterized by intimal hyperplasia. Vascular smooth muscle (VSM) cells in the medial wall of blood vessels are normally quiescent and express a differentiated phenotype that serves to generate and maintain vascular tone [123]. In response to de-endotheliazation and increased exposure to cytokines and growth factors, VSM may dedifferentiate, migrate across the elastic lamina, and proliferate to form a neointimal layer and to secrete extracellular matrix components that form the bulk of the neointimal tissue [135]. The intimal hyperplasia results in vessel narrowing, and clinically manifests itself in repeat adverse events such as myocardial infarction or repeat intervention. The molecular mechanisms underlying the VSM response to injury and the signaling pathways that control the integration of cytokines and growth factors to regulate VSM gene expression are not well-understood. Overall, the complete etiology of intimal hyperplasia remains unknown.
Accumulating evidence point to vascular injury-induced production of reactive oxygen (ROS) and nitrogen (RNS) species as one important mechanism for the regulation of VSM migration and proliferation in the vascular wall. Recent advances have focused on the identification of non-phagocytic NADPH oxidases (Nox) that generate ROS and the realization that endogenously derived ROS regulate signaling pathways in all primary cellular components of the vascular wall. The contribution of nitric oxide synthase (NOS)-derived NO to vascular injury has been studied in greater details and the convergence between ROS and RNS pathways has been focused on specific chemical interactions such as the reaction of NO with superoxide (O2·−) [11] and lipid peroxyl radicals [130].
The alternative that ROS regulate NO production through regulation of iNOS expression has been poorly explored. Surely, the existing literature is pointing out to the importance of both superoxide and hydrogen peroxide in regulating signaling pathways by influencing the activity of multiple kinases and phosphatases and modulating transcription factors. The nuclear transcription factor κB, a primary regulator of iNOS expression, is redox-regulated at multiple levels including the binding of NFκB to κB motifs [154], regulation of the IκB kinase complex [88], and potentially very upstream through control of the endosomal targeting of cytokine receptors and Nox [101]. Direct evidence that the redox environment may regulate iNOS expression in vascular smooth muscle cells has been obtained: increased levels of the antioxidant enzyme catalase increase NFκB activation and iNOS expression after cytokine stimulation of vascular smooth muscle cells [42,60]. These studies indicated that in this context cytokine-induced hydrogen peroxide production negatively regulates iNOS expression. The physiological significance and molecular mechanism underlying these observations are unknown, but would suggest that iNOS expression may be regulated by cytokine-mediated activation of Nox either at the level or upstream of transcription factors. The primary purpose of this article is to review potential interactions between Nox signaling and iNOS expression in vascular smooth muscle. We summarize and contrast what is known regarding the distribution and roles of iNOS and Nox in the vasculature during injury. Given the complexity of iNOS transcriptional regulation, it would be impossible to cover all potentially relevant signaling pathways that might regulate iNOS expression in a redox-sensitive manner. Instead, we focus on one specific signaling molecule, protein kinase C, to exemplify some of the molecular mechanisms that may underline the redox regulation of iNOS expression and cross-talks between NOX and iNOS in vascular smooth muscle.
Inducible nitric oxide synthase: where, when, and how much?
Mammalian cells synthesize NO through the five-electron oxidation of one of the two-guanidino nitrogen of L-arginine [114]. A family of enzymes generically called nitric oxide synthase (NOS) catalyzes this reaction from which three major classes have been described: neuronal-NOS (nNOS, type I), inducible-NOS (iNOS, type II) and endothelial-NOS (eNOS, type III). All three isoforms are expressed in the vasculature. The predominant isoform of NOS detectable in VSM in response to inflammatory cytokines is iNOS [45,84,170] and nNOS upregulation is induced in VSM by shear stress, hypoxia, and growth factors [116,124,159]. In the healthy vessel, the endothelium serves as the main source of NO production through eNOS activity to maintain vascular tone, and regulate platelet aggregation and leukocyte adhesion [91,114,126]. Disruption of the endothelial layer and initial loss of eNOS is a hallmark of the development of atherosclerosis as well as restenosis. Traditionally, the upregulation of iNOS is perceived to compensate for the loss of a functional endothelium and eNOS during injury and atherosclerosis [61], although the presence of excess NO and ROS coincidentally may lead to additional tissue damage and dysfunction [84].
Early studies of the role of NOS and possible compensation for endothelial dysfunction focused on iNOS expression in plaque initiation and progression. The presence of iNOS mRNA and protein expression has been described in atherosclerotic human plaques in macrophages as well as endothelial and smooth muscle cells [106,162]. In support of a role for iNOS in promoting pathogenesis, iNOS expression was found in the majority of samples as early as the fatty streak stage and in all of the advanced stages of plaques coinciding with an increase in oxidized LDL and nitrated proteins [106]. The suggestion that iNOS expression and activity are correlated with lipid oxidation within the plaque is also supported by the observation that macrophage derived iNOS colocalizes with oxidized lipid and protein derivatives found in atherosclerotic plaques, as well as nitro-tyrosine in advanced atherosclerosis [28]. More recent studies support a dual role for iNOS in the development of the atherosclerotic plaque. In the apoE−/− mouse model, iNOS is expressed in both macrophages and smooth muscle cells of the developing plaque, although smooth muscle cells are not present in early lesions [112]. In the advanced atherosclerotic plaques smooth muscle and macrophage derived iNOS may serve to continually promote a pathogenic environment by enhancing oxidative and nitrosative stress.
The rapid upregulation of iNOS in response to vascular injury is also a characteristic feature of balloon angioplasty. Upregulation of iNOS at the mRNA level is observed in the rat carotid artery by 24 hours post injury and it is sustained throughout 14 days [61]. Based on immunohistochemical analysis, the increase in iNOS expression at 24 hours post injury localizes in the smooth muscle cells of the medial layer of the swine carotid artery [9]. The expression of iNOS in the media disappears at 5 days post injury, but exhibits intense expression at the luminal surface of the neointima that was sustained through 21 days post injury [9]. In some cases, iNOS protein is expressed as early as 6 hours post injury in the medial layer of the rat artery due to removal of platelets from the preparation [49]. By contrast, in response to periarterial collar placement in the rabbit, no iNOS expression in the injured artery is detected until the neointima forms approximately 7 days post injury where iNOS expression is detected in the smooth muscle [5]. Overall, these observations indicate not only a role for endothelial denudation in the induction of iNOS expression in the vessel wall, but also a role for platelets in inhibiting iNOS expression. A more recent study in the rabbit demonstrates the co-expression of iNOS with CuZn-superoxide dismustase (SOD) in the vessel wall in response to balloon injury supporting a role for CuZn-SOD to limit the effects of NO and superoxide-derived peroxynitrite, although nitrotyrosine immunoreactivity is still detectable [83].
The essential role of iNOS during vascular injury: good or bad….once again
Early studies using either pharmacological strategies aimed at modulating NOS activity or gene transfer of different NOS isoforms have provided direct evidence that additional local increase in vascular NO production inhibits smooth muscle cell proliferation by modulating VSMC phenotype and cell cycle [22,84]. Since the last comprehensive review on the role of iNOS in vascular injury [84], many studies have taken advantage of transgenic animals to evaluate the implications of iNOS expression in vascular proliferative diseases (Table 1). Genetic deficiency of iNOS in itself does not change atherosclerotic lesion size in diet-induced atherosclerosis [122]. In contrast, all studies but one using the apolipoprotein E-deficient (apoE−/−) mouse model of atherosclerosis have shown decreased atherogenesis in iNOS/apoE double knockout animals [23,33,85,93,112]. Perivascular cuffing of the carotid artery in iNOS knockout mouse is also associated with decreased neointima formation indicating a contribution of iNOS to the development of vascular injury [4,26]. The apoE−/− and perivascular cuffing models are characterized by an important contribution from monocytes and T lymphocytes [18]. In this context, iNOS may directly contribute to injury by promoting inflammation and necrosis at least in advanced lesions. iNOS may also participate in the establishment of initial lesions by regulating the expansion of cloned Th1 cells [25]. However, Koglin and coworkers found no apparent change in T cell response in iNOS knockout animals in a model of transplant arteriosclerosis [86]. In fact, in this model a significant increase in the number of smooth muscle cells within the neointima was observed in iNOS knockout animals suggesting that a primary contribution of iNOS in this case was to reduce neointimal SMC accumulation. Similarly, neointimal formation was found to be increased in iNOS knockout animals in a carotid ligation model [139]. Another study using the same model found no change in neointima formation [172], although constrictive remodeling was found to be larger in iNOS-deficient animals compared to wild-type. Increased medial hyperplasia rather than intimal hyperplasia was also described in a model employing wire injury to the endothelium in iNOS-deficient mice [115].
Table 1.
Effect of molecular manipulation of iNOS in vascular injury models in vivo
| Reference | Knockout | Manipulation | Effect on Area Lesion Increase/Decrease |
|---|---|---|---|
| Koglin et al., 1998 [86] | iNOS | Transplant arteriosclerosis | ↑ |
| Chyu et al., 1999 [26] | iNOS | Perivascular cuff | ↓ |
| Detmers et al., 2000 [33] | apoE/iNOS | Western diet | ↓ |
| Knowles et al., 2000 [85] | apoE/iNOS | Normal chow | No effect |
| Yogo et al., 2000 [172] | iNOS | Carotid ligation | ↑1 |
| Kuhlencordt et al., 2001 [93] | apoE/iNOS | Western diet | ↓ |
| Niu et al., 2001 [122] | iNOS | Atherogenic diet | No effect |
| Chen et al., 2003 [23] | apoE/iNOS | Western diet | ↓ |
| Sirsjö et al., 2003 [139] | iNOS | Carotid ligation | ↑ |
| Anazawa et al., 2004 [4] | iNOS | Perivascular cuff | ↓ |
| Moore and Hui, 2005 [115] | iNOS | Wire injury | ↑ |
| Miyoshi et al., 2006 [112] | apoE/iNOS | Western diet | ↓ |
Increase in constrictive remodeling but no change in neointima formation.
Granted genetic variations between animal strains [62,92], the studies outlined above indicate that the benefits or drawbacks from inhibiting iNOS vary with the type of vascular injury. This is reminiscent of other pathologies in which the role of iNOS as a detrimental or beneficial protein during inflammation has been already highlighted [90]. For the differences observed in the various vascular injuries, a possibility is that differences in the immune response may underline the outcome associated with inhibition of iNOS. In contrast to diet-induced injuries, mechanical injuries are characterized by early and transient lymphocyte and monocyte infiltration with very little detectable immune cells at the injured site days after the injury. In this case, the primary cells expressing iNOS are VSM cells consistent with a role for VSM-derived iNOS in inhibiting VSM proliferation in vascular injury models primarily dominated by strong VSM migration and proliferation. In models in which the tissue responds with an overwhelming inflammatory cell contribution throughout the course of the disease, the balance may be tilted towards iNOS-mediated tissue dysfunction and damage in as much as these models may be also accompanied by large production of ROS. Most investigators have also assumed an identical role for iNOS independent of the vascular bed and the multiplicity of cell types expressing iNOS. The development of cell or tissue specific iNOS –deficient mice should represent an important approach in order to tease out the different roles of iNOS in complex vascular diseases where both VSM proliferation and the immune response participate.
Regulation of iNOS activity and expression
The role of iNOS in vascular injury has been traditionally linked to the level of protein expression attained in the injured vessels and to the amounts of NO produced by the enzyme itself. Recent biochemical studies indicate that iNOS product release varies with the environment and kinetics of the enzyme such that the long-standing dichotomy between the beneficial effects of low eNOS-derived and high iNOS-derived NO concentrations may be unjustifiable, at least at the level of enzyme activity [144]. For example, medial VSM cells in the arterial vessel wall exist in a chronic state of hypoxia that is increased during vascular diseases [132]. This would be inconsistent with the production of large amounts of iNOS-derived NO because the Km of iNOS for molecular oxygen approximates 130 µM [1]. The bioavailability of L-arginine is also an important regulator of iNOS activity in vivo. Recent studies indicate that this may involve the catabolism of L-arginine to ornithine and urea by arginase, a metalloenzyme, which expression has been documented in several cell types of the vasculature including smooth muscle cells [37]. Increased expression of arginase during vascular injury may shunt L-arginine metabolism to ornithine and polyamine synthesis, inhibit iNOS-derived NO production, and promote cell proliferation [37]. Similarly, the requirement of iNOS activity for tetrahydriobiopterin (H4B) is well established and the production of superoxide and peroxynitrite by iNOS in vitro under suboptimal conditions has been described [58,164]. H4B deficiency has been recently investigated as a cause of eNOS dysregulation and endothelial dysfunction in several clinical settings including artherosclerosis [150] and the implications of changes in H4B bioavailability for iNOS activity and vascular injury have yet to be described.
In contrast to eNOS, little is known about posttranscriptional modifications that control the localization and activity of iNOS. Recent studies have shown palmitoylation of iNOS during processing and activation [77,118,119]. Jones et al. have indicated the importance of calcium/calmodulin-dependent protein kinase II in regulating iNOS trafficking and activity in VSM cells [77]. Activation of the inducible kinin B1 receptor stimulates the production of NO in cytokine-treated human lung microvascular endothelial cells [131] and Zhang and coworkers have recently identified ser745 as a physiologically relevant - ERK-dependent – phosphorylation site on human iNOS that enhances NO production [174]. These studies would indicate acute regulation of iNOS activity in human cells that express low levels of iNOS. Beyond this, the regulation of mRNA and protein iNOS stability also represents an important mechanism by which iNOS levels and activity may be regulated and may explain some of the differences in iNOS induction observed between different species. For example, the 3’-UTR region of the human iNOS mRNA contains several AU-rich elements that have been shown to destabilize reporter mRNAs [129]. Recent advances also indicate that epigenetic factors including DNA methylation and histone H3 lysine 9 methylation silence or repress human iNOS expression in human endothelial and smooth muscle cell cultures [21]. Overall, much of the research efforts on the regulation of iNOS function have focused on the elucidation of the transcriptional regulation of iNOS expression because iNOS expression requires protein synthesis and is induced by cytokines and lipopolysaccharides.
The molecular mechanisms regulating iNOS expression were first characterized by cloning the murine iNOS promoter from the macrophage cell line, RAW 264.7 [105,165]. These studies have shown that 1,000 bases out of the 1500 bp long promoter confers full activation of iNOS transcription upon stimulation with IFNγ and LPS. The human iNOS promoter, however, is considerably larger. The first 3.8kb upstream of the iNOS gene only confers basal activity, 3 to 5 fold induction is found in promoter segments containing between 5.8kb and 7.0kb upstream, and 10-fold activation is found in a promoter segment containing 16kb of the upstream 5’ region [30,141]. The rat VSMC iNOS promoter was later cloned, and it was demonstrated that 3.2kb of the 5kb region of the iNOS promoter is required for maximum activation of the promoter upon stimulation with LPS or a mixture of IL-1β, TNFα and IFNγ [38,173]. The mechanisms by which transcription factors and promoter elements regulate iNOS expression have been extensively reviewed [43]. Analysis of the human, mouse, and rat iNOS promoters revealed multiple consensus sites for transcription factors that are activated by inflammatory cytokines and lipopolysaccharides. Although these studies show there are large species- and cell type-dependent variations in the regulation of iNOS expression, they all show an important role for the NF-κB proteins in the regulation of iNOS transcription.
The transcription factor NF-κB is persistently activated in advanced atherosclerotic lesions and its activation is linked to a wide variety of processes including inflammation, proliferation, differentiation and apoptosis [16,127]. Cytokines such as IL-1β activate NF-κB in many cell types including VSMC and activation of NF-κB is a requirement for iNOS expression [63,75]. A classical NF-κB activation signaling pathway from the IL-1 receptor (IL-1RI) has been well characterized using overexpression in human fibroblasts [121] and the components of this pathway are only beginning to be characterized in VSM cells [82] (see Figure 1). IL-1 binding to the receptor leads to recruitment of several adaptor proteins including MyD88 [161]. These activate the IL-1 receptor associated kinase (IRAK) which binds TNFα receptor associated factor 6 (TRAF6) [17,76]. These two proteins are involved in the activation of the TGFβ activated kinase 1 (TAK1) which phosphorylates the NF-κB inducing kinase (NIK) in the NF-κB signalsome [102,121], which also contains the IκB kinases IKKα and IKKβ as well as the IκK adaptor protein (IKAP). NIK activates the IKKs which phosphorylate the two NF-κB inhibitory proteins, IκBα and IκBβ [102]. These proteins bind NF-κB in the cytosol under basal conditions. Upon phosphorylation, they are degraded by a ubiquitin-mediated proteosomal pathway and release active NF-κB dimers which translocate to the nucleus to activate transcription [148]. The inhibitory proteins are under complex temporal regulation allowing a transient phase of NF-κB activation mediated by the quick degradation and subsequent recovery of IκBα followed by a persistent phase of activation mediated by the slower, long term degradation of IκBβ [66,75].
Fig. 1.
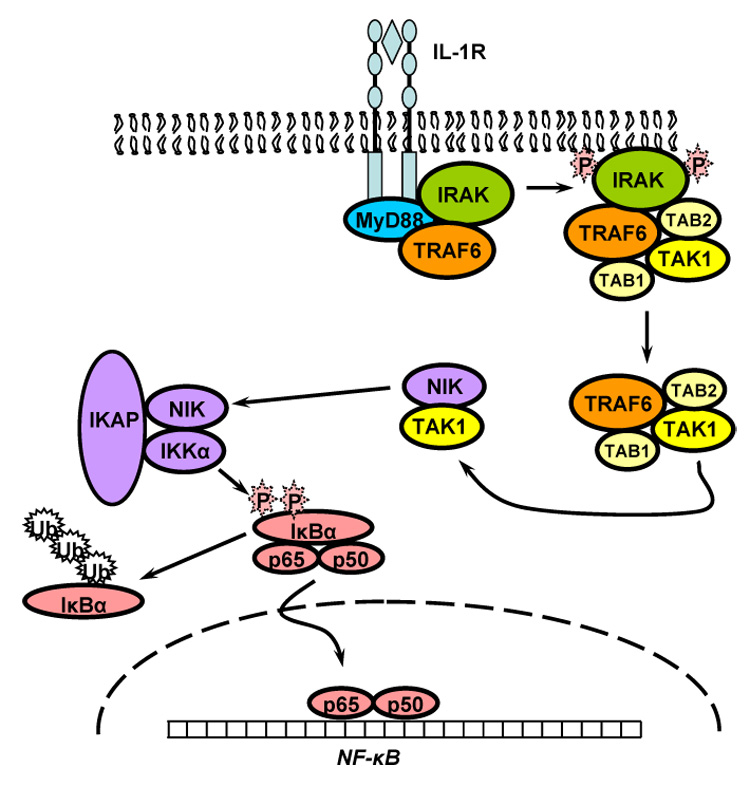
Classical pathway of interleukin-1β stimulated activation of NF-κB. See text for details.
There is a large body of literature demonstrating that the activation of the transcription factor NF-κB is regulated by ROS [69]. In vitro, NF-κB binding can be inhibited by N-ethylmaleimide and diamide [154]. Early work also showed that cytokine-induced activation of NF-κB is inhibited by metal chelators and antioxidants in cells [78]. The response of NF-κB to hydrogen peroxide has been studied in many cell types with divergent results showing context dependent regulation [69]. Using transformed alveolar type II cells, Korn and coworkers have shown that cytokine-induced activation of NF-κB is inhibited by hydrogen peroxide through direct oxidation and inactivation of IκB kinase [88]. In contrast, activation of the redox-sensitive PI3K/Akt pathway stimulates NF-κB by promoting the dissociation of phosphorylated IκBα [13]. In the human breast cancer cell-line MCF-7, the recruitment of NIK to TRAF6 in response to IL-1β stimulation requires endosomal targeting of Nox2, indicating that in this cell type the primary site of redox regulation of NF-κB occurs very upstream and involves Nox [101].
In rat thoracic VSM cells, pharmacological antioxidants have been shown to modulate NF-κB activation [64,70,88]. We as well as others have shown that NF-κB activity is potentiated by increased protein levels of catalase, although in this case the mechanisms of action have not been delineated [42,60]. As described in the next sections, the fact that cytokine-induced iNOS expression in rodent SMC requires the activation of ERK would also suggest that redox-regulation of NF-κB in this system would occur at the level or upstream of mitogen activated protein kinases.
The role of MAP kinase and Protein kinase C in regulating iNOS expression
Increasing evidence indicates that the mitogen activated protein (MAP) kinase family of protein kinases (ERK1/2, c-Jun Kinase, p38), are important modulators of pro-inflammatory cytokine-dependent expression of inducible nitric oxide synthase (iNOS) in multiple cell types [29,68,97]. Of particular importance in vascular smooth muscle cells (VSM) is the role and mechanisms that couple ERK1/2 to iNOS expression in response to proinflammatory cytokines. An early study by Jiang et al reported that stimulation with IL-1β results in a coordinate activation of ERK1/2 and NF-κB leading to increased expression of iNOS in VSM cells [71]. Furthermore, this study showed that this IL-1β-dependent activation of NF-κB is dependent upon ERK1/2 through the ability of ERK1/2 to phosphorylate I-κBβ resulting in its degradation and subsequent translocation of the p65 and p50 subunits to the nucleus to initiate gene expression. Further studies showed that ERK1/2 selectively phosphorylates I-κBβ rather than I-κBα resulting in sustained NF-κB activity which is required for iNOS gene expression [72,73,168]. The intermediate between ERK1/2 and I-κBβ is ribosomal S6 kinase (RSK) 1 [168].
In VSM, the addition of growth factors such as PDGF and EGF enhances ERK1/2-dependent I-κBβ phosphorylation and corresponding increases in NF-κB activity and iNOS expression [72]. Conversely, Angiotensin II (Ang II) has been reported to suppress iNOS expression due to its ability to activate p38 MAPK which suppresses activation of ERK1/2 [74,117]. Taken together, these studies support a critical role for ERK1/2 and sustained NF-κB activity in IL-1β-dependent iNOS expression in VSM cells. Other proinflammatory cytokines have also been reported to mediate a potent immune response after vasculature injury that involves ERK1/2. For example, TNF-α initiates an ERK1/2-dependent expression of several transcription factors in VSM cells in a vascular injury model [48] and has also been shown to stimulate the expression of iNOS in an ERK1/2-dependent manner in cultured VSM cells [35].
While the studies reviewed above strongly support a role for ERK1/2 and NF-κB in iNOS expression, they provide little insight into the signaling molecules upstream of ERK1/2. In studies designed to elucidate the mechanism by which the proinflammatory cytokine IL-1β induces ERK1/2 activity in VSM cells, our laboratory investigated the effects of reactive oxygen species (ROS) on IL-1β-induced iNOS expression. The results from these studies indicate that along with iNOS expression, IL-1β induces an increase in ROS production in VSM cells and this increase in ROS suppresses ERK1/2 activity and iNOS expression [60]. Furthermore, stimulation with IL-1β also results in increased p38MAPK activity, which suppresses ERK1/2 activity and iNOS expression in VSM cells. Thus, this report describes in VSM cells the intriguing ability of IL-1β to enhance iNOS expression through activation of ERK1/2 and NF-κB and to suppress iNOS expression through two distinct mechanisms involving p38MAPK and increased levels of ROS.
As already stated, little is known regarding the signaling molecules that are activated proximal to IL-1β stimulation in VSM cells. Early studies by Finder et al indicated that IL-1β induced iNOS expression in VSM cells is mediated in a positive manner by proteins that are farnesylated and in a negative manner by proteins that are geranylgeranylated [41]. These findings are difficult to interpret in light of the studies discussed previously because they did not report a role for ERK1/2 in IL-1β-dependent increases in iNOS expression. The studies described in these reports were done in pulmonary VSM cells while the studies discussed earlier were carried out in thoracic aortic VSM cells suggesting the possibility that the signaling pathways IL-1β or other proinflammatory cytokines utilize may be smooth muscle cell type specific. Recently, our laboratory reported that IL-1β-dependent activation of ERK1/2 and increases in iNOS expression in VSM cells were positively mediated by PLCγ and PKCδ [47]. Thus, PLCγ is activated upon IL-1β stimulation and is required for increases in PKCδ activity, activation of ERK1/2, and expression of iNOS. This study confirmed an earlier report in gingival fibroblasts which also provided evidence supporting a requirement for PLCγ in IL-1β-dependent activation of ERK1/2 [158]. Not only is there evidence for the involvement of PLCγ in IL-1β-dependent activation of ERK1/2 in gingival fibroblasts, a series of studies by McCulloch et al also report the involvement of the traditional mediators of IL-1β signaling such as IRAK along with the non-receptor tyrosine kinase focal adhesion kinase (FAK) [104] [108] and the tyrosine phosphatase SHP2 [107]. While little is known concerning the IL-1 receptor and its adaptor molecules in VSM cells, it is tempting to speculate that the mechanism by which IL-1β stimulation results in a PLCγ- and PKCδ-dependent activation of ERK1/2 and subsequent increases in iNOS expression in VSM cells is similar.
It is not surprising that PKCδ is involved in this cytokine-dependent activation of ERK1/2. Other studies have reported a role for PKCδ in mediating ERK1/2 activity in VSM cells [46] [94]. Interestingly, PKCδ has also been implicated in the activation of MEKK1, a Mek kinase involved in the activation of ERK1/2 and JNK [166,167]. Several studies have reported a role for MEKK1 in the regulation of NFκB activity through its ability to mediate IKK activation [99,171]. This raises the interesting possibility that PKCδ may mediate IL-1β-dependent increases in iNOS expression through its ability to drive ERK1/2 along with its ability to increase NF-κB activity in a more direct manner. In light of our study indicating a role for ROS in mediating IL-1β-dependent increases in iNOS expression and multiple reports describing a role for ROS in regulating PKCδ activity and function, it is important to determine if PKCδ may serve as an integration point for ROS in the IL-1β-dependent signaling pathway [60].
Our studies also indicate that IL-1β dependent increase in ERK1/2 activity in VSM cells does not involve the canonical signaling molecules Ras and RAF [47]. While Ras’ involvement in IL-1-dependent ERK1/2 activation hasn’t been studied elsewhere in VSM cells, reports in other cell systems suggest that Ras is required for IL-1β dependent activation of ERK1/2 and subsequent increases in iNOS expression [152]. Given the discrepancies of these published studies and the possibility that IL-1β may utilize a non-canonical signaling pathway to ERK1/2, more work is required to elucidate the signal transduction pathways that result in cytokine-dependent activation of ERK1/2 and iNOS expression in VSM cells. Finally, it is clear that a unique signaling pathway (most probably TRAF6/TAK1/IKK) is necessary for the initial activation of NFκB and for the subsequent persistent activation of NF-κB, which is ERK-dependent. Recent studies have placed other PKC isoforms upstream of TAK1 [138]. One exciting possibility is that -in VSM cells- PKCδ itself lies very upstream in the IL-1-signaling pathway regulating both TRAF6/TAK1/IKK and ERK/RSK1.
NADPH oxidase expression and function in vascular injury
Although several sources of ROS coexist within the vasculature, NADPH oxidases (Nox) have emerged as essential components in redox signaling and oxidative stress in the vessel wall. To date, five distinct Nox isoforms (Nox1,2,3,4 and 5) and two homologous oxidases that contain an additional peroxidase domain, termed Duox 1 and 2 (for dual oxidases 1 and 2), have been discovered [95]. In vivo, macrophage-induced expression of phagocytic gp91phox (Nox2) as well as the catalytic subunit p22phox have been characterized within the lesion of atherosclerotic plaques in human coronary arteries as well as an increase in smooth muscle Nox 4 expression within advanced lesions [140]. Sorescu et al. observed the increase in p22phox within the medial layer of the vessel wall as well as the plaque, which agree with earlier studies by Azumi and colleagues [6,140]. The novel observation proved to be that gp91phox expression is found in the plaque and adventitial macrophages, particularly the core of the plaque, where Nox 4 expression is complementary to gp91phox located in the medial smooth muscle layer and luminal surface of the plaque smooth muscle cells.
Little is known about Nox protein expression and function in neointimal formation in response to vascular injury. Using the balloon injury model in the rat, Griendling and colleagues observed that Nox1 as well as p22phox mRNA was upregulated 3 days post injury and sustained through 15 days post injury while there was an increase in gp91phox at 7 days post injury which also sustained through 15 days [149]. In addition Nox4 mRNA remained unchanged until 15 days post injury where an increase in the mRNA was observed. The same authors used immunohistochemistry to confirm that p22phox expression was detected basally in the adventitia of the vessel, but appeared in the medial smooth muscle layer by 7 days post injury and then most abundantly in the neointima by 10 days post injury, where Nox4 was only detected in medial and neointimal cells. However, they were not able to establish the site of Nox1 expression [149]. Moe et al. investigated Nox expression in human aortic smooth muscle cells in response to the pro-inflammatory cytokine TNF-α. They show evidence to support an increase in Nox4 expression, but no change in Nox1 levels [113]. This is in contrast to rodent VSM cells in which an increase in Nox1 and a decrease in Nox4 expression has been observed [98].
Given that atherosclerosis is a chronic disease that progresses over years if not decades in human, it is hard to match the expression of proteins to stages of disease progression. Nevertheless, iNOS and Nox protein expression seem to coincide with localized oxidative stress in lesion progression, particularly in the macrophage and smooth muscle cells in the media and plaque of the vessel. It is difficult to ascertain, however, if the expression of one protein precedes the other although it has been shown that iNOS protein is detectable in all stages of plaque development, where the literature only discusses Nox expression in more advanced atherosclerotic lesions. Overall, the current observations support an early increase in iNOS mRNA and protein levels in the media while Nox protein expression occurs later, mostly when the neointima has already begun to develop between 3 and 7 days post injury. Both iNOS and Nox proteins are expressed at the same time within the same cell type in neointimal hyperplasia, but iNOS expression seems to disappear by day 14 post injury while the smooth muscle cells see another wave of Nox protein expression when Nox4 is upregulated at day 15. This not only suggests different roles for iNOS and Nox proteins, but that perhaps different Nox isoforms have separate roles within the same cells.
In spite of the lack of specific Nox pharmacological inhibitors, studies using knockout or transgene animals targeting Noxs are lacking. Deletion of the NADPH organizer protein p47phox in the ApoE−/−mouse significantly reduced lesion area indicating that one or several NOX isoforms play a role in this animal model [10]. Similarly, p47phox−/− mouse have reduced intimal hyperplasia following transluminal wire injury of the femoral artery [157]. The effects of p47phox deficiency in mouse models of vascular injury point to a role for gp91phox. As already stated, upregulation of gp91phox in human atherosclerotic plaques and in animal models has been demonstrated. Pharmacological and molecular manipulation of gp91phox activity and protein levels also indicate a role for gp91phox in Ang-II-mediated smooth muscle cell hypertrophy [103,128].
Nox1 is also an integral component of the signaling pathways associated with angiotensin II in smooth muscle [98] and recent studies using Nox1-deficient or transgenic mice support an important role for Nox1 in angiotensin II-induced hypertension [34,110]. The Nox4 isoform has been generally considered to be constitutively active. It was recently found that the insulin receptor [156], toll-like receptor 4 [125], transforming growth factor β1 [145,146], and possibly interleukin 1β [39,57,59] all required Nox4 activity. In adipocytes, overexpression of Nox4 was not sufficient to increase basal levels of ROS but required insulin stimulation, arguing for a regulated Nox4 activity. Functionally, Nox4 may be required for the maintenance of the differentiated smooth muscle phenotype [27] and may play an important role in the vascular remodeling associated with the development of pulmonary hypertension [111].
Regulation of NADPH oxidase
The molecular mechanisms regulating Nox activity have been recently reviewed [20,96]. In general terms, Noxs are multimeric complexes composed of one or two membrane proteins (Nox and p22phox) as well as cytosolic components including p47phox, NOXO1, p40phox, p67phox, NOXOA1, Rac1 and Rac2. In human and rodent VSM cells, Nox1 and Nox4 expression have been documented [65,80,98,140,163], and Nox2 expression has also been reported in some human VSM cells [79,155]. Nox5 may be expressed in human VSM cells but not in rodent [8].
In the case of the phagocytic Nox2, the fully active enzyme requires assembly of Nox2 and p22phox with p47phox, p67phox, and Rac. Activation requires stimulus-induced serine phosphorylation of the C-terminal region of p47phox that ultimately leads to the targeting of p67phox to the membrane complex [3,100,147]. The N-terminal region of p67phox interacts with GTP-bound Rac and the activation domain of p67phox allows electron flow in the p22phox/Nox2 complex required for superoxide production [56,133]. The fully active NADPH oxidase is also under complex regulation by lipid metabolites that interact with the Phox-homology (PX) domains of p47phox and p40phox [2,67].
The proteins NOXO1 (Nox organizer 1) and NOXA1 (Nox activator 1) are novel homologs of p47phox and p67phox, respectively, that regulate the activity of Nox1 and Nox3 [7,24,44,151]. NOXO1 contains tandem SH3 domains that interact with p22phox like p47phox, but has no autoinhibitory domain like p47phox. Instead, direct intramolecular interaction of the tandem SH3 domains with a prolin rich region may allow for regulation of its binding to NOXOA1 and p22 [169]. NOXO1 also contains a PX domain, which regulates lipid-protein and protein-protein interactions and unlike the PX domain of p47phox, showed specificity for monophosphorylated phosphoinoside lipids which are present in nonstimulated cells [24]. These proteins are expressed primarily in the colon epithelium where ROS production is elevated even in the absence of any apparent stimuli. Nox1 can also interact with p47phox in vascular smooth muscle cells, which would render it susceptible to stimulated activation [155]. The function of NOXO1 and NOXOA1 has been described primarily in overexpression systems. No studies to date have examined the role of these novel proteins in physiological conditions. Though the activation mechanisms of Nox1 are still poorly characterized, even less is known about the regulation of Nox4 activity which is not enhanced by Rac or any of the known activating or organizing proteins [81,109]. Recent studies indicate different pharmacological profiles between Nox isoforms that may allow distinction between isoforms including Nox4 and 1 [136,137].
Redox regulation of protein kinase C
As mentioned previously, cytokine stimulation of VSM cells results in NADPH oxidase derived increases in ROS. A signaling molecule that is particularly redox sensitive is protein kinase C (PKC) and it may provide a good example to illustrate some of the molecular mechanisms that may underline the redox regulation of iNOS expression and cross-talks between NOX and iNOS in vascular smooth muscle.
PKC activity can be altered in response to increases in ROS either by direct oxidation of PKC itself or indirectly through oxidant-dependent regulation of kinases or phosphatases that modulate PKC activity. Reports suggest that PKC activity can be either inhibited or enhanced when exposed to oxidants [55]. The reason for these opposing effects is due to the selective oxidation of cysteine residues in either the regulatory or catalytic domain of PKC. The precise molecular mechanisms resulting in either an increase or decreases in PKC activity have been reviewed in detail [120]. Briefly, PKCs are comprised of a regulatory and catalytic domain. The regulatory domain consists of a pseudosubstrate region which interacts with the catalytic domain (which autoinhibits kinase activity) along with domains that bind the necessary cofactors for PKC activation, lipids and/or calcium. Upon binding of these cofactors, the conformation of the regulatory domain of PKC changes relieving autoinhibition and allowing for the binding of ATP and autophosphophorylation of serine638/641/643 residues in the catalytic domain to occur, which are required for complete activation of the enzyme [120]. There are several excellent reviews for a more complete description of PKC structure and function [120,143,153]. Oxidation of the cysteine residues in the regulatory domain have been shown to induce the conformational changes necessary to relieve autoinhibition of PKC and render the kinase constitutively active [51] while oxidation of the cysteine residues in the catalytic domain have been shown to induce [160] as well as inhibit [50] kinase activity. There is still much we do not understand regarding the effects of the direct oxidation of the cysteine residues on PKC. One of the first studies to examine the role of oxidation on PKC activity clearly shows that treatments with low levels of the oxidant periodate modified the regulatory domain of PKC resulting in a loss of PKC activity while treatment with higher concentrations modified the catalytic domain resulting in an increase of PKC activity [52]. Later studies have reported that exposure to redox active compounds such as hydrogen peroxide [54], organic peroxides [36], and selenocompounds [53] result in the direct oxidation of PKC and change its activity. PKCs are a large family serine/threonine protein kinases subdivided into three subtypes based on their requirements for activation and are ubiquitously expressed [120]. Modulation of PKC activity by direct oxidation as described above is presumably not subtype or PKC isoform specific or tissue dependent. There is no reported evidence suggesting that oxidation of cysteine residues is an important modulator of PKC activity in VSM cells although the most likely method would be through Nox-derived ROS. In support to this contention, our recent studies would indicate that Nox1 and 4 negatively regulate IL-1β-mediated activation of ERK1/2 [59], which is downstream of PKCδ [47].
As mentioned earlier, redox-dependent regulation of PKC activity can also occur in an indirect manner. It is known that increases in H2O2 result in the oxidation of cysteine residues on tyrosine phosphatases such as SHP2 [32] and serine/threonine phosphatases such as PP2A [134]. The inhibition of tyrosine phosphatase activity results in unregulated activation of tyrosine kinases such as Src [19]. It has been reported that Src-dependent phosphorylation of tyrosine residues on PKCs catalytic domain can augment its activity. Although in vitro studies have shown that multiple PKCs can be activated in this manner [87], the only member of the PKC family that has been shown to be tyrosine phosphorylated in vivo in VSM cells is PKCδ [31]. PKCδ has multiple tyrosine residues on its regulatory and catalytic domain and in vivo studies have reported that phosphorylation of the tyrosine residues occur exclusively in the regulatory domain [89]. The precise nature of these tyrosine phosphorylations is not clear but they have been implicated in regulating PKCδ activity [12], as signals for protein degradation [14], and in inducing apoptotic events [15]. Interestingly, PKCs including PKCδ have been implicated in the regulation of NADPH oxidase activity [40]. These data identify PKC not only as being regulated in an oxidant-dependent manner but also as an important mediator of intracellular oxidant levels. Another possibility that PKC activity and function may be altered by increases in an oxidant-dependent manner is through regulation of serine/threonine phosphatases such as PP2A. PP2A, which is redox sensitive [134], is an important regulator of PKC activity because of its ability to remove phosphates from PKC’s catalytic domain [142].
Summary and concluding remarks
The studies summarized in the present review provide support to the hypothesis that ROS production in general, and NADPH oxidases in particular, have a role during vascular injury in regulating the expression and function of iNOS (see Figure 2). In vascular injury, iNOS expression has been proposed to be beneficial, as NO inhibits VSM cell proliferation. Clearly, some have questioned the benefit of iNOS expression on injured vessels since large quantities of NO may interact with ROS and form cytotoxic species. However, recent studies do show that iNOS deficiency results in increased neointimal formation in some animal models of vascular injury. In contrast, Nox-derived ROS act as cell signaling mediators which may enhance the ability of VSM cells to proliferate into the intima of blood vessels leading to the thickening of the vascular wall. Since NO opposes this function by limiting VSMC proliferation, a novel and attractive possibility is that one or several Nox isoforms may negatively regulate some of the cell signaling pathways leading to iNOS expression.
Fig. 2.
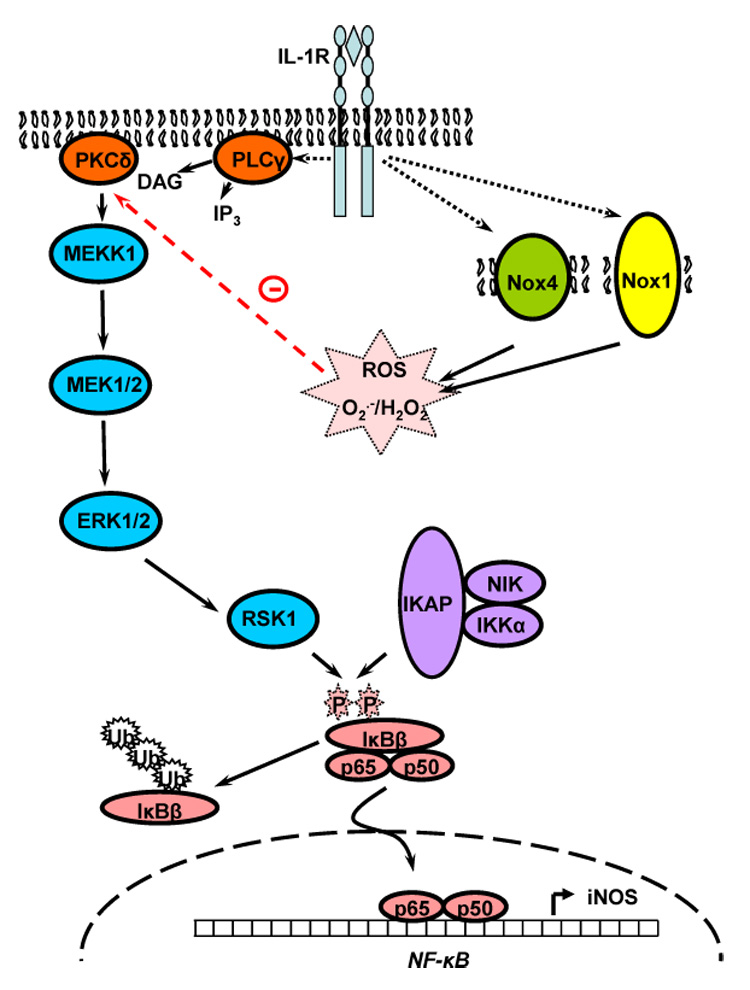
Possible interplay between interleukin-1β (IL-1β), protein kinase Cδ (PKCδ), and NADPHoxidase (Nox) in the cytokine-mediated expression of iNOS in vascular smooth muscle cells. Activation of the MAP kinases ERK1/2 is independent of Ras and Raf, but dependent on PLCγ and PKCδ. The exact nature of the MAPKKK involved is unclear but may be MEKK1. ROS negatively regulate the IL-1β-mediated expression of iNOS [60]. Most likely sources of ROS activated by IL-1β are Nox1 and 4 [59]. ROS-dependent inhibition of PKCδ may be a critical mechanism by which Nox may regulate iNOS expression. The exact mechanism by which ERK1/2 may be regulating iNOS expression are still under investigation and involves ribosomal S6 kinase 1 (RSK1; [168]). See text for details.
There are two primary issues that need to be addressed in order for this research to go forward. The first one is to elucidate the specific mechanisms that lead to selective activation of different Nox isoforms by cytokines. The second one is to determine how cytokine receptor-mediated Nox regulation of relevant signaling molecules such as PKC regulates gene expression. The implications of Nox-dependent regulation of PKC during cytokine stimulation would obviously extend beyond the regulation of iNOS. The transcription of several other genes besides iNOS is regulated by the combination of inflammatory cytokines and it is possible that the expression of these genes is under the same regulatory mechanisms.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute RO1-HL-40992 and RO1-HL-49426 (to H.A. Singer) and T-32-HL-07194 (to B.J. Guikema, pre-doctoral training grant), the National Cancer Institute CA-89366 (to D. Jourd’heuil), and Philip Morris USA Inc. and Philip Morris International (to D. Jourd’heuil).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abu-Soud HM, Ichimori K, Nakazawa H, Stuehr DJ. Regulation of inducible nitric oxide synthase by self-generated NO. Biochemistry. 2001;40:6876–6881. doi: 10.1021/bi010066m. [DOI] [PubMed] [Google Scholar]
- 2.Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc. Natl. Acad. Sci. USA. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ago T, Nunoi H, Ito T, Sumimoto H. Mechanism for Phosphorylation-induced Activation of the Phagocyte NADPH Oxidase Protein p47phox. TRIPLE REPLACEMENT OF SERINES 303, 304, AND 328 WITH ASPARTATES DISRUPTS THE SH3 DOMAIN-MEDIATED INTRAMOLECULAR INTERACTION IN p47phox, THEREBY ACTIVATING THE OXIDASE. J. Biol. Chem. 1999;274:33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- 4.Anazawa T, Dimayuga PC, Li H, Tani S, Bradfield J, Chyu KY, Kaul S, Shah PK, Cercek B. Effect of exposure to cigarette smoke on carotid artery intimal thickening: the role of inducible NO synthase. Arterioscler Thromb Vasc Biol. 2004;24:1652–1658. doi: 10.1161/01.ATV.0000139925.84444.ad. [DOI] [PubMed] [Google Scholar]
- 5.Arthur JF, Yin ZL, Young HM, Dusting GJ. Induction of nitric oxide synthase in the neointima induced by a periarterial collar in rabbits. Arterioscler Thromb Vasc Biol. 1997;17:737–740. doi: 10.1161/01.atv.17.4.737. [DOI] [PubMed] [Google Scholar]
- 6.Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 7.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 8.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 9.Banning AP, Groves PH, Buttery LD, Wharton J, Wharton J, Rutherford RA, Black P, Winkler F, Polak JM, Lewis MJ, Drexler H. Reciprocal changes in endothelial and inducible nitric oxide synthase expression following carotid angioplasty in the pig. Atherosclerosis. 1999;145:17–32. doi: 10.1016/s0021-9150(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 10.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ETH, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE−/− mice. J. Clin. Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci.USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benes C, Soltoff SP. Modulation of PKCdelta tyrosine phosphorylation and activity in salivary and PC-12 cells by Src kinases. Am J Physiol Cell Physiol. 2001;280:C1498–C1510. doi: 10.1152/ajpcell.2001.280.6.C1498. [DOI] [PubMed] [Google Scholar]
- 13.Beraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc. Natl. Acad Sci U. S. A. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake RA, Garcia-Paramio P, Parker PJ, Courtneidge SA. Src promotes PKCdelta degradation. Cell Growth Differ. 1999;10:231–241. [PubMed] [Google Scholar]
- 15.Blass M, Kronfeld I, Kazimirsky G, Blumberg PM, Brodie C. Tyrosine phosphorylation of protein kinase Cdelta is essential for its apoptotic effect in response to etoposide. Mol Cell Biol. 2002;22:182–195. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bu Dx, Erl W, de Martin R, Hansson GK, Yan Zq. IKKβ-dependent NF- κB pathway controls vascular inflammation and intimal hyperplasia. The FASEB journal. 2005:04-264fje. doi: 10.1096/fj.04-2645fje. [DOI] [PubMed] [Google Scholar]
- 17.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Moons L, Collen D. Mouse models of angiogenesis, arterial stenosis, atherosclerosis and hemostasis. Cardiovasc. Res. 1998;39:8–33. doi: 10.1016/s0008-6363(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 19.Catarzi S, Biagioni C, Giannoni E, Favilli F, Marcucci T, Iantomasi T, Vincenzini MT. Redox regulation of platelet-derived-growth-factor-receptor: role of NADPH-oxidase and c-Src tyrosine kinase. Biochim. Biophys. Acta. 2005;1745:166–175. doi: 10.1016/j.bbamcr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox. Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 21.Chan GC, Fish JE, Mawji IA, Leung DD, Rachlis AC, Marsden PA. Epigenetic basis for the transcriptional hyporesponsiveness of the human inducible nitric oxide synthase gene in vascular endothelial cells. J Immunol. 2005;175:3846–3861. doi: 10.4049/jimmunol.175.6.3846. [DOI] [PubMed] [Google Scholar]
- 22.Channon KM, Qian H, George SE. Nitric Oxide Synthase in Atherosclerosis and Vascular Injury : Insights From Experimental Gene Therapy. Arterioscler Thromb Vasc Biol. 2000;20:1873–1881. doi: 10.1161/01.atv.20.8.1873. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Kuhlencordt P, Urano F, Ichinose H, Astern J, Huang PL. L-Arginine on Atherosclerosis in ApoE Knockout and ApoE/Inducible NO Synthase Double-Knockout Mice. Arterioscler Thromb Vasc Biol. 2003;23:97–103. doi: 10.1161/01.atv.0000040223.74255.5a. [DOI] [PubMed] [Google Scholar]
- 24.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol. Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 25.Choy JC, Wang Y, Tellides G, Pober JS. Induction of inducible NO synthase in bystander human T cells increases allogeneic responses in the vasculature. Proc. Natl. Acad. Sci. USA. 2007;104:1313–1318. doi: 10.1073/pnas.0607731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chyu KY, Dimayuga P, Zhu J, Nilsson J, Kaul S, Shah PK, Cercek B. Decreased Neointimal Thickening After Arterial Wall Injury in Inducible Nitric Oxide Synthase Knockout Mice. Circ. Res. 1999;85:1192–1198. doi: 10.1161/01.res.85.12.1192. [DOI] [PubMed] [Google Scholar]
- 27.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cromheeke KM, Kockx MM, De Meyer GR, Bosmans JM, Bult H, Beelaerts WJ, Vrints CJ, Herman AG. Inducible nitric oxide synthase colocalizes with signs of lipid oxidation/peroxidation in human atherosclerotic plaques. Cardiovasc. Res. 1999;43:744–754. doi: 10.1016/s0008-6363(99)00148-0. [DOI] [PubMed] [Google Scholar]
- 29.da SJ, Pierrat B, Mary JL, Lesslauer W. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J. Biol. Chem. 1997;272:28373–28380. doi: 10.1074/jbc.272.45.28373. [DOI] [PubMed] [Google Scholar]
- 30.de Vera ME, Shapiro RA, Nussler AK, Mudgett JS, Simmons RL, Morris SM, Jr, BILLIAR TR, Geller DA. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: Initial analysis of the human NOS2 promoter. Proc. Natl. Acad. Sci. USA. 1996;93:1054–1059. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denning MF, Dlugosz AA, Threadgill DW, Magnuson T, Yuspa SH. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C delta. J Biol Chem. 1996;271:5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 32.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 33.Detmers PA, Hernandez M, Mudgett J, Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock J, Weikel R, Bergstrom JD, Shevell DE, Hermanowski-Vosatka A, Sparrow CP, Chao YS, Rader DJ, Wright SD, Pure E. Deficiency in Inducible Nitric Oxide Synthase Results in Reduced Atherosclerosis in Apolipoprotein E-Deficient Mice. J Immunol. 2000;165:3430–3435. doi: 10.4049/jimmunol.165.6.3430. [DOI] [PubMed] [Google Scholar]
- 34.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San MA, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 35.Doi M, Shichiri M, Katsuyama K, Marumo F, Hirata Y. Cytokine-activated p42/p44 MAP kinase is involved in inducible nitric oxide synthase gene expression independent from NF-kappaB activation in vascular smooth muscle cells. Hypertens. Res. 2000;23:659–667. doi: 10.1291/hypres.23.659. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly TE, Jr, Pelling JC, Anderson CL, Dalbey D. Benzoyl peroxide activation of protein kinase C activity in epidermal cell membranes. Carcinogenesis. 1987;8:1871–1874. doi: 10.1093/carcin/8.12.1871. [DOI] [PubMed] [Google Scholar]
- 37.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp. Pharmacol Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eberhardt W, Kunz D, Hummel R, Pfeilschifter J. Molecular Cloning of the Rat Inducible Nitric Oxide Synthase Gene Promoter. Biochem. Biophys. Res. Commun. 1996;223:752–756. doi: 10.1006/bbrc.1996.0968. [DOI] [PubMed] [Google Scholar]
- 39.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Fan CY, Katsuyama M, Yabe-Nishimura C. PKCdelta mediates up-regulation of NOX1, a catalytic subunit of NADPH oxidase, via transactivation of the EGF receptor: possible involvement of PKCdelta in vascular hypertrophy. Biochem J. 2005;390:761–767. doi: 10.1042/BJ20050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finder JD, Litz JL, Blaskovich MA, McGuire TF, Qian Y, Hamilton AD, Davies P, Sebti SM. Inhibition of protein geranylgeranylation causes a superinduction of nitric-oxide synthase-2 by interleukin-1beta in vascular smooth muscle cells. J Biol Chem. 1997;272:13484–13488. doi: 10.1074/jbc.272.21.13484. [DOI] [PubMed] [Google Scholar]
- 42.Fries DM, Paxinou E, Themistocleous M, Swanberg E, Griendling KK, Salvemini D, Slot JW, Heijnen HFG, Hazen SL, Ischiropoulos H. Expression of Inducible Nitric-oxide Synthase and Intracellular Protein Tyrosine Nitration in Vascular Smooth Muscle Cells: ROLE OF REACTIVE OXYGEN SPECIES. J. Biol.Chem. 2003;278:22901. doi: 10.1074/jbc.M210806200. [DOI] [PubMed] [Google Scholar]
- 43.Ganster RW, Geller DA. Molecular regulation of inducible nitric oxide synthase. In: Ignarro LJ, editor. Nitric Oxide Biology and Pathobiology. Academic Press; 2000. pp. 129–156. [Google Scholar]
- 44.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins Homologous to p47phox and p67phox Support Superoxide Production by NAD(P)H Oxidase 1 in Colon Epithelial Cells. J. Biol. Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 45.Geller DA, Lowenstein CJ, Shapiro RA, Nussler AK, Silvio MD, Wang SC, Nakayama DK, Simmons RL, Snyder SH, Billiar TR. Molecular Cloning and Expression of Inducible Nitric Oxide Synthase from Human Hepatocytes. Proc. Natl.Acad. Sci. USA. 1993;90:3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginnan R, Singer HA. PKC-delta-dependent pathways contribute to PDGF-stimulated ERK1/2 activation in vascular smooth muscle. Am. J. Physiol Cell Physiol. 2005;288:C1193–C1201. doi: 10.1152/ajpcell.00499.2004. [DOI] [PubMed] [Google Scholar]
- 47.Ginnan R, Guikema BJ, Singer HA, Jourd'heuil D. PKC-{delta} mediates activation of ERK1/2 and induction of iNOS by IL-1beta in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C1583–C1591. doi: 10.1152/ajpcell.00390.2005. [DOI] [PubMed] [Google Scholar]
- 48.Goetze S, Kintscher U, Kaneshiro K, Meehan WP, Collins A, Fleck E, Hsueh WA, Law RE. TNFalpha induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases 1/2. Atherosclerosis. 2001;159:93–101. doi: 10.1016/s0021-9150(01)00497-x. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Fernandez F, Lopez-Farre A, Rodriguez-Feo JA, Farre J, Guerra J, Fortes J, Millas I, Garcia-Duran M, Rico L, Mata P, de Miguel LS, Casado S. Expression of inducible nitric oxide synthase after endothelial denudation of the rat carotid artery: role of platelets. Circ Res. 1998;83:1080–1087. doi: 10.1161/01.res.83.11.1080. [DOI] [PubMed] [Google Scholar]
- 50.Gopalakrishna R, Anderson WB. Susceptibility of protein kinase C to oxidative inactivation: loss of both phosphotransferase activity and phorbol diester binding. FEBS Lett. 1987;225:233–237. doi: 10.1016/0014-5793(87)81164-x. [DOI] [PubMed] [Google Scholar]
- 51.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc. Natl.Acad Sci U. S. A. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gopalakrishna R, Anderson WB. Reversible oxidative activation and inactivation of protein kinase C by the mitogen/tumor promoter periodate. Arch. Biochem Biophys. 1991;285:382–387. doi: 10.1016/0003-9861(91)90377-u. [DOI] [PubMed] [Google Scholar]
- 53.Gopalakrishna R, Chen ZH, Gundimeda U. Selenocompounds induce a redox modulation of protein kinase C in the cell, compartmentally independent from cytosolic glutathione: its role in inhibition of tumor promotion. Arch. Biochem Biophys. 1997;348:37–48. doi: 10.1006/abbi.1997.0335. [DOI] [PubMed] [Google Scholar]
- 54.Gopalakrishna R, Chen ZH, Gundimeda U. Modifications of cysteine-rich regions in protein kinase C induced by oxidant tumor promoters and enzyme-specific inhibitors. Methods Enzymol. 1995;252:132–146. doi: 10.1016/0076-6879(95)52016-3. [DOI] [PubMed] [Google Scholar]
- 55.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic.Biol. Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 56.Gorzalczany Y, Alloul N, Sigal N, Weinbaum C, Pick EA. Prenylated p67phox-Rac1 Chimera Elicits NADPH-dependent Superoxide Production by Phagocyte Membranes in the Absence of an Activator and of p47phox. CONVERSION OF A PAGAN NADPH OXIDASE TO MONOTHEISM. J. Biol. Chem. 2002;277:18605–18610. doi: 10.1074/jbc.M202114200. [DOI] [PubMed] [Google Scholar]
- 57.Grange L, Nguyen MV, Lardy B, Derouazi M, Campion Y, Trocme C, Paclet MH, Gaudin P, Morel F. NAD(P)H oxidase activity of Nox4 in chondrocytes is both inducible and involved in collagenase expression. Antioxid. Redox. Signal. 2006;8:1485–1496. doi: 10.1089/ars.2006.8.1485. [DOI] [PubMed] [Google Scholar]
- 58.Gross SS, Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine- induced nitric oxide generation by vascular smooth muscle. J. Biol. Chem. 1992;267:25722–25729. [PubMed] [Google Scholar]
- 59.Guikema B, Ginnan R, Singer HA, Jourd'heuil D. The NADPH oxidase homologs Nox1 and Nox4 attenuate interleukin-1 beta induced niric oxide synthase expression in vascular smooth muscle cells (abstract). Free Radic. Biol. Med. 2005;39 [Google Scholar]
- 60.Guikema BJ, Ginnan R, Singer HA, Jourd'heuil D. Catalase potentiates interleukin-1beta-induced expression of nitric oxide synthase in rat vascular smooth muscle cells. Free Radic. Biol. Med. 2005;38:597–605. doi: 10.1016/j.freeradbiomed.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 61.Hansson GK, Geng YJ, Holm J, Hardhammar P, Wennmalm A, Jennische E. Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. J Exp. Med. 1994;180:733–738. doi: 10.1084/jem.180.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harmon KJ, Couper LL, Lindner V. Strain-Dependent Vascular Remodeling Phenotypes in Inbred Mice. Am J Pathol. 2000;156:1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hecker M, Cattaruzza M, Wagner AH. Regulation of inducible nitric oxide synthase gene expression in vascular smooth muscle cells. Gen. Pharmac. 1999;32:9–16. doi: 10.1016/s0306-3623(98)00082-2. [DOI] [PubMed] [Google Scholar]
- 64.Hecker M, Preiss C, Schini-Kerth VB, Busse R. Antioxidants differentially affect nuclear factor kappa B-mediated nitric oxide synthase expression in vascular smooth muscle cells. FEBS Lett. 1996;380:224–228. doi: 10.1016/0014-5793(96)00046-4. [DOI] [PubMed] [Google Scholar]
- 65.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 66.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 67.Honbou K, Minakami R, Yuzawa S, Takeya R, Suzuki NN, Kamakura S, Sumimoto H, Inagaki F. Full-length p40phox structure suggests a basis for regulation mechanism of its membrane binding. EMBO J. 2007;26:1176–1186. doi: 10.1038/sj.emboj.7601561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hua LL, Zhao ML, Cosenza M, Kim MO, Huang H, Tanowitz HB, Brosnan CF, Lee SC. Role of mitogen-activated protein kinases in inducible nitric oxide synthase and TNFalpha expression in human fetal astrocytes. J. Neuroimmunol. 2002;126:180–189. doi: 10.1016/s0165-5728(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 69.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic.Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 70.Jiang B, Brecher P. N-Acetyl-L-cysteine potentiates interleukin-1beta induction of nitric oxide synthase : role of p44/42 mitogen-activated protein kinases. Hypertension. 2000;35:914–918. doi: 10.1161/01.hyp.35.4.914. [DOI] [PubMed] [Google Scholar]
- 71.Jiang B, Brecher P, Cohen RA. Persistent activation of nuclear factor-kappaB by interleukin-1beta and subsequent inducible NO synthase expression requires extracellular signal-regulated kinase. Arterioscler. Thromb. Vasc. Biol. 2001;21:1915–1920. doi: 10.1161/hq1201.099424. [DOI] [PubMed] [Google Scholar]
- 72.Jiang B, Xu S, Brecher P, Cohen RA. Growth factors enhance interleukin-1 beta-induced persistent activation of nuclear factor-kappa B in rat vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2002;22:1811–1816. doi: 10.1161/01.atv.0000037679.60584.3f. [DOI] [PubMed] [Google Scholar]
- 73.Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohenx RA. Temporal control of NF-kappaB activation by ERK differentially regulates interleukin-1beta-induced gene expression. J. Biol. Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 74.Jiang B, Xu S, Hou X, Pimentel DR, Cohen RA. Angiotensin II differentially regulates interleukin-1-beta-inducible NO synthase (iNOS) and vascular cell adhesion molecule-1 (VCAM-1) expression: role of p38 MAPK. J Biol Chem. 2004;279:20363–20368. doi: 10.1074/jbc.M314172200. [DOI] [PubMed] [Google Scholar]
- 75.Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal Control of NF-{kappa}B Activation by ERK Differentially Regulates Interleukin-1{beta}- induced Gene Expression. J. Biol. Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 76.Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) Receptor-Associated Kinase-Dependent IL-1-Induced Signaling Complexes Phosphorylate TAK1 and TAB2 at the Plasma Membrane and Activate TAK1 in the Cytosol. Mol. Cell. Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones RJ, Jourd'heuil D, Salerno JC, Smith SME, Singer HA. iNOS regulation by calcium/calmodulin-dependent protein kinase II in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2634–H2642. doi: 10.1152/ajpheart.01247.2006. [DOI] [PubMed] [Google Scholar]
- 78.Jourd'heuil D, Morise Z, Conner EM, Kurose I, Grisham MB. Oxidant-regulation of gene expression in the chronically inflamed intestine. Keio J. Med. 1997;46:10–15. doi: 10.2302/kjm.46.10. [DOI] [PubMed] [Google Scholar]
- 79.Kalinina N, Agrotis A, Tararak E, Antropova Y, Kanellakis P, Ilyinskaya O, Quinn MT, Smirnov V, Bobik A. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2002;22:2037–2043. doi: 10.1161/01.atv.0000040222.02255.0f. [DOI] [PubMed] [Google Scholar]
- 80.Katsuyama M, Fan C, Yabe-Nishimura C. NADPH oxidase is involved in prostaglandin F2alpha-induced hypertrophy of vascular smooth muscle cells: induction of NOX1 by PGF2alpha. J Biol Chem. 2002;277:13438–13442. doi: 10.1074/jbc.M111634200. [DOI] [PubMed] [Google Scholar]
- 81.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point Mutations in the Proline-rich Region of p22phox Are Dominant Inhibitors of Nox1- and Nox2-dependent Reactive Oxygen Generation. J. Biol. Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 82.Kawamura A, Baitsch D, Telgmann R, Feuerborn R, Weissen-Plenz G, Hagedorn C, Saku K, Brand-Herrmann SM, von Eckardstein A, Assmann G, Nofer JR. Apolipoprotein E Interrupts Interleukin-1{beta} Signaling in Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2007;27:1610–1617. doi: 10.1161/ATVBAHA.106.129957. [DOI] [PubMed] [Google Scholar]
- 83.Kennedy S, Preston AA, McPhaden AR, Miller AM, Wainwright CL, Wadsworth RM. Correlation of changes in nitric oxide synthase, superoxide dismutase and nitrotyrosine with endothelial regeneration and neointimal hyperplasia in the ballooninjured rabbit subclavian artery. Coron. Artery Dis. 2004;15:337–346. doi: 10.1097/00019501-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 84.Kibbe M, Billiar T, Tzeng E. Inducible nitric oxide synthase and vascular injury. Cardiovasc. Res. 1999;43:650–657. doi: 10.1016/s0008-6363(99)00130-3. [DOI] [PubMed] [Google Scholar]
- 85.Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS-/-Apoe-/- mice are ameliorated by enalapril treatment. J. Clin. Invest. 2000;105:451–458. doi: 10.1172/JCI8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koglin Jo, Glysing-Jensen T, Mudgett JS, Russell ME. Exacerbated Transplant Arteriosclerosis in Inducible Nitric OxideûDeficient Mice. Circulation. 1998;97:2059–2065. doi: 10.1161/01.cir.97.20.2059. [DOI] [PubMed] [Google Scholar]
- 87.Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, Ohmae K, Kikkawa U, Nishizuka Y. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc. Natl. Acad. Sci.USA. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Korn SH, Wouters EFM, Vos N, Janssen-Heininger YMW. Cytokine-induced Activation of Nuclear Factor-kappa B Is Inhibited by Hydrogen Peroxide through Oxidative Inactivation of Ikappa B Kinase. J. Biol. Chem. 2001;276:35693–35700. doi: 10.1074/jbc.M104321200. [DOI] [PubMed] [Google Scholar]
- 89.Kronfeld I, Kazimirsky G, Lorenzo PS, Garfield SH, Blumberg PM, Brodie C. Phosphorylation of protein kinase Cdelta on distinct tyrosine residues regulates specific cellular functions. J Biol Chem. 2000;275:35491–35498. doi: 10.1074/jbc.M005991200. [DOI] [PubMed] [Google Scholar]
- 90.Kubes P. Inducible nitric oxide synthase: a little bit of good in all of us. Gut. 2000;47:6–9. doi: 10.1136/gut.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuhel DG, Zhu B, Witte DP, Hui DY. Distinction in Genetic Determinants for Injury-Induced Neointimal Hyperplasia and Diet-Induced Atherosclerosis in Inbred Mice. Arterioscler Thromb Vasc Biol. 2002;22:955–960. doi: 10.1161/01.atv.0000017994.77066.75. [DOI] [PubMed] [Google Scholar]
- 93.Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation. 2001;103:3099–3104. doi: 10.1161/01.cir.103.25.3099. [DOI] [PubMed] [Google Scholar]
- 94.Kumar S, Avraham S, Bharti A, Goyal J, Pandey P, Kharbanda S. Negative regulation of PYK2/related adhesion focal tyrosine kinase signal transduction by hematopoietic tyrosine phosphatase SHPTP1. J. Biol. Chem. 1999;274:30657–30663. doi: 10.1074/jbc.274.43.30657. [DOI] [PubMed] [Google Scholar]
- 95.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 96.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LaPointe MC, Isenovic E. Interleukin-1beta regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33:276–282. doi: 10.1161/01.hyp.33.1.276. [DOI] [PubMed] [Google Scholar]
- 98.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91phox homologues in vascular smooth muscle cells. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 99.Lee FS, Peters RT, Dang LC, Maniatis T. MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leto TL, Adams AG, de Mendez I. Assembly of the Phagocyte NADPH Oxidase: Binding of Src Homology 3 Domains to Proline-Rich Targets. Proc. Natl. Acad. Sci. USA. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ling L, Cao Z, Goeddel DV. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc. Natl. Acad Sci U. S. A. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J, Ormsby A, Oja-Tebbe N, Pagano PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res. 2004;95:587–594. doi: 10.1161/01.RES.0000142317.88591.e6. [DOI] [PubMed] [Google Scholar]
- 104.Lo YY, Luo L, McCulloch CA, Cruz TF. Requirements of focal adhesions and calcium fluxes for interleukin-1-induced ERK kinase activation and c-fos expression in fibroblasts. J. Biol. Chem. 1998;273:7059–7065. doi: 10.1074/jbc.273.12.7059. [DOI] [PubMed] [Google Scholar]
- 105.Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage Nitric Oxide Synthase Gene: Two Upstream Regions Mediate Induction by Interferon {gamma} and Lipopolysaccharide. Proc. Natl. Acad.Sci. USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luoma JS, Yla-Herttuala S. Expression of inducible nitric oxide synthase in macrophages and smooth muscle cells in various types of human atherosclerotic lesions. Virchows Arch. 1999;434:561–568. doi: 10.1007/s004280050384. [DOI] [PubMed] [Google Scholar]
- 107.MacGillivray M, Herrera-Abreu MT, Chow CW, Shek C, Wang Q, Vachon E, Feng GS, Siminovitch KA, McCulloch CA, Downey GP. The protein tyrosine phosphatase SHP-2 regulates interleukin-1-induced ERK activation in fibroblasts. J. Biol. Chem. 2003;278:27190–27198. doi: 10.1074/jbc.M213083200. [DOI] [PubMed] [Google Scholar]
- 108.MacGillivray MK, Cruz TF, McCulloch CA. The recruitment of the interleukin-1 (IL-1) receptor-associated kinase (IRAK) into focal adhesion complexes is required for IL-1beta -induced ERK activation. J. Biol. Chem. 2000;275:23509–23515. doi: 10.1074/jbc.M003186200. [DOI] [PubMed] [Google Scholar]
- 109.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cellular Signalling. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 110.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 111.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MAR, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HHHW, Weissmann N. Hypoxia-Dependent Regulation of Nonphagocytic NADPH Oxidase Subunit NOX4 in the Pulmonary Vasculature. Circul. Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 112.Miyoshi T, Li Y, Shih DM, Wang X, Laubach VE, Matsumoto AH, Helm GA, Lusis AJ, Shi W. Deficiency of inducible NO synthase reduces advanced but not early atherosclerosis in apolipoprotein E-deficient mice. Life Sci. 2006;79:525–531. doi: 10.1016/j.lfs.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 113.Moe KT, Aulia S, Jiang F, Chua YL, Koh TH, Wong MC, Dusting GJ. Differential upregulation of Nox homologues of NADPH oxidase by tumor necrosis factor-alpha in human aortic smooth muscle and embryonic kidney cells. J. Cell. Mol.Med. 2006;10:231–239. doi: 10.1111/j.1582-4934.2006.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide:physiology,pathophysiology,and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 115.Moore ZWQ, Hui DY. Apolipoprotein E inhibition of vascular hyperplasia and neointima formation requires inducible nitric oxide synthase. J. Lipid Res. 2005;46:2083–2090. doi: 10.1194/jlr.M500177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakata S, Tsutsui M, Shimokawa H, Tamura M, Tasaki H, Morishita T, Suda O, Ueno S, Toyohira Y, Nakashima Y, Yanagihara N. Vascular Neuronal NO Synthase Is Selectively Upregulated by Platelet-Derived Growth Factor: Involvement of the MEK/ERK Pathway. Arterioscler Thromb Vasc Biol. 2005;25:2502–2508. doi: 10.1161/01.ATV.0000190663.88143.97. [DOI] [PubMed] [Google Scholar]
- 117.Nakayama I, Kawahara Y, Tsuda T, Okuda M, Yokoyama M. Angiotensin II inhibits cytokine-stimulated inducible nitric oxide synthase expression in vascular smooth muscle cells. J Biol Chem. 1994;269:11628–11633. [PubMed] [Google Scholar]
- 118.Navarro-Lerida I, Corvi MM, Barrientos AA, Gavilanes F, Berthiaume LG, Rodriguez-Crespo I. Palmitoylation of Inducible Nitric-oxide Synthase at Cys-3 Is Required for Proper Intracellular Traffic and Nitric Oxide Synthesis. J. Biol. Chem. 2004;279:55682–55689. doi: 10.1074/jbc.M406621200. [DOI] [PubMed] [Google Scholar]
- 119.Navarro-Lerida I, varez-Barrientos A, Rodriguez-Crespo I. N-terminal palmitoylation within the appropriate amino acid environment conveys on NOS2 the ability to progress along the intracellular sorting pathways. J. Cell. Sci. 2006;119:1558–1569. doi: 10.1242/jcs.02878. [DOI] [PubMed] [Google Scholar]
- 120.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 121.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 122.Niu XL, Yang X, Hoshiai K, Tanaka K, Sawamura S, Koga Y, Nakazawa H. Inducible Nitric Oxide Synthase Deficiency Does Not Affect the Susceptibility of Mice to Atherosclerosis but Increases Collagen Content in Lesions. Circulation. 2001;103:1115–1120. doi: 10.1161/01.cir.103.8.1115. [DOI] [PubMed] [Google Scholar]
- 123.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 124.Papadaki M, Tilton RG, Eskin SG, McIntire LV. Nitric oxide production by cultured human aortic smooth muscle cells: stimulation by fluid flow. Am J Physiol Heart Circ Physiol. 1998;274:H616–H626. doi: 10.1152/ajpheart.1998.274.2.h616. [DOI] [PubMed] [Google Scholar]
- 125.Patel DN, Bailey SR, Gresham JK, Schuchman DB, Shelhamer JH, Goldstein BJ, Foxwell BM, Stemerman MB, Maranchie JK, Valente AJ. TLR4-NOX4-AP-1 signaling mediates lipopolysaccharide-induced CXCR6 expression in human aortic smooth muscle cells. Biochemical and Biophysical Research Communications. 2006;347:1113–1120. doi: 10.1016/j.bbrc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 126.Radomski MW, Palmer RMJ, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. U. S A. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rainer dM, Hoeth M, Hofer-Warbinek R, Schmid JA. The Transcription Factor NF-{kappa}B and the Regulation of Vascular Cell Function. Arterioscler Thromb Vasc Biol. 2000;20:e83–e88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 128.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2 and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 129.Rodriguez-Pascual F, Hausding M, Ihrig-Biedert I, Furneaux H, Levy AP, Forstermann U, Kleinert H. Complex contribution of the 3′-untranslated region to the expressional regulation of the human inducible nitric-oxide synthase gene. Involvement of the RNA-binding protein HuR. J Biol Chem. 2000;275:26040–26049. doi: 10.1074/jbc.M910460199. [DOI] [PubMed] [Google Scholar]
- 130.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 131.Sangsree S, Brovkovych V, Minshall RD, Skidgel RA. Kininase I-type carboxypeptidases enhance nitric oxide production in endothelial cells by generating bradykinin B1 receptor agonists. Am J Physiol Heart Circ Physiol. 2003;284:H1959–H1968. doi: 10.1152/ajpheart.00036.2003. [DOI] [PubMed] [Google Scholar]
- 132.Santilli SM, Tretinyak AS, Lee ES. Transarterial wall oxygen gradients at the deployment site of an intra-arterial stent in the rabbit. Am J Physiol Heart Circ Physiol. 2000;279:H1518–H1525. doi: 10.1152/ajpheart.2000.279.4.H1518. [DOI] [PubMed] [Google Scholar]
- 133.Sarfstein R, Gorzalczany Y, Mizrahi A, Berdichevsky Y, Molshanski-Mor S, Weinbaum C, Hirshberg M, Dagher MC, Pick E. Dual Role of Rac in the Assembly of NADPH Oxidase, Tethering to the Membrane and Activation of p67phox: A STUDY BASED ON MUTAGENESIS OF p67phox-Rac1 CHIMERAS. J. Biol. Chem. 2004;279:16007–16016. doi: 10.1074/jbc.M312394200. [DOI] [PubMed] [Google Scholar]
- 134.Sasaki K, Murata M, Yasumoto T, Mieskes G, Takai A. Affinity of okadaic acid to type-1 and type-2A protein phosphatases is markedly reduced by oxidation of its 27- hydroxyl group. Biochem J. 1994;298(Pt 2):259–262. doi: 10.1042/bj2980259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schwartz SM. Perspectives series: cell adhesion in vascular biology. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;99:2814–2816. doi: 10.1172/JCI119472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 138.Shinohara H, Yasuda T, Aiba Y, Sanjo H, Hamadate M, Watarai H, Sakurai H, Kurosaki T. PKC{beta} regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J. Exp. Med. 2005;202:1423–1431. doi: 10.1084/jem.20051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sirsjo A, Lofving A, Hansson GK, Wagsater D, Tokuno S, Valen G. Deficiency of nitric oxide synthase 2 results in increased neointima formation in a mouse model of vascular injury. J Cardiovasc. Pharmacol. 2003;41:897–902. doi: 10.1097/00005344-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 140.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 141.Spitsin SV, Koprowski H, Michaels FH. Characterization and functional analysis of the human inducible nitric oxide synthase gene promoter. Mol. Med. 1996;2:226–235. [PMC free article] [PubMed] [Google Scholar]
- 142.Srivastava J, Goris J, Dilworth SM, Parker PJ. Dephosphorylation of PKCdelta by protein phosphatase 2Ac and its inhibition by nucleotides. FEBS Lett. 2002;516:265–269. doi: 10.1016/s0014-5793(02)02500-0. [DOI] [PubMed] [Google Scholar]
- 143.Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem. J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on Mechanism and Catalytic Regulation in the NO Synthases. J. Biol. Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 145.Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1543–L1555. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- 146.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 147.Sumimoto H, Kage Y, Nunoi H, Sasaki H, Nose T, Fukumaki Y, Ohno M, Minakami S, Takeshige K. Role of Src Homology 3 Domains in Assembly and Activated of the Phagocyte NADPH Oxidase. Proc. Natl. Acad. Sci. USA. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun Z, Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 149.Szöcs K, Lassègue B, Sorescu D, Hilenski LL, Valppu L, Cousse TL, Wilcox JN, Quinn MT, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler. Thromb. Vasc. Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 150.Takaya T, Hirata Ki, Yamashita T, Shinohara M, Sasaki N, Inoue N, Yada T, Goto M, Fukatsu A, Hayashi T, Alp NJ, Channon KM, Yokoyama M, Kawashima S. A Specific Role for eNOS-Derived Reactive Oxygen Species in Atherosclerosis Progression. Arterioscler Thromb Vasc Biol. 2007;27:1632–1637. doi: 10.1161/ATVBAHA.107.142182. [DOI] [PubMed] [Google Scholar]
- 151.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel Human Homologues of p47phox and p67phox Participate in Activation of Superoxide-producing NADPH Oxidases. J. Biol. Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 152.Tannous M, Amin R, Popoff MR, Fiorentini C, Kowluru A. Positive modulation by Ras of interleukin-1beta-mediated nitric oxide generation in insulin-secreting clonal beta (HIT-T15) cells. Biochem Pharmacol. 2001;62:1459–1468. doi: 10.1016/s0006-2952(01)00818-8. [DOI] [PubMed] [Google Scholar]
- 153.Toker A. Signaling through protein kinase C. Front Biosci. 1998;3:D1134–D1147. doi: 10.2741/a350. [DOI] [PubMed] [Google Scholar]
- 154.Toledano MN, Leonard WJ. Modulation of transcription factor NF-kB binding activity by oxidation-reduction in vitro. Proc. Natl. Acad. Sci. USA. 1991;88:4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 156.Ushio-Fukai M, Maziar Zafari A, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 157.Vendrov AE, Madamanchi NR, Hakim ZS, Rojas M, Runge MS. Thrombin and NAD(P)H Oxidase-Mediated Regulation of CD44 and BMP4-Id Pathway in VSMC, Restenosis, and Atherosclerosis. Circul. Res. 2006;98:1254–1263. doi: 10.1161/01.RES.0000221214.37803.79. [DOI] [PubMed] [Google Scholar]
- 158.Wang Q, Downey GP, Herrera-Abreu MT, Kapus A, McCulloch CA. SHP-2 modulates interleukin-1-induced Ca2+ flux and ERK activation via phosphorylation of phospholipase Cgamma1. J. Biol. Chem. 2005;280:8397–8406. doi: 10.1074/jbc.M410462200. [DOI] [PubMed] [Google Scholar]
- 159.Ward ME, Toporsian M, Scott JA, Teoh H, Govindaraju V, Quan A, Wener AD, Wang G, Bevan SC, Newton DC, Marsden PA. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J. Clin. Invest. 2005;115:3128–3139. doi: 10.1172/JCI20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ward NE, Gravitt KR, O'Brian CA. Covalent modification of protein kinase C isozymes by the inactivating peptide substrate analog N-biotinyl-Arg-Arg-Arg-Cys-Leu-Arg-Arg-Leu. Evidence that the biotinylated peptide is an active-site affinity label. J Biol Chem. 1996;271:24193–24200. doi: 10.1074/jbc.271.39.24193. [DOI] [PubMed] [Google Scholar]
- 161.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 162.Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA. Expression of Multiple Isoforms of Nitric Oxide Synthase in Normal and Atherosclerotic Vessels. Arterioscler Thromb Vasc Biol. 1997;17:2479–2488. doi: 10.1161/01.atv.17.11.2479. [DOI] [PubMed] [Google Scholar]
- 163.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the reninangiotensin system in vitro and in vivo. Free Radic. Biol Med. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 164.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cell injury. Proc. Natl. Acad. Sci. USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calciumindependent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu S, Cobb MH. MEKK1 binds directly to the c-Jun N-terminal kinases/stress-activated protein kinases. J Biol Chem. 1997;272:32056–32060. doi: 10.1074/jbc.272.51.32056. [DOI] [PubMed] [Google Scholar]
- 167.Xu S, Robbins D, Frost J, Dang A, Lange-Carter C, Cobb MH. MEKK1 phosphorylates MEK1 and MEK2 but does not cause activation of mitogen-activated protein kinase. Proc. Natl. Acad Sci U. S. A. 1995;92:6808–6812. doi: 10.1073/pnas.92.15.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu S, Bayat H, Hou X, Jiang B. Ribosomal S6 kinase-1 modulates interleukin-1betainduced persistent activation of NF-{kappa}B through phosphorylation of I{kappa}Bbeta. Am J Physiol Cell Physiol. 2006;291:C1336–C1345. doi: 10.1152/ajpcell.00552.2005. [DOI] [PubMed] [Google Scholar]
- 169.Yamamoto A, Kami K, Takeya R, Sumimoto H. Interaction between the SH3 domains and C-terminal proline-rich region in NADPH oxidase organizer 1 (Noxo1) Biochem. Biophys. Res Commun. 2007;352:560–565. doi: 10.1016/j.bbrc.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 170.Yan Zq, Hansson GK. Overexpression of Inducible Nitric Oxide Synthase by Neointimal Smooth Muscle Cells. Circul. Res. 1998;82:21–29. doi: 10.1161/01.res.82.1.21. [DOI] [PubMed] [Google Scholar]
- 171.Yin MJ, Christerson LB, Yamamoto Y, Kwak YT, Xu S, Mercurio F, Barbosa M, Cobb MH, Gaynor RB. HTLV-I Tax protein binds to MEKK1 to stimulate IkappaB kinase activity and NF-kappaB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 172.Yogo K, Shimokawa H, Funakoshi H, Kandabashi T, Miyata K, Okamoto S, Egashira K, Huang P, Akaike T, Takeshita A. Different Vasculoprotective Roles of NO Synthase Isoforms in Vascular Lesion Formation in Mice. Arterioscler Thromb Vasc Biol. 2000;20:96e–100e. doi: 10.1161/01.atv.20.11.e96. [DOI] [PubMed] [Google Scholar]
- 173.Zhang H, Chen X, Teng X, Snead C, Catravas JD. Molecular Cloning and Analysis of the Rat Inducible Nitric Oxide Synthase Gene Promoter in Aortic Smooth Muscle Cells. Biochem. Pharmacol. 1998;55:1873–1880. doi: 10.1016/s0006-2952(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Y, Brovkovych V, Brovkovych S, Tan F, Lee BS, Sharma T, Skidgel RA. Dynamic receptor-dependent activation of inducible nitric-oxide synthase by ERK-mediated phosphorylation of Ser745. J Biol Chem. 2007;282:32453–32461. doi: 10.1074/jbc.M706242200. [DOI] [PubMed] [Google Scholar]


